Abstract
Few reliable tests are available for screening people rapidly for vestibular disorders although such tests would be useful for a variety of testing situations. Balance testing is widely performed but of unknown value for screening. The goal of this study was to determine the value of tests of walking balance for screening people with vestibular impairments. We tested three groups of patients with known vestibular impairments: benign paroxysmal positional vertigo, unilateral vestibular weakness, and post-acoustic neuroma resection. We compared them to normal subjects. All subjects were independently ambulatory without gait aids. Subjects were tested on tandem walking (TW) with eyes open and eyes closed for 10 steps, walking with no additional head motions and with augmented head rotations in yaw for 7 m (WwHT), and an obstacle avoidance task, the Functional Mobility Test (FMT). Subjects wore a 3-D motion sensor centered at mid-torso to capture kinematic measures. Patients and normals differed significantly on some behavioral measures, such as the number of steps to perform TW, and on some but not all kinematic measures. ROC analyses, however, were at best only moderate, and failed to find strong differences and cut-points that would differentiate the groups. These findings suggest that although patients and normals differ in performance of these tests in some interesting ways the groups are not sufficiently different on these tests for easy use as screening tests to differentiate the populations.
Keywords: Balance testing, tandem gait, vestibular screening, neurology testing, clinical examination
1. Introduction
Balance testing of people with vestibular disorders has a long history. Balance tests are often cited as being useful for screening people suspected of having vestibular impairments [11]. For example, we have shown that patients with vestibular disorders have decreased performance compared to normal people on an obstacle avoidance test, the Functional Mobility Test, and that version of the Functional Mobility Test combined with the Sensory Organization Test (Equitest) subtest of eyes closed/ sway referenced platform motion has good sensitivity to patients with vestibular impairments [3]. Unfortunately the equipment for FMT requires considerable space, which might not be available in the average clinic or screening facility. Therapists who practice vestibular rehabilitation may also create clinic-specific obstacle courses for evaluating patients [20] but those courses are not standardized for testing. The cost of the Equitest might also be beyond the budget of the average clinic or screening facility.
Other tests have often been used to screen people. These screening tests include walking with head turns (WwHT), which therapists have used as part of the Dynamic Gait Index balance battery [17] and independently [20] for many years, and various versions of the tandem walking test (TW) [5,7]. Allum and his colleagues studied trunk sway and time on several balance tests, including tandem gait on the floor for 8 steps and a variation of WwHT: walking 5 steps with yaw head rotations [1,8]. They found greater trunk sway in patients with vestibular impairments than normals and greater sway in older than younger subjects for walking with head turns [1] but unfortunately did not report values for walking on the floor.
TW has been used for many years to assess balance in people with vestibular disorders [6,9]; originally developed for use on narrow rails with eyes open eventually it became standardized with eyes closed [7]. Although TW is widely used, Longridge and Mallinson recently reported that less than 30% of patients or normals could perform 5 steps of tandem walking with eyes closed [15]. Kammerlind et al. [13] tested TW for 15 steps with eyes open and showed that patients as well as normals could perform a mean of 13 consecutive steps, but they did not test the eyes closed condition. Similarly, Vereeck et al. [19] showed that with eyes open normals could perform 20 tandem steps, but performance declined over age 60. Despite its widespread use no studies have determined if TW distinguishes patients from normals.
To determine which, if any, of the screening tests mentioned above are most useful for screening people for vestibular impairments, we tested normal people and people with known vestibular disorders on WwHT, modified from the related subscale on the Dynamic Gait Index, Tandem walking, and shortened FMT. All of these screening tests are easy to administer and require minimal equipment and space. For all tests we measured kinematics of head and trunk motions as well as standard, easily observed behavioral measures.
2. Methods
2.1. Subjects
Normal subjects were screened with a brief health history to exclude people who had histories of neurologic, orthopedic, visual, or otologic disorders. They were tested with Dix-Hallpike maneuvers to left and right, head shaking in yaw rotations, and head thrusts to left and right and they had negative responses on all tests. All subjects wore comfortable clothes and performed all tests without shoes. For good hygiene, all subjects wore socks. All subjects were ambulatory without use of canes or other gait aids. No subjects had had joint replacements or other significant orthopedic limitations, and no subjects complained of pain while walking.
During pilot testing, 14 normal adults (mean age 34.5, SD 9.1, range 24.3 to 46.5, 8 females, 6 males) performed each test, with repeated trials on all tests, to learn about the effects of repeated trials. This question influenced the number of trials used on subsequent testing. During the main study we used 127 subjects, including a second group of 61 normal adults who were not previously tested, who had no history of otologic, neurologic, or orthopedic disorders, and who had negative responses on head thrusts, Dix-Hallpike maneuvers and head shaking. We tested a group of patients with three types of vestibular impairments: 21 patients with benign paroxysmal vertigo as indicated by unilateral positive responses to Dix-Hallpike maneuvers and no other significant vestibular impairments (BP-PV), 27 patients with unilateral vestibular impairment (UW) as indicated by ≥20% difference between right and left side on bi-thermal caloric testing, and 18 postoperative acoustic neuroma patients (AN). Every patient was diagnosed by a board-certified otolaryngologist or neurologist. See Table 1 for demographic details.
Table 1.
Demographic details of study sample. Mean age (yrs) (SD, range), mean length of illness (yrs) (SD, range) for subjects with vestibular impairments, number per gender
| Group | Age | Length of illness | Females/Males |
|---|---|---|---|
| Normals | 49.6 (16.0, 23.3 to 77.0) | 30 F, 31 M | |
| BPPV | 58.8 (11.7, 34.7 to 78.8) | 0.28 (0.45, 0.03 to 1.63) | 11 F, 10 M |
| AN | 55.6 (10.7, 35.2 to 72.9) | 5.5 (5.8, 0.27 to 27) | 12 F, 6 M |
| UW | 54.9, (17.9, 21.4 to 75.2) | 4.0 (9.0, 0.07 to 40) | 18 F, 9 M |
Patient subgroups: BPPV, benign paroxysmal positional vertigo. AN, acoustic neuroma. UW, unilateral weakness
This study was approved by the Institutional Review Board for Human Subjects Research for Baylor College of Medicine and Affiliated Hospitals. Subjects gave informed consent prior to participation.
2.2. Instrumentation
Every subject wore a lightweight vest with an inertial motion sensor (IMU; Xsens North America Inc., Los Angeles, CA) centered on the back at the mid-thoracic. The IMU was 5.25 × 3.75 × 2 cm and weighed 28.3 g. It was used to measure kinematic data, as described below. For WwHT the 0.33 Hz signal to cue head turns was provided via an iPod attached to amplifiers on a desktop.
2.3. Tests
Three tests of walking balance were given to all subjects, in random order. BPPV patients were pre-tested, treated with standard canalith repositioning maneuvers [2], and post-tested one week after the initial test, to learn the effect of treatment on performance. Each subject was tested by one of three technicians, with 7 to 25 years of experience performing vestibular diagnostic testing and research testing, including balance tests. Staff members were naïve to subjects’ diagnoses.
For WwHT, subjects were asked to walk 7 m under 2 conditions: either without augmented head movements, at the subject’s preferred pace (head still); and with yaw rotations of the head at 0.33 Hz but walking at the subject’s preferred pace (head yaw). Behavioral measures were the number of steps taken, the time to complete the walk as measured with a stopwatch, and the level of disequilibrium or gait ataxia (described here as ataxia), measured on a 4-point scale in which 1 was normal, 2 was mild, 3 was severe, 4 was loss of balance and unable to walk unassisted.
For TW subjects were asked to walk for 10 steps, heel-to-toe without spaces between the steps, under two conditions: eyes open (EO) and eyes closed (EC). The dependent measure was the maximum number of correct consecutive steps. Errors included taking a side step, making a space between the feet, and opening the eyes if eyes were closed.
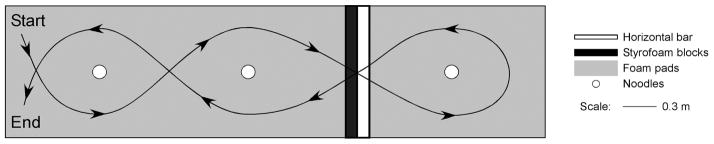
The shortened FMT was an obstacle avoidance task based on the original FMT [16]. Subjects were asked to walk through a course, on 10 cm compliant foam, 0.94 m × 4.12 m, (Sunmate; Dynamic Systems, Inc., Leicester, NC) that involved stepping around 3 vertical, foam tubes, 6.35 cm, and stepping through a portal that consisted of a horizontal foam tube at shoulder height and a 40 cm high Styrofoam block. See Fig. 1. Subjects performed the task twice, once without distraction and once while naming girls and boys names for each letter of the alphabet in order (distraction), to learn if distraction with a cognitive task affected performance. The dependent behavioral measures were the time to complete the course and the number of obstacles bumped.
Fig. 1.
Plan view of the Functional Mobility Test.
2.4. Kinematic and behavioral analyses
For kinematic analyses the following root mean square values of the IMU variables for the trunk segment were quantified and used for further analysis: acceleration along the fore-aft axis (TAX), acceleration along the medio-lateral axis for the trunk (TAY), resultant acceleration (TAR), angular velocity about the roll axis (TRV), angular velocity about the pitch axis (TPV), angular velocity about the yaw axis (TYV). For WwHT the root mean square values of the resultant acceleration for the trunk are not reported because the subjects did not walk with bending of the segments at the hip during this test.
Behavioral measures included the number of steps walked, time to perform a task as measured with a stopwatch, level of ataxia, and number of obstacles bumped on FMT. All behavioral and kinematic measurements began from standing still and ended when the subject stopped walking.
2.5. Statistical analyses
The study used four groups of people (normals, BP-PV, AN, UW), who were tested with three different tests (TW, WwHT, FMT) under different conditions for each screening test: TW, eyes closed eyes open; WwHT: head yaw, head still; FMT: distraction, no distraction. At each test and under each condition, two sets of dependent measures were assessed for each subject: behavioral and kinematic as detailed above. Multilevel statistical methods [18] were employed to describe changes in the dependent variables of interest. A separate model was produced and fitted to each dependent variable. Within each model, we examined the significance of effects within subjects (within subject over conditions of head, eye, or distraction) and between subjects (between the 4 study groups). Interaction effects were included in each model and tested. Changes across head and eye conditions were compared among groups by using a likelihood ratio statistic that follows a chi-square distribution. Adjustments were made for multiple comparisons. To determine if any test is useful in identifying people with vestibular disorders and to determine the optimal cut off on that test, we performed logistic regression and Receiver Operating Characteristic (ROC) analysis for interval data, and provided corresponding sensitivity and specificity values for various cut offs. To examine inter-rate reliability of various behavioral measures two separate raters independently measured all behavioral variables for a random sample of 89 subjects. The level of association was assessed by correlation coefficients. P < 0.05 was considered as statistically significant. All analyses were performed using SAS Statistical software (SAS, Carry, NC).
3. Results
3.1. Tandem walking
All patiens took significantly more consecutive steps with eyes open than eyes closed (p < 0.0001). Normals took significantly more consecutive steps than patients, regardless of visual condition (p < 0.0001) although differences between patients and normals were more evident with eyes closed than with eyes open. The patient groups did not differ significantly with eyes open or closed. See Table 2. For both the eyes open and eyes closed conditions, inter-rater reliability was high, r = 0.97.
Table 2.
Tandem walking. Number of consecutive steps per condition. Mean (median, ranges).
| Eyes Open | Eyes Closed | |
|---|---|---|
| Normals | 9.4 (10, 3 to 10) | 5.9 (5, 1 to 10) |
| Mean patients overall | 8.7 (10, 0 to 10) | 3.6 (3, 0 to 10) |
| BPPV | 8 (10, 2 to 10) | 4 (3, 0 to 10) |
| AN | 8.1 (10, 0 to 10) | 2.3 (2, 0 to 8) |
| UW | 7.9 (10, 4 to 10) | 3.9 (3, 0 to 10) |
Patient subgroups: BPPV, benign paroxysmal positional vertigo. AN, acoustic neuroma. UW, unilateral weakness
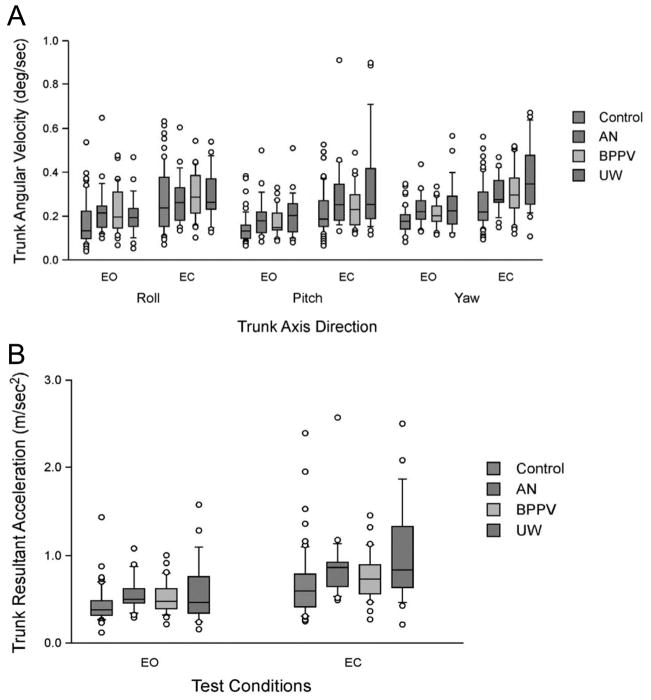
Normals and patients had significantly lower TRV, TPV TYV, and TAR with eyes open than closed, p < 0.0001. No differences were found among the subgroups. UW subjects had significantly higher TRV with eyes closed than normals, p =0.0004. Patients also had significantly higher TYV than normals, p = 0.0002. There was a trend for interaction between group and conditions (p = 0.08). UW subjects had higher scores than normals with eyes closed (p < 0.0001) but BPPV and AN subjects did not differ significantly from normals with eyes closed. Normals had significantly lower TAR with eyes open than eyes closed, p < 0.0001. With eyes closed UW subjects had significantly higher TAR than normals, p = 0.0026. Thus patients had greater instability in all planes than normals during TW in the eyes closed condition. See Fig. 2.
Fig. 2.
Tandem walking kinematics. EO, eyes open; EC, eyes closed. A. Trunk angular velocity by group and condition. B. Trunk resultant acceleration by group and condition. Center horizontal bars are medians, rectangle ends are interquartile ranges, error bars are 10th and 90th deciles and circles are outliers. (Colours are visible in the online version of the article; http://dx.doi.org/10.3233/VES-2012-0443)
For eyes open, the ROC analysis was only moderate (ROC = 0.64, 95% CI = 0.56 to 0.71); to identify more than 95% of normals the cut-off must be 5 steps. That level, however, only identified 14% of patients. ROC analyses showed that for eyes closed, to identify more than 92% of normals the cut-off must be less than 2 steps but that level only correctly identified 23% of patients (ROC = 0.71, 95% CI = 0.61 to 0.80). See Table 3.
Table 3.
Tandem walking. ROC analyses for number of steps. Sensitivity and specificity at several scores for patients compared to normals
| Eyes open | Sensitivity (to patients) | Specificity (to normals) | Eyes closed | Sensitivity to patients | Specificity to normals |
|---|---|---|---|---|---|
| 2 steps | 0.23 | 0.92 | |||
| 3 steps | 0.05 | 1.00 | 3 steps | 0.45 | 0.82 |
| 5 steps | 0.14 | 0.99 | 5 steps | 0.68 | 0.66 |
| 7 steps | 0.27 | 0.92 | 7 steps | 0.83 | 0.41 |
| 8 steps | 0.36 | 0.89 | 8 steps | 0.86 | 0.38 |
3.2. Walking with head turns
For WwHT normals and patients all walked significantly more quickly with head still than with head yaw (p < 0.0001). Walking speed did not differ significantly between groups. When the groups were analyzed separately, at head still the BPPV group was significantly slower than normals (p = 0.04) but the other groups did not differ significantly from normals. At head yaw, the UW group approached being different from normals (p = 0.059), but the other patient groups did not. On ataxia, with head still the patient and normal groups did not differ significantly. Normals did not differ between conditions on ataxia, but patients had significantly more ataxia with head yaw than head still (p < 0.0001), and with head yaw patients had significantly higher ataxia scores than normals (p < 0.0001). With head yaw UW and AN groups had significantly more ataxia than normals but BPPV patients did not differ from normals. Patient subjects took significantly more steps than normals in both conditions (p =0.001). All subjects took significantly more steps with head yaw (p < 0.0001). With head still the BPPV group approached differing from normals (p = 0.06), but the other patient groups did not differ from normals. With head yaw the UW group took significantly more steps than normals, but the other patient groups did not differ from normals. For the number of steps the groups differed significantly with head still (p = 0.005) and with head yaw (p = 0.006). See Table 4.
Table 4.
Walking with Head Turns, mean step speed (steps/sec; SD, range), mean number of steps (median, range), mean ataxia (median, range).
| Speed (steps/sec)
|
Steps
|
Ataxia
|
||||
|---|---|---|---|---|---|---|
| Head still | Head yaw | Head still | Head yaw | Head still | Head yaw | |
| Normals | 1.8 (0.2, 1.0 to 2.2) | 1.6 (0.3, 0.8 to 2.1) | 12.3 (12, 10 to 15) | 13.6 (13, 10 to 22) | 1 (1, all 1) | 1.1 (1, 1 to 2) |
| BPPV | 1.7 (0.3, 0.7 to 2.0) | 1.6 (0.2, 1.0 to 2.0) | 13 (13, 10 to 19) | 14 (14, 11 to 21) | 1 (1, 1 to 2) | 1 (1, 1 to 3) |
| AN | 1.9 (9.2, 1.4 to 2.2) | 1.6 (0.3, 1.0 to 2.1) | 13.1 (13, 11 to 19) | 14.8 (14, 11 to 24) | 1.1 (1, 1 to 2) | 1.4 (1, 1 to 2) |
| UW | 1.7 (0.2, 1.4 to 2.0) | 1.5 (0.4, 0.8 to 1.9) | 13.2 (13, 11 to 16) | 15.2 (15, 12 to 23) | 1.0 (1, 1 to 2) | 1 (1.5, 1 to 3) |
Patient subgroups: BPPV, benign paroxysmal positional vertigo. AN, acoustic neuroma. UW, unilateral weakness
Inter-rater reliability varied by test measure. It was moderate for ataxia with head still, r =0.70, and higher with head yaw, r = 0.83. Inter-rater reliability was high for other measures: walking time head still, r = 0.94; walking time head yaw, r = 0.99; number of steps head still, r = 0.98; number of steps head yaw, r = 0.97.
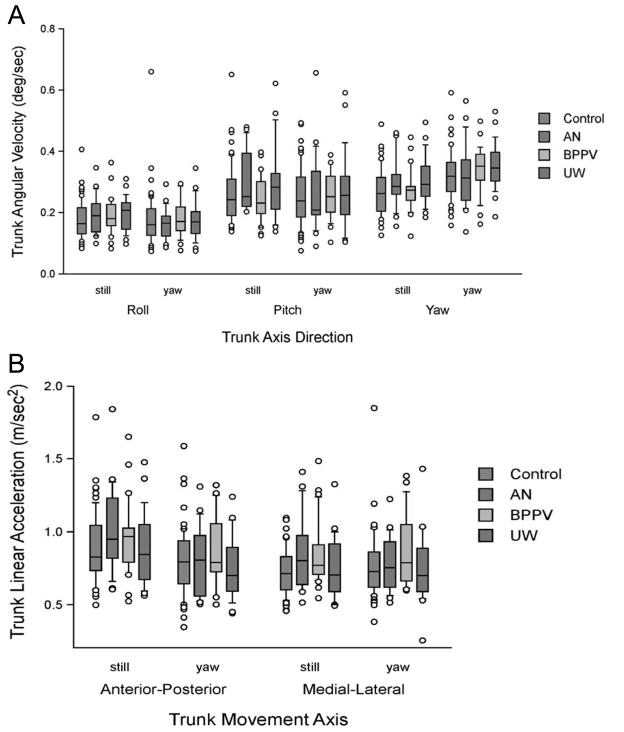
Normals and patients did not differ significantly for TRV and TPV, i.e., no group or condition effect was found, indicating that patients are similar to normals, and scores are similar regardless of head movement condition. No differences were found between normals and patients for TYV; scores were lower for head still than head yaw in both groups (p < 0.0001). TAX was significantly higher in head still than in head yaw (p < 0.0001), regardless of group. Normals and patients did not differ significantly. For TAY with groups collapsed, scores did not differ significantly between head movement conditions. Scores for normal subjects were significantly lower than scores for BPPV subjects (p =0.05). No other differences were found among the groups. TAY, TRV and TPV showed no differences between conditions. TYV was different at head still than head yaw (p < 0.0001). No group differences were observed for TYV. For TAY the difference between normals and BPPV subjects approached significance (p = 0.052). See Fig. 3.
Fig. 3.
Walking with Head Turns kinematics A. Trunk angular velocity (deg/sec). B. Trunk linear acceleration. Center horizontal bars are medians, rectangle ends are interquartile ranges, error bars are 10th and 90th deciles and circles are outliers. (Colours are visible in the online version of the article; http://dx.doi.org/10.3233/VES-2012-0443)
For walking speed during head yaw, ROC was moderately low (ROC = 0.60, 95% CI: 0.50 to 0.70). No cut-off points were optimal. For the number of steps taken during walking with head yaw ROC was moderately low (ROC = 0.65, 95% CI: 0.55 to 0.74). Similar to walking speed, no optimal cut-off points were found.
3.3. Functional mobility test
Normals and patients did not differ significantly on the number of obstacles touched. Normals and patients bumped more obstacles with no distraction than with distraction (p < 0.0001). Distraction had no such effect on time to traverse the course; trials without and with distraction did not differ significantly. Patients took significantly longer than normals under both conditions, (p < 0.001). The UW group took significantly longer than normals with no distraction (p = 0.03) and with distraction (p = 0.05). No other differences were found between the groups. See Table 5 For time without distraction and number of obstacles without distraction, inter-rater reliability was high, r = 0.99 and r = 0.93, respectively.
Table 5.
Functional Mobility Test. Time with no distraction and with distraction, mean (SD, ranges); obstacles with no distraction and with distraction, mean (median, ranges.
| Time, no distraction | Time, distraction | Obstacles, no distraction | Obstacles, distraction | |
|---|---|---|---|---|
| Normals | 19.19 (8.01, 9.34 to 48.85) | 20.77 (9.61, 8.56 to 65.93) | 2.38 (2, 0 to 8) | 1.64 (1, 0 to 6) |
| BPPV | 25.60 (15.61, 9.37 to 69.89) | 25.79 (16.54, 9.34 to 79.41) | 3.05 (3, 0 to 13) | 2.0 (0, 0 to 12) |
| AN | 28.86 (21.80, 10.25 to 91.9) | 26.65 (17.77, 10.25 to 71.30) | 3.22 (2, 0 to 10) | 2.61 (1, 0 to 10) |
| UW | 28.33 (16.06, 9.23 to 71.39) | 29.35 (17.14, 11.36 to 84.18) | 2.78 (2, 0 to 13) | 2 (1, 0 to 10) |
Patient subgroups: BPPV, benign paroxysmal positional vertigo. AN, acoustic neuroma. UW, unilateral weakness
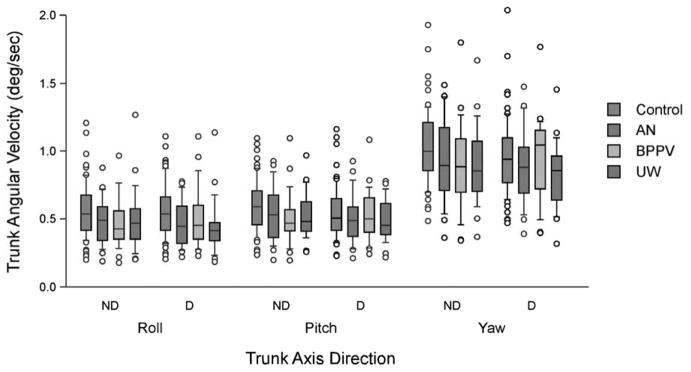
Kinematic analyses of FMT for patients compared to normals showed minimal effects. Normals had higher TPV and TYV during no-distraction trials than distraction, p = 0.02 and p = 0.002, respectively. No other differences were found. TAR did not differ significantly between normals and patients and TAR did not differ significantly between no-distraction and distraction trials. See Fig. 4.
Fig. 4.
Functional Mobility Test kinematics. Trunk angular velocity (deg/sec). ND, no distraction; D, Distraction. Center horizontal bars are medians, rectangle ends are interquartile ranges, error bars are 10th and 90th deciles and circles are outliers. (Colours are visible in the online version of the article; http://dx.doi.org/10.3233/VES-2012-0443)
The ROC analysis for patients compared to normals for time without distraction was only low to moderate (ROC = 0.67, 95% CI = 0.57 to 0.76). For obstacles without distraction for patients compared to normals ROC was low (ROC = 0.54, 95% CI = 0.44 to 0.64) and for obstacles with distraction ROC was also low (ROC = 0.51, 95% CI = 0.41 to 0.61). No clear cut-points could be found for any measures. The best cut-point was time = 22.8 sec, but the sensitivity and specificity were only moderate. See Table 6.
Table 6.
Results of ROC analysis of FMT time. Sensitivity to subjects with vestibular impairments compared to specificity to normals
| Time (sec) | Sensitivity to patients | Specificity to normals |
|---|---|---|
| 50.4 | 0.136 | 1.0 |
| 18.90 | 0.61 | 0.63 |
| 9.6 | 0.97 | 0.02 |
4. Discussion
These results, in general, suggest that the balance tests assessed in this study, i.e., tandem walking, walking with head turns, and the Functional Mobility Test, are not useful for screening people for vestibular impairments. Some patient groups did differ from normals on some measures. ROC analyses, however, showed no clear cut-points that can be used to distinguish groups clearly. These screening tests may be useful for assessing performance over time among patients with known diagnoses. They are not, however, useful for screening members of the general population quickly or for initial assessment of someone who presents to a clinic complaining of “dizziness” or a balance problem.
4.1. Individual tests
TW has been widely used for many years but other reports have not included ROC analyses. Although normals and patients do differ significantly on some measures, the ROC analyses show that neither the eyes open condition nor the eyes closed condition differentiates normals and patients well. Therefore the clinician should use this test with caution, and perhaps only as an adjunct to other tests. Thus we agree with Longridge and Mallinson [15] that tandem walking with eyes closed is not useful in screening patients for vestibular disease, when the dependent measure is the number of steps taken. Despite it’s long history, high reliability, and widespread use this test is so challenging for some normals or so easy for some known patients that it does not differentiate normals from patient groups. The kinematic analyses show some differences between normals and patient groups, which are manifested as qualitative differences in gait during the task, but those differences are not readily quantifiable to the naked eye.
WwHT appeared more promising until we examined the ROC values. Ataxia, number of steps and speed were all reliable measures and were significantly different between normals and patients. ROC values were moderate, however, so no optimal cut-off between normals and patients could be found. This finding suggests that this test is not useful for differentiating patients from normals. This idea is supported by the lack of differences in kinematic measures.
On FMT only the UW group took more time than normals. Kinematic measures suggested that, compared to normals, patients walked with stiffer gaits and less variability in movement strategies in pitch and yaw planes while they navigated a course that required bending at the hip to avoid horizontal obstacles and rotating the trunk to avoid vertical obstacles. This reduced variability may have been caused by attempts to limit the degrees of freedom during performance of a challenging task. Nevertheless, despite previous results showing that an obstacle avoidance task on compliant foam is sensitive to patients with vestibular disorders [3] the shortened version of FMT that we used in this study was not sensitive to vestibular disorders or peripheral neuropathies. Therefore, the clinician who seeks to use an obstacle avoidance test for screening should norm that specific test to determine if it will be useful with the patient population of interest. The version of FMT used in this study was shortened from the original to be more practical for use in smaller spaces, but in so doing the value of the test for patients with chronic vestibular impairments was lost. Subjects might have bumped fewer obstacles during distraction trials due to a practice effect because they performed the distraction trials after they performed the no distraction trials. Therefore, they may have learned the course well enough on the first trial.
4.2. General comments
The results from comparisons of patient subgroups to normals should be considered as pilot data. The lack of differences among subgroups may be related to small samples, especially where the patient group, as a whole, differs from normals. Because subgroup analyses were not the intent of the study those analyses are additional information. Larger samples of subgroups from the population of patients with vestibular impairments might have yielded slightly different results. Aside from patients who were not interested in spending the time to participate we excluded subjects who had had joint replacements, who could not walk unassisted without a cane or walker, or who had other neurologic or otologic diagnoses. Those exclusion criteria necessarily limited the available sample. We tested all subjects without shoes but while wearing socks. Therefore these results may not be strictly comparable to the clinical situation in which a patient is tested while wearing shoes.
The finding that AN subjects did not differ from normals on some measures was not surprising. These participants were all past the acute stage of recovery, generally considered the first three post-operative months [4, 10,12]. They were all well-compensated, as indicated by lack of complaints of vertigo and return to most activities of daily living to normal or near-normal levels. Therefore, the lack of differences indicates the plasticity of their nervous systems and of their behavioral skills in developing compensatory strategies for performing these challenging tasks. We cannot draw conclusions about underlying neural mechanisms but the data suggest that their ability to develop useful behavioral strategies is impressive.
If tests are to be useful for screening people who do not know they have disorders then the tests should be sensitive and specific enough to indentify performance impairments consistent with vestibular disorders, regardless of the final or definitive diagnosis made by a specialty care physician. Thus, these data suggest that some commonly used screening tests, which are often used by clinicians, are not useful for epidemiologic screening. Investigators and clinicians should use these tests with caution. Although the clinician may learn something clinically helpful about an individual patient’s balance skills by using these tests, none of these tests should be considered as reliably increasing the probability of a given diagnosis.
Acknowledgments
Supported by NIH grant R01DC009031 (HSC) and grants from the National Space Biomedical Research Institute through NASA NCC 9-58 (APM and JJB). We thank Christopher Miller, Wyle Integrated Science and Engineering Group, and the staff of the Center for Balance Disorders, Baylor College of Medicine, for invaluable technical assistance.
References
- 1.Allum JHJ, Adkin AL, Carpenter MG, Held-Ziolkow M, Honegger F, Pierchala K. Trunk sway measures of postural stability during clinical balance tests: effects of a unilateral vestibular deficit. Gait Posture. 2001;14:227–237. doi: 10.1016/s0966-6362(01)00132-1. [DOI] [PubMed] [Google Scholar]
- 2.Cohen HS, Kimball KT. Effectiveness of treatments for benign paroxysmal positional vertigo of the posterior canal. Otol Neurotol. 2005;26:1034–1040. doi: 10.1097/01.mao.0000185044.31276.59. [DOI] [PubMed] [Google Scholar]
- 3.Cohen HS, Kimball KT. Usefulness of some current balance tests for indentifying individuals with disequilibrium due to vestibular impairments. J Vestib Res. 2008;18:295–303. [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen HS, Kimball KT, Jenkins HA. Factors affecting recovery after acoustic neuroma resection. Acta Otolaryngologica. 2002;122:841–850. [PubMed] [Google Scholar]
- 5.Fregly AR. Vestibular ataxia and its measurement in man. In: Kornhuber HH, editor. Handbook of Sensory Physiology. VI/2. Springer Verlag; Berlin: 1974. pp. 321–360. [Google Scholar]
- 6.Fregly AR, Graybiel A. Labyrinthine defects as shown by ataxia and caloric tests. Acta Otolaryngol. 1970;69:216–222. doi: 10.3109/00016487009123356. [DOI] [PubMed] [Google Scholar]
- 7.Fregly AR, Graybiel A, Smith MJ. Walk on Floor Eyes Closed (WOFEC): a new addition to an ataxia test battery. Aerosp Med. 1972;43:395–399. [PubMed] [Google Scholar]
- 8.Gill J, Allum JHJ, Carpenter MG, Held-Ziolkowska M, Adkin AL, Honegger F, Pierchala K. Trunk sway measures of postural stability during clinical balance test: effects of age. J Gerontol A Biol Sci Med Sci. 2001;56A:M438–M447. doi: 10.1093/gerona/56.7.m438. [DOI] [PubMed] [Google Scholar]
- 9.Graybiel A, Fregly AR. A new quantitative ataxia test battery. Acta Otolaryngol. 1966;61:292–312. [PubMed] [Google Scholar]
- 10.Honrubia V, Jenkins HA, Baloh RW, Yee RD, Lau CGY. Vestibulo-ocular reflexes in peripheral labyrinthine lesions: I. Unilateral dysfunction. American Journal of Otolaryngology. 1984;5:15–26. doi: 10.1016/s0196-0709(84)80016-2. [DOI] [PubMed] [Google Scholar]
- 11.Hullar TE, Zee DS, Minor LB. Evaluation of the patient with dizziness. In: Flint PW, Haughey BH, Lund VJ, Niparko JK, Richardson MA, Robbins KT, Thomas JR, editors. Cummings Otolaryngology Head and Nek Surgery. Vol. 3. Mosby Elsevier; Philadelphia: 2010. pp. 2304–2327. [Google Scholar]
- 12.Jenkins HA, Cohen HS, Kimball KT. Long-term vestibulo-ocular reflex changes in patients with vestibular ablation. Acta Otolaryngologica. 2000;120:187–191. doi: 10.1080/000164800750000883. [DOI] [PubMed] [Google Scholar]
- 13.Kammerlind AS, Larsson PB, Ledin TEA, Skargren EIB. Reliability of clinical balance tests and subjective ratings in dizziness and disequilibirum. Adv Physiother. 2005;7:96–107. [Google Scholar]
- 14.Lempert T, Bronstein AM. Management of common central vestibular disorders. Curr Opin Otolaryngol Head Neck Surg. 2010;18:436–440. doi: 10.1097/MOO.0b013e32833dbd69. [DOI] [PubMed] [Google Scholar]
- 15.Longridge NS, Mallinson AI. Clinical Romberg testing does not detect vestibular disease. Otol Neurotol. 2010;31:803–810. doi: 10.1097/MAO.0b013e3181e3deb2. [DOI] [PubMed] [Google Scholar]
- 16.Mulavara AP, Cohