Abstract
Refractory nephrotic syndrome continues to be a therapeutic challenge despite advances in immunosuppression and blockade of the renin–angiotensin–aldosterone cascade. Adrenocorticotropic hormone (ACTH), a pituitary neuroimmunoendocrine polypeptide, was widely used in the 1950s as an effective therapy for childhood nephrotic syndrome, but has since been replaced by synthetic glucocorticoid analogues. In addition to controlling steroidogenesis, ACTH also acts as an important physiological agonist of the melanocortin system. Clinical and experimental evidence now suggests that ACTH has antiproteinuric, lipid-lowering and renoprotective properties, which are not fully explained by its steroidogenic effects. ACTH therapy is effective in inducing remission of nephrotic syndrome in patients with a variety of proteinuric nephropathies, even those resistant to steroids and other immunosuppressants. This Perspectives article describes the biophysiology of ACTH, with emphasis on its melanocortin actions, particularly in renal parenchymal cells, which could potentially fulfill the therapeutic effects of ACTH in nephrotic glomerulopathies.
Introduction
Adrenocorticotropic hormone (ACTH), or corticotropin, is a pituitary polypeptide hormone consisting of 39 amino acids (ACTH1–39).1 It is an important component of the hypothalamic–pituitary–adrenal (HPA) axis and has a pivotal role in sustaining homeostasis of the neuroimmunoendocrine system.1 In the 1950s and early 1960s, ACTH was widely used for the treatment of childhood nephrotic syndrome.2–4 Subsequent clinical studies demonstrated that ACTH therapy induces remission of proteinuria in patients with nephrotic syndrome who were refractory to glucocorticoid and/or immunosuppressive therapies,5, 6 suggesting that ACTH has prominent antiproteinuric and renoprotective effects that are not entirely explained by its steroidogenic actions. Nevertheless, the exact mechanism underlying this antiproteinuric action remains largely unknown and the precise pathobiology underlying the function of ACTH as a renoprotective agent in patients with proteinuric nephropathies remains elusive. This Perspectives article presents a comprehensive overview of the biophysiology of ACTH, the effects of ACTH on proteinuric renal diseases, and the molecular mechanisms likely to be involved in its therapeutic action, with emphasis on the melanocortin effects. As a potent physiological agonist of melanocortin system that could directly target renal parenchymal cells, like podocytes, ACTH might serve as a promising therapy for nephrotic glomerulopathies.
Biophysiology of ACTH
The melanocortin system
ACTH is one of several physiologically active peptides (Figure 1a) that are synthesized by proteolytic cleavage from the precursor peptide pre-pro-opiomelanocortin (pre-POMC).1 As a major component of the HPA axis, ACTH is produced by corticotrophs in the anterior lobe of the pituitary gland.1 ACTH stimulates the adrenal cortex to produce cortisol in response to stress and, as such, transmits vital information from the brain to the rest of the mammalian body (Figure 1b). Besides governing steroidogenesis, ACTH is also an important physiological agonist of the melanocortin system.7, 8 This system comprises multiple components, including five class A guanine nucleotide-binding protein (G protein)-coupled melanocortin receptors (MCRs) MC1R–MC5R; peptide agonists derived from POMC; and endogenous antagonists (Table 1, Box 1).7, 8 The five MCRs each have a distinct tissue distribution, convey signaling of different melanocortins and exert varying biological activities (Table 1).8 Receptor-binding studies revealed that all five MCRs show a strong affinity for ACTH, thereby establishing the potential for this hormone to activate these receptors.9 MCRs are expressed in kidney cells,10–15 which indicates that the kidney is a target organ for the effects of ACTH.
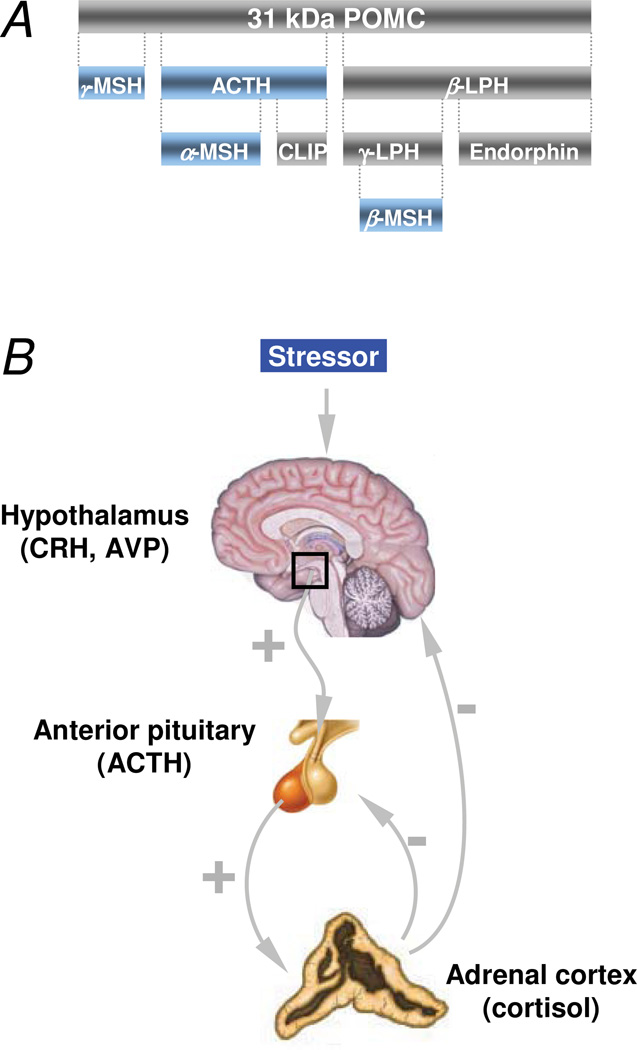
Figure 1.
Biosynthesis of ACTH and its regulation by the hypothalamic–pituitary–adrenal axis. a | ACTH is one of several physiologically active peptides that are biosynthesized from the precursor peptide pre-POMC by corticotrophs in the anterior lobe of the pituitary gland. POMC undergoes a series of post-translational modifications before it is proteolytically cleaved by prohormone convertase enzymes to yield a chemically and biogenetically related family of polypeptides with varying physiological activity, including ACTH and other natural melanocortins (blue), like α- MSH, β-MSH, γ-MSH, and LPH as well as CLIP. b | Stress induces synthesis and release of ACTH from corticotrophs in the anterior lobe of the pituitary gland through various hormones, including corticotropin-releasing hormone and arginine vasopressin, which are secreted by the hypothalamus. ACTH synthesis and release are controlled by slow, intermediate or fast negative feedback at multiple levels by multiple substances secreted within the HPA axis, including cortisol, which is released from the adrenal cortex following ACTH-mediated activation of the melanocortin 2 receptor (MC2R) on adrenocortical cells. Abbreviations: ACTH, adrenocorticotropic hormone; CLIP, corticotrophin-like intermediate peptide; HPA, hypothalamic–pituitary–adrenal; LPH, lipotropic hormone; MSH, melanocyte-stimulating hormone; POMC, pro-opiomelanocortin.
Table 1.
Sites of expression of melanocortin receptors and their biophysiological functions, agonists and antagonists
| Receptor | Major sites of expression |
Agonist preference and affinity |
Biological function | Antagonists |
|---|---|---|---|---|
| MC1R | Melanoma cells, melanocytes, skin glands, hair follicles, testes, Sertoli cells, immune and inflammatory cells, endothelial cells, kidney | ACTH = α-MSH > β-MSH > γ-MSH | Pigmentation, antipyresis, antimicrobial immunity, anti-inflammatory responses, Immunomodulation | Agouti signaling protein |
| MC2R | Adrenal gland (zona fasciculata and zona glomerulosa), adipocytes, kidney | ACTH only | Steroidogenesis | NA |
| MC3R | Brain, placenta, stomach, duodenum, pancreas, kidney | ACTH = α-MSH = β-MSH = γ-MSH | Energy homeostasis, modulation of feeding behavior | Agouti-related protein |
| MC4R | Brain, spinal cord, kidney | ACTH = α-MSH > β-MSH > γ-MSH | Energy homeostasis, appetite regulation, modulation of sexual behavior | Agouti-related protein, Agouti signaling protein |
| MC5R | Adrenal gland, adipose tissue, leukocytes, lung, lymph nodes, mammary glands, ovaries, testes, uterus, brain, skeletal muscle, exocrine tissues, kidney | ACTH = α-MSH > β-MSH > γ-MSH | Modulation of exocrine function, immunomodulation | Agouti-related protein |
Abbrevations: ACTH, adrenocorticotropic hormone; MSH, melanocyte stimulatory hormone.
Box 1: The melanocortin system.
The melanocortin system is a hormone system that has an integral role in the homeostatic control of a diverse array of physiological functions, including pigmentation, inflammation, immunomodulation, steroidogenesis, energy homeostasis, sexual function and exocrine secretion.
The melanocortin system is comprised of: the melanocortin peptide ligands derived from pro-opiomelanocortin, including melanocyte-stimulating hormone (α- MSH, β-MSH, γ-MSH) and ACTH; a family of five class A seven-transmembrane guanine protein-coupled melanocortin receptors, MC1R–MC5R; and the endogenous melanocortin antagonists: agouti signaling protein and agouti related protein.
The five MCRs have distinct tissue distribution, convey signaling of different melanocortins and exert varying biological activities.
Despite discrepancies, multiple types of MCR have been detected in kidney cells, including podocytes, tubular epithelial cells and endothelial cells, underscoring kidney as a direct effector organ of ACTH and other melanocortin peptides.
Melanocortins, including ACTH and α-MSH, have been reproducibly shown to have kidney protective effects in acute kidney injury, chronic kidney disease and proteinuric glomerulopathies.
Abbreviations: ACTH, adrenocorticotropic hormone; MCR, melanocortin receptor; MSH, melanocyte stimulatory hormone
Physiological function of ACTH
In addition to the adrenal effects of ACTH, that is, its steroidogenic and adrenotropic activities, ACTH possesses a multifaceted extra-adrenal action that is mediated by the different MCRs present in the peripheral tissues and central nervous system. ACTH has a lipostatic effect and stimulates lipolysis.16 Indeed, ACTH deficiency leads to visceral obesity and causes conversion of brown adipose tissue to white adipose tissue.16 Moreover, ACTH administration to both healthy humans and patients with dyslipidemia lowers the levels of plasma lipids, including total cholesterol, LDL-cholesterol, phospholipids and triglycerides,17, 18 which suggests a lipid-lowering effect. Additionally, administration of the complete ACTH molecule (ACTH1–39) produces a marked and rapid increase in the levels of plasma insulin,19 which is consistent with an insulin secretagogue effect. This insulinotropic action is exerted by corticotrophin-like intermediate peptide (CLIP), also known as the ACTH18–39 fragment (Figure 1a).19 ACTH also has various other peripheral endocrine activities: regulation of skin and hair pigmentation, modulation of sebaceous gland function, anti-inflammatory and immunomodulatory functions (Table 1).
Synthetic versus natural ACTH
Currently, two products that contain ACTH are commercially available. The first is an ACTH gel (HP Acthar Gel®, Questcor Pharmaceuticals, NY, USA), which is a proprietary mixture isolated from porcine pituitary extracts. The major component of this gel is ACTH1–39, which is likely to be the active principle, but it also contains a number of other potentially bioactive POMC-derived peptides (personal communication with Questcor). ACTH gel is the only long-acting ACTH product available in the USA and is approved by the FDA to induce diuresis or remission of proteinuria in patients with nephrotic syndrome that is not associated with either idiopathic uremia or systemic lupus erythematosus. The second product is a synthetic, truncated ACTH analogue, referred to as tetracosactide or tetracopeptide, which consists of the first 24 amino acids1 of the native hormone (ACTH1–24). This synthetic analogue is available as a short-acting formulation (cosyntropin) that is used solely to assess adrenal gland function. Outside the USA, ACTH1–24 is also available in a long-acting formulation (synacthen). Although synthetic ACTH1–24 has often been presumed to possesses the same therapeutic profile as ACTH gel, a growing body of evidence suggests that these synthetic analogues are different from natural ACTH1–39 in terms of pharmacokinetics, pharmacodynamics and physiologic actions.20, 21 For example, the β-cell tropic and insulinotropic effect of the endogenous hormone resides in the C-terminus of ACTH1–39,19 which is omitted from the synthetic analogue but is retained in ACTH gel. Moreover, other pharmacologically active molecules derived from POMC, including other melanocortin peptides, might also exist in the naturally derived ACTH gel but be absent from the synthetic formulation and this warrants further investigation.
Therapeutic effects of ACTH
Preclinical evidence
The renoprotective effect of melanocortins on the kidney, in particular α-melanocyte-stimulating hormone (MSH) (Box 1), has been reproducibly demonstrated in various animal models of acute kidney injury (AKI),22 including ischemic reperfusion injury,23 renal toxic effects24 and ureteral obstruction25. Indeed, my research group’s work indicates that pretreatment with ACTH gel could substantially attenuate tumor necrosis factor (TNF)-induced AKI in rats.26 Even at low doses, ACTH gel pretreatment normalized systemic and renal hemodynamics, significantly improved kidney function and increased survival. Renal histological injury was also prevented by ACTH gel.26 Mechanistically, the beneficial effect of ACTH gel was associated with a reduction in renal inflammation and apoptosis—two important pathogenetic processes that are involved in AKI.26 Thus, ACTH gel might represent a novel therapeutic strategy to prevent or treat AKI.
In animal models of chronic kidney disease (CKD), ACTH also has a potent role in controlling proteinuria, ameliorating histological signs of renal injury and retarding the progression of kidney dysfunction. For example, rats with passive Heymann nephritis treated with synthetic ACTH1–24 show a significant reduction in proteinuria, which correlated with improved glomerular morphology and podocyte ultrastructure.10 Our group has consistently demonstrated evidence for a direct protective effect of ACTH gel on podocytes in subtotally nephrectomized rats—a standard model of secondary focal segmental glomerulosclerosis (FSGS) and progressive CKD.11 After subtotal renal ablation, these rats received ACTH gel every other day for 5 weeks. This treatment markedly diminished urinary protein excretion (by ~50%) and preserved kidney function as measured by increased renal plasma flow, improved insulin clearance rate and reduced serum creatinine levels.11 Histologically, glomerulosclerosis and tubulointerstitial fibrosis, renal inflammation, tubular atrophy, and tubular epithelial–mesenchymal transdifferentiation all improved.11 As glucocorticoid therapy exacerbates proteinuria and glomerulosclerosis in this model,27 these beneficial effects of ACTH gel are unlikely to result from steroidogenesis. Instead, my research group found evidence of direct protective effects of ACTH gel treatment on podocytes in the remnant glomeruli. These effects included notable reductions in foot-process effacement and podocyte apoptosis, and amelioration of the decline in glomerular expression of podocyte markers, including vimentin, nephrin, podocin and the transcription factor WT1.11 These findings suggest that ACTH gel has a direct protective activity on podocytes in progressive glomerulopathies. Overall, therefore, these preclinical studies indicate that ACTH has a kidney-protective effect in experimental models of AKI and CKD.
Clinical observations
Several studies have demonstrated that ACTH gel and ACTH1–24 improve proteinuria in adult patients with nephrotic syndrome owing to proteinuric nephropathies, including idiopathic membranous nephropathy (IMN).5, 6,10, 18, 28–33 The antiproteinuric effect of ACTH1–24 treatment was highlighted in a dose-escalation study conducted in adults with nephrotic syndrome owing to IMN. The optimal dose was estimated to be 1 mg twice per week, taking both the therapeutic effects and the modest side effects of ACTH into consideration.8 Patients on ACTH1–24 therapy attained significant improvements in serum lipoprotein profiles, urine protein excretion and glomerular function. An open-label, prospective, randomized, controlled trial has been conducted to assess the efficacy and safety of a 12-month course of synthetic ACTH1–24 therapy in 32 patients with nephrotic syndrome.30 The ACTH1–24 therapy was compared with methylprednisolone alternated with a cytotoxic agent (chlorambucil or cytoxan) in patients with biopsy-proven IMN. Comparable response rates were observed: 75% of patients treated with the immunosuppressive regimen and 83% of ACTH1–24-treated patients experienced complete or partial remission of nephrotic syndrome, and both groups had a comparable magnitude of dyslipidemia correction. In an observational study, sustained remission of nephrotic syndrome was found in five patients with IMN treated with ACTH1–24 for 12 months with a follow-up period of up to 26 months.32 The results of a study published as an abstract in 2009 showed that ACTH-induced remission of proteinuria in patients with IMN was associated with a reduction in the levels of autoantibody against M-type phospholipase A2 receptor (PLA2R),34 which is a major target antigen in this disease.35 Striking antiproteinuric effects of ACTH1–24 have also been found in patients with nephrotic kidney diseases other than IMN, including FSGS, proliferative glomerulonephritis and hereditary nephropathy,28 implying that the therapeutic effect of ACTH1–24 is not dependent on the type of glomerular injury, but is probably mediated by a general glomeruloprotective mechanism.
Similar antiproteinuric and renoprotective properties were also observed with ACTH gel in patients with various proteinuric nephropathies. One study included 21 patients with treatment-refractory nephrotic syndrome associated with a variety of diagnoses, including membranous nephropathy, membranoproliferative glomerulonephritis, FSGS, minimal change disease, lupus nephritis (class V) and IgA nephropathy.33 The most frequent treatment regimen was 80 IU of ACTH gel injected subcutaneously twice per week for 6 months. A total of 11 patients (52%) achieved complete or partial remission of proteinuria. Of note, 82% of the patients with membranous nephropathy achieved remission (27% complete remission and 55% partial remission), despite having previously failed to respond to a mean of 2.4 immunosuppressive treatment regimens, denoting that ACTH gel is a viable therapy for resistant nephrotic syndrome owing to membranous nephropathy.33
In summary, accumulating clinical evidence indicates that ACTH therapy has antiproteinuric, lipid-lowering and kidney-protective effects in patients with nephrotic syndrome caused by a variety of immune-mediated and nonimmune-mediated glomerulopathies. ACTH therapy could potentially attain remission of all nephrotic symptoms: reduction of urinary protein excretion,4–6, 18, 28–32 correction of dyslipidemia,18, 29, 30 induction of diuresis2, 4 and attenuation of edema.2–4 Most of these findings, however, were made in single-center, observational studies with small sample sizes and limited use of appropriate controls. In-depth, large-scale, randomized, multicenter studies are merited to verify these findings and to further define the exact mechanisms of action of ACTH therapy.
Adverse effects of ACTH therapy [H2]
Long-term (6–12 months) ACTH therapy is well tolerated in most patients with nephrotic syndrome. Noted adverse effects tend to be mild and are generally similar to those associated with glucocorticoid treatment.18, 29, 30 Most patients require potassium supplementation and some develop hypertension. Serious allergic reactions (anaphylaxis) to ACTH therapy are rare, but have occurred in patients treated with ACTH gel for asthma and allergic conditions36. Skin pigmentation (bronze coloration) has been noted in some patients treated with synthetic ACTH1–2418, 29, 30 and a few treated with ACTH gel (A. S. Bomback, personal communication). Although the results of ongoing studies will help to elucidate potential adverse events associated with prolonged use of ACTH, a case series published in 201113 reported that very few patients with nephrotic syndrome developed hyperglycemia (2 out of 21 patients), had significant weight gain or exhibited evidence of accelerated bone loss after ACTH gel therapy. In most cases, adverse effects were tolerable and completely disappeared after the cessation of therapy.
Renoprotective mechanisms of ACTH
ACTH was initially postulated to exert its beneficial effects indirectly, through its adrenal MC2R-mediated steroidogenic action,3 as administration of glucocorticoids sometimes also generate a similar remission of nephrotic syndrome. Emerging data, however, support the view that ACTH might have a protective effect on the kidney that is independent of its steroidogenic action. This notion is based on the observation that in glomerular diseases, including IMN, treatment with steroids alone (even when administered at high doses) is unable to modify the natural course of the disease and has unproven benefit.37 By contrast, ACTH monotherapy is effective in reducing proteinuria.10, 29, 30 Additionally, in patients with steroid-resistant nephrotic syndrome, ACTH treatment induces satisfactory remission of proteinuria,29, 31, 32, thereby suggesting the involvement of steroid-independent mechanisms.
Direct effects on kidney cells
MCRs have been extensively studied in many animal and human organ systems. Little is known, however, about kidney-specific MCR expression. Results in rodent studies vary: MC3R, MC4R and MC5R are expressed in the murine kidney,14 whereas only MC1R and MC3R are expressed in the rat kidney.15 My team’s latest data11 also clearly demonstrate the expression of MC5R in rodent podocytes, both in vivo and in vitro (Figure 2). In the human kidney, strong expression of MC5R and weak expression of MC2R have been demonstrated using PCR amplification of human kidney-specific cDNA.12 This finding is, to a certain extent, consistent with observations in sea bass, in which MC5R is abundantly expressed in both the anterior kidney and posterior kidney.13 By contrast, MC1R expression (and, to a much lesser extent, MC4R expression) were detected by quantitative reverse transcriptase PCR in the human kidney and in cultured human podocytes, glomerular endothelial cells and tubular epithelial cells.10 This discrepancy could reflect species differences but most probably relates to the potential pitfalls inherent in these detection technologies. As the MCRs are the product of small, intronless genes, hence, genomic contamination complicates expression analysis based on reverse transcriptase PCR.38 Nevertheless, if expression of MCRs in podocytes is verified, the antiproteinuric effect of ACTH might be justified by its direct protective effect on these cells.11
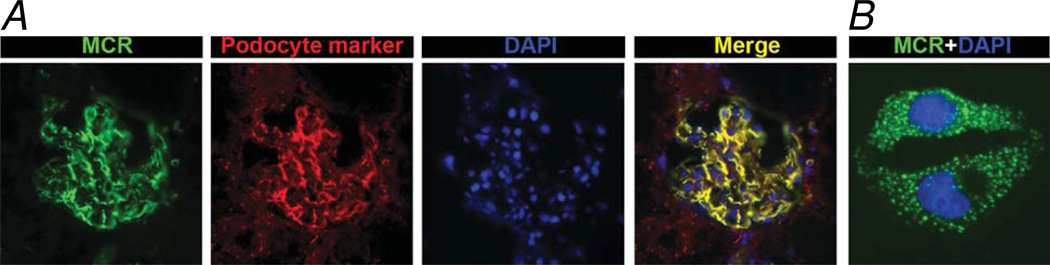
Figure 2.
Expression of melanocortin receptors in podocytes. a | In frozen sections of rat kidney, immunofluorescent staining for MCR (green) is colocalized with a podocyte marker (red), and demonstrates perfect superposition of these markers in a merged image (yellow). Nuclear counterstaining was obtained with DAPI (blue). b | In cultured murine podocytes, immunofluorescent staining for MCR reveals a punctate, cytoplasmic membrane distribution of this receptor (green). Abbreviations: 4,6 diamidino 2 phenylindole; MCR, melanocortin receptor.
Tubular expression of MCRs also implies the possible efficacy of ACTH in AKI26 or tubulointerstitial injury associated with glomerular diseases.11 In addition, expression of MCRs in glomerular endothelial cells might also explain the potential efficacy of ACTH in patients with thrombotic microangiopathy caused by hemolytic-uremic syndrome39 and might also suggest the potential use of ACTH in other diseases of the glomerular endothelium, including pre-eclampsia, thrombotic thrombocytopenic purpura and transplant glomerulopathy.
Regardless of which types of MCR are expressed in this organ, a burgeoning body of evidence suggests that the kidney might be a direct effector organ of ACTH and other melanocortin peptides. ACTH might directly activate MCRs expressed on renal parenchymal cells and trigger downstream cellular effects (Table 1) which exerts downstream renoprotective effects,7 including inhibition of apoptosis, promotion of cell survival, reduction of inflammation40 and immunomodulation.40
Immunomodulatory effects of ACTH
Renal inflammation is an invariable pathologic finding in patients with various proteinuric glomerulopathies.41 Epidemiologic data suggest that renal inflammation is one of the few modifiable risk factors for long-term prognosis and progression to end-stage renal failure in most forms of proteinuric nephropathy.41 ACTH and other melanocortins, such as α-MSH, have potent anti-inflammatory activities in their target organs (including the kidney) through multiple mechanisms.40 First, inhibition of proinflammatory NF-κB activation followed by reduced expression of NF-κB-dependent proinflammatory molecules, including interleukins, chemokines and adhesion molecules.40 Second, inhibition of migration and infiltration of inflammatory cells, in particular macrophages.11, 26 Third, suppression of inflammatory cell proliferation, possibly through inhibition of NF-κB signaling40 or through destabilization of c-Myc via the cAMP–PKA–Akt–glycogen synthase kinase 3β pathway42, 43 and, fourth, an antioxidant effect.44 Additionally, ACTH can modulate leukocyte function and impair lymphocyte blastogenesis.45, 46 Evidence also indicates that in human peripheral blood leukocytes, MCRs are abundantly expressed by B lymphocytes,47 CD4+ T helper cells,48 natural killer cells,48 monocytes and granulocytes.48 ACTH can directly bind to immunocompetent cells and modulate their immunoreactivity through functional MCRs.49 These findings suggest that the effect of ACTH on immune-complex-mediated glomerulopathies (such as IMN) and podocytopathies (including minimal change disease and FSGS) could result from its systemic immunomodulatory activity. These activities include reinstating the immune balance between subsets of T helper lymphocytes; reducing autoantibody production; and diminishing the release of lymphokines that increase glomerular permeability.50
The cholinergic anti-inflammatory pathway51 might also have a role in the anti-inflammatory effects of ACTH. In this pathway, vagus nerve signals modulate inflammation at various locations in the body through nicotinic acetylcholine receptors, which are expressed by peripheral cells (including kidney cells).52 Data demonstrating a systemic protective effect of ACTH mediated through the central nervous system.53 suggest that an MCR-mediated, vagal, cholinergic pathway is likely to contribute, at least in part, to the renoprotective effect of ACTH.
Correction of dyslipidemia
Dyslipidemia has an important role in the development and progression of nephrotic glomerulopathies.54 Lipid-lowering drugs, such as statins, can also contribute to amelioration of proteinuria, as shown by controlled trials in patients with IMN.55–58 Moreover, depletion of apolipoprotein J is a cause of proteinuric glomerulopathies, including membranous neuropathy and FSGS.59 Consistent with this finding, supplementation with apolipoproteins can prevent glomerular permeability to albumin induced in vitro by serum from patients with FSGS.60
ACTH1–24 has a pronounced lipid-lowering effect even in healthy individuals.17, 61 The mechanism responsible for this lipid-modifying activity has not been elucidated, but in vitro studies support the hypothesis that ACTH directly regulates hepatic lipoprotein metabolism.17, 61 A possible interpretation is that by modifying apolipoprotein metabolism, ACTH restores levels of certain apolipoproteins, including apolipoprotein E or apolipoprotein J.30, 61 These apolipoproteins could neutralize the activity of circulating permeability factors in patients with FSGS and thereby induce remission of proteinuria.60 In addition, experimental studies demonstrated that apolipoprotein J competes with the terminal components of complement (C5b–9) for the same receptor—namely megalin—in podocytes,59, 62 thereby attenuating glomerular injury and proteinuria in rat models of membranous neuropathy.
Conclusions
Clinical and experimental evidence supports a role for ACTH, with its antiproteinuric, lipid lowering and renoprotective activities, as a novel therapy for treatment-refractory nephrotic syndrome caused by a variety of glomerulopathies. ACTH might induce remission of nephrotic syndrome through a variety of potential mechanisms (Figure 3), including (but not limited to): corticosteroid-mediated pathways; kidney protection by dyslipidemia correction or through neurogenic anti-inflammatory effects on the central nervous system; direct MCR-mediated immunomodulation; as well as direct MCR-mediated protection of kidney cells, particularly podocytes. Although the existing findings are encouraging, most were made in single-center, observational studies with small sample sizes, limited controls and short-term follow-up. Thus, a multicenter, randomized, controlled, double-blind trial is the essential next step, in which the efficacy of ACTH therapy in patients with nephrotic syndrome must be appropriately tested. An extended follow-up duration is merited to assess whether ACTH therapy can actually retard the progression of chronic renal disease and stabilize kidney function, in addition to its proteinuria-reducing effect.
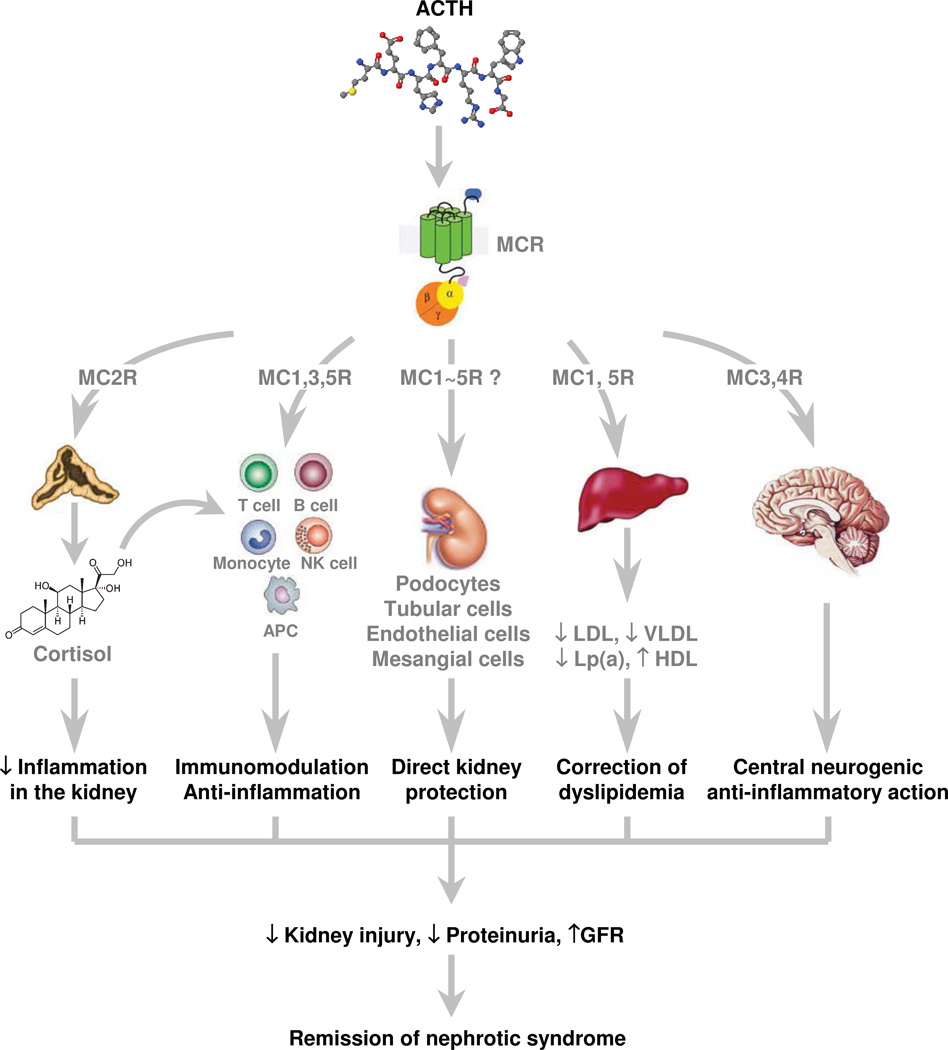
Figure 3.
Potential mechanisms underlying the therapeutic efficacy of ACTH in proteinuric nephropathies. ACTH could induce remission of proteinuria and exert renoprotective effects through a variety of potential mechanisms, including (but not limited to): corticosteroid-mediated systemic immunosuppression and anti-inflammatory actions in the kidney subsequent to ACTH-induced steroidogenesis; direct MCR-mediated systemic immunomodulation and anti-inflammatory effects; direct MCR-mediated protective effects on kidney cells, particularly podocytes; kidney protection secondary to correction of dyslipidemia mediated by MCR on hepatic cells; and renal protection via neurogenic anti-inflammatory effects mediated by MCRs expressed in the central nervous system. Abbreviations: ACTH, adrenocorticotropic hormone; APC, antigen-presenting cells; GFR, glomerular filtration rate; Lp(a), lipoprotein (a); MCR, melanocortin receptor; NK, natural killer.
ACTH, a well-known treatment for lipoid nephrosis, is experiencing a revival and attracting much attention after 50 years of neglect. Future studies will help to elucidate how these mechanisms contribute to the efficacy of ACTH as an alternative option for patients with treatment-refractory steroid-sensitive and steroid-resistant nephrotic syndrome.
Acknowledgments
Research work by R. Gong has been supported by research grants from Questcor, Foundation for Health, and NIH Grant R01DK092485.
Footnotes
Competing interests
R. Gong declares an association with the following company: Questcor. See the article online for full details of the relationship.
References
- 1.Dores RM. Adrenocorticotropic hormone, melanocyte-stimulating hormone, and the melanocortin receptors: revisiting the work of Robert Schwyzer: a thirty-year retrospective. Ann N Y Acad Sci. 2009;1163:93–100. doi: 10.1111/j.1749-6632.2009.04434.x. [DOI] [PubMed] [Google Scholar]
- 2.Arneil GC, Wilson HE. A.C.T.H. in nephrosis. Arch Dis Child. 1953;28:372–380. doi: 10.1136/adc.28.141.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piel CF. Management of nephrosis; the use of long continued hormone therapy. Calif Med. 1956;85:152–156. [PMC free article] [PubMed] [Google Scholar]
- 4.Lauson HD, Forman CW, Mc NH, Mattar G, Barnett HL. The effect of corticotropin (ACTH) on glomerular permeability to albumin in children with the nephrotic syndrome. J Clin Invest. 1954;33:657–664. doi: 10.1172/JCI102936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berg AL, Arnadottir M. ACTH revisited--potential implications for patients with renal disease. Nephrol Dial Transplant. 2000;15:940–942. doi: 10.1093/ndt/15.7.940. [DOI] [PubMed] [Google Scholar]
- 6.Ponticelli C. Membranous nephropathy. J Nephrol. 2007;20:268–287. [PubMed] [Google Scholar]
- 7.Cone RD. Studies on the physiological functions of the melanocortin system. Endocr Rev. 2006;27:736–749. doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]
- 8.Voisey J, Carroll L, van Daal A. Melanocortins and their receptors and antagonists. Curr Drug Targets. 2003;4:586–597. doi: 10.2174/1389450033490858. [DOI] [PubMed] [Google Scholar]
- 9.Penhoat A, Naville D, Begeot M. The adrenocorticotropic hormone receptor. Curr Opin Endocrinol Diabetes. 2001;8:112–117. [Google Scholar]
- 10.Lindskog A, et al. Melanocortin 1 receptor agonists reduce proteinuria. J Am Soc Nephrol. 2010;21:1290–1298. doi: 10.1681/ASN.2009101025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong R, Dworkin LD. ACTH(Acthar Gel) prevents proteinuria and renal injury in the remnant kidney: Evidence for direct podocyte protection. J Am Soc Nephrol. 2010;21:548A. [Google Scholar]
- 12.Chhajlani V. Distribution of cDNA for melanocortin receptor subtypes in human tissues. Biochem Mol Biol Int. 1996;38:73–80. [PubMed] [Google Scholar]
- 13.Sanchez E, Rubio VC, Cerda-Reverter JM. Characterization of the sea bass melanocortin 5 receptor: a putative role in hepatic lipid metabolism. J Exp Biol. 2009;212:3901–3910. doi: 10.1242/jeb.035121. [DOI] [PubMed] [Google Scholar]
- 14.Ni XP, Bhargava A, Pearce D, Humphreys MH. Modulation by dietary sodium intake of melanocortin 3 receptor mRNA and protein abundance in the rat kidney. Am J Physiol Regul Integr Comp Physiol. 2006;290:R560–R567. doi: 10.1152/ajpregu.00279.2005. [DOI] [PubMed] [Google Scholar]
- 15.Lee YS, Park JJ, Chung KY. Change of melanocortin receptor expression in rat kidney ischemia-reperfusion injury. Transplant Proc. 2008;40:2142–2144. doi: 10.1016/j.transproceed.2008.07.101. [DOI] [PubMed] [Google Scholar]
- 16.Schwandt P, Richter WO. Effects of pituitary peptides on fat mobilisation. Int J Obes. 1982;6(Suppl 1):49–54. [PubMed] [Google Scholar]
- 17.Berg AL, Hansson P, Nilsson-Ehle P. ACTH 1–24 decreases hepatic lipase activities and low density lipoprotein concentrations in healthy men. J Intern Med. 1991;229:201–203. doi: 10.1111/j.1365-2796.1991.tb00332.x. [DOI] [PubMed] [Google Scholar]
- 18.Berg AL, Nilsson-Ehle P. ACTH lowers serum lipids in steroid-treated hyperlipemic patients with kidney disease. Kidney Int. 1996;50:538–542. doi: 10.1038/ki.1996.346. [DOI] [PubMed] [Google Scholar]
- 19.Beloff-Chain A, Morton J, Dunmore S, Taylor GW, Morris HR. Evidence that the insulin secretagogue, beta-cell-tropin, is ACTH22-39. Nature. 1983;301:255–258. doi: 10.1038/301255a0. [DOI] [PubMed] [Google Scholar]
- 20.Schoneshofer M, Goverde HJ. Corticotropin in human plasma. General considerations. Surv Immunol Res. 1984;3:55–63. doi: 10.1007/BF02918598. [DOI] [PubMed] [Google Scholar]
- 21.Wan L, Chen YH, Chang TW. Improving pharmacokinetic properties of adrenocorticotropin by site-specific lipid modification. J Pharm Sci. 2003;92:1882–1892. doi: 10.1002/jps.10442. [DOI] [PubMed] [Google Scholar]
- 22.Kohda Y, Chiao H, Star RA. alpha-Melanocyte-stimulating hormone and acute renal failure. Curr Opin Nephrol Hypertens. 1998;7:413–417. doi: 10.1097/00041552-199807000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Chiao H, et al. Alpha-melanocyte-stimulating hormone protects against renal injury after ischemia in mice and rats. J Clin Invest. 1997;99:1165–1172. doi: 10.1172/JCI119272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kolgazi M, Arbak S, Alican I. The effect of alpha-melanocyte stimulating hormone on gentamicin-induced acute nephrotoxicity in rats. J Appl Toxicol. 2007;27:183–188. doi: 10.1002/jat.1191. [DOI] [PubMed] [Google Scholar]
- 25.Li C, et al. alpha-MSH prevents impairment in renal function and dysregulation of AQPs and Na-K-ATPase in rats with bilateral ureteral obstruction. Am J Physiol Renal Physiol. 2006;290:F384–F396. doi: 10.1152/ajprenal.00282.2004. [DOI] [PubMed] [Google Scholar]
- 26.Gong R, Ge Y, Tolbert EM, Zhuang S, Dworkin LD. Renoprotection by adrenocorticotropin in experimental acute kidney injury. J Am Soc Nephrol. 2009;20:510A. [Google Scholar]
- 27.Garcia DL, Rennke HG, Brenner BM, Anderson S. Chronic glucocorticoid therapy amplifies glomerular injury in rats with renal ablation. J Clin Invest. 1987;80:867–874. doi: 10.1172/JCI113145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berg AL, Arnadottir M. ACTH-induced improvement in the nephrotic syndrome in patients with a variety of diagnoses. Nephrol Dial Transplant. 2004;19:1305–1307. doi: 10.1093/ndt/gfh110. [DOI] [PubMed] [Google Scholar]
- 29.Berg AL, Nilsson-Ehle P, Arnadottir M. Beneficial effects of ACTH on the serum lipoprotein profile and glomerular function in patients with membranous nephropathy. Kidney Int. 1999;56:1534–1543. doi: 10.1046/j.1523-1755.1999.00675.x. [DOI] [PubMed] [Google Scholar]
- 30.Ponticelli C, et al. A randomized pilot trial comparing methylprednisolone plus a cytotoxic agent versus synthetic adrenocorticotropic hormone in idiopathic membranous nephropathy. Am J Kidney Dis. 2006;47:233–240. doi: 10.1053/j.ajkd.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 31.Rauen T, Michaelis A, Floege J, Mertens PR. Case series of idiopathic membranous nephropathy with long-term beneficial effects of ACTH peptide 1–24. Clin Nephrol. 2009;71:637–642. doi: 10.5414/cnp71637. [DOI] [PubMed] [Google Scholar]
- 32.Picardi L, et al. ACTH therapy in nephrotic syndrome induced by idiopathic membranous nephropathy. Clin Nephrol. 2004;62:403–404. doi: 10.5414/cnp62403. [DOI] [PubMed] [Google Scholar]
- 33.Bomback AS, et al. Treatment of resistant nephrotic syndrome with adrenocorticotropic hormone (ACTH) gel. Drug Design, Development and Therapy. 2011;5:147–153. doi: 10.2147/DDDT.S17521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beck LH, Berg AL, Beck DM, Bonegio RGB, J SD. Treatment of idiopathic membranous nephropathy with ACTH is associated with a decline in anti-PLA2R antibody. J Am Soc Nephrol. 2009;20:306A. [Google Scholar]
- 35.Beck LH, Jr, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenblum AH, Rosenblum P. Anaphylactic Reactions to Adrenocorticotropic Hormone in Children. J Pediatr. 1964;64:387–295. doi: 10.1016/s0022-3476(64)80191-8. [DOI] [PubMed] [Google Scholar]
- 37.Glassock RJ. The treatment of idiopathic membranous nephropathy: a dilemma or a conundrum? Am J Kidney Dis. 2004;44:562–566. [PubMed] [Google Scholar]
- 38.Catania A. The melanocortin system in leukocyte biology. J Leukoc Biol. 2007;81:383–392. doi: 10.1189/jlb.0706426. [DOI] [PubMed] [Google Scholar]
- 39.Symmers WS. Thrombotic microangiopathic haemolytic anaemia (thrombotic microangiopathy) Br Med J. 1952;2:897–903. doi: 10.1136/bmj.2.4790.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Catania A, Gatti S, Colombo G, Lipton JM. Targeting melanocortin receptors as a novel strategy to control inflammation. Pharmacol Rev. 2004;56:1–29. doi: 10.1124/pr.56.1.1. [DOI] [PubMed] [Google Scholar]
- 41.Remuzzi G, Bertani T. Pathophysiology of progressive nephropathies. N Engl J Med. 1998;339:1448–1456. doi: 10.1056/NEJM199811123392007. [DOI] [PubMed] [Google Scholar]
- 42.Johnson EW, Blalock JE, Smith EM. ACTH receptor-mediated induction of leukocyte cyclic AMP. Biochem Biophys Res Commun. 1988;157:1205–1211. doi: 10.1016/s0006-291x(88)81002-7. [DOI] [PubMed] [Google Scholar]
- 43.Lepique AP, et al. c-Myc protein is stabilized by fibroblast growth factor 2 and destabilized by ACTH to control cell cycle in mouse Y1 adrenocortical cells. J Mol Endocrinol. 2004;33:623–638. doi: 10.1677/jme.1.01485. [DOI] [PubMed] [Google Scholar]
- 44.Wang H, Jia Z, Liu G, Yang T. Adrenocorticotropic hormone attenuates diabetic nephropathy in Zucker diabetic fatty rats via inhibition of oxidative and inflammatory response. J Am Soc Nephrol. 2010;21:825A. [Google Scholar]
- 45.Roth JA, Kaeberle ML, Hsu WH. Effects of ACTH administration on bovine polymorphonuclear leukocyte function and lymphocyte blastogenesis. Am J Vet Res. 1982;43:412–416. [PubMed] [Google Scholar]
- 46.Smith E, Hammarstrom L, Moller E, Matell G. The effect of ACTH treatment on lymphocyte subpopulations in patients with myasthenia gravis. Neurology. 1976;26:915–918. doi: 10.1212/wnl.26.10.915. [DOI] [PubMed] [Google Scholar]
- 47.Cooper A, et al. Alpha-melanocyte-stimulating hormone suppresses antigen-induced lymphocyte proliferation in humans independently of melanocortin 1 receptor gene status. J Immunol. 2005;175:4806–4813. doi: 10.4049/jimmunol.175.7.4806. [DOI] [PubMed] [Google Scholar]
- 48.Andersen GN, et al. Quantitative measurement of the levels of melanocortin receptor subtype 1, 2, 3 and 5 and pro-opio-melanocortin peptide gene expression in subsets of human peripheral blood leucocytes. Scand J Immunol. 2005;61:279–284. doi: 10.1111/j.1365-3083.2005.01565.x. [DOI] [PubMed] [Google Scholar]
- 49.Gonzalez-Rey E, Chorny A, Delgado M. Regulation of immune tolerance by anti-inflammatory neuropeptides. Nat Rev Immunol. 2007;7:52–63. doi: 10.1038/nri1984. [DOI] [PubMed] [Google Scholar]
- 50.Koyama A, Fujisaki M, Kobayashi M, Igarashi M, Narita M. A glomerular permeability factor produced by human T cell hybridomas. Kidney Int. 1991;40:453–460. doi: 10.1038/ki.1991.232. [DOI] [PubMed] [Google Scholar]
- 51.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 52.Yeboah MM, et al. Cholinergic agonists attenuate renal ischemia-reperfusion injury in rats. Kidney Int. 2008;74:62–69. doi: 10.1038/ki.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guarini S, et al. Adrenocorticotropin reverses hemorrhagic shock in anesthetized rats through the rapid activation of a vagal anti-inflammatory pathway. Cardiovasc Res. 2004;63:357–365. doi: 10.1016/j.cardiores.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 54.Cases A, Coll E. Dyslipidemia and the progression of renal disease in chronic renal failure patients. Kidney Int Suppl. 2005:S87–S93. doi: 10.1111/j.1523-1755.2005.09916.x. [DOI] [PubMed] [Google Scholar]
- 55.Rayner BL, Byrne MJ, van Zyl Smit R. A prospective clinical trial comparing the treatment of idiopathic membranous nephropathy and nephrotic syndrome with simvastatin and diet, versus diet alone. Clin Nephrol. 1996;46:219–224. [PubMed] [Google Scholar]
- 56.Rabelink AJ, Hene RJ, Erkelens DW, Joles JA, Koomans HA. Partial remission of nephrotic syndrome in patient on long-term simvastatin. Lancet. 1990;335:1045–1046. doi: 10.1016/0140-6736(90)91118-t. [DOI] [PubMed] [Google Scholar]
- 57.Thomas ME, et al. Simvastatin therapy for hypercholesterolemic patients with nephrotic syndrome or significant proteinuria. Kidney Int. 1993;44:1124–1129. doi: 10.1038/ki.1993.358. [DOI] [PubMed] [Google Scholar]
- 58.Chan PC, et al. Lovastatin in glomerulonephritis patients with hyperlipidaemia and heavy proteinuria. Nephrol Dial Transplant. 1992;7:93–99. doi: 10.1093/oxfordjournals.ndt.a092102. [DOI] [PubMed] [Google Scholar]
- 59.Ghiggeri GM, et al. Depletion of clusterin in renal diseases causing nephrotic syndrome. Kidney Int. 2002;62:2184–2194. doi: 10.1046/j.1523-1755.2002.00664.x. [DOI] [PubMed] [Google Scholar]
- 60.Candiano G, et al. Apolipoproteins prevent glomerular albumin permeability induced in vitro by serum from patients with focal segmental glomerulosclerosis. J Am Soc Nephrol. 2001;12:143–150. doi: 10.1681/ASN.V121143. [DOI] [PubMed] [Google Scholar]
- 61.Berg AL, Nilsson-Ehle P. Direct effects of corticotropin on plasma lipoprotein metabolism in man--studies in vivo and in vitro. Metabolism. 1994;43:90–97. doi: 10.1016/0026-0495(94)90162-7. [DOI] [PubMed] [Google Scholar]
- 62.Rastaldi MP, et al. Glomerular clusterin is associated with PKC-alpha/beta regulation and good outcome of membranous glomerulonephritis in humans. Kidney Int. 2006;70:477–485. doi: 10.1038/sj.ki.5001563. [DOI] [PubMed] [Google Scholar]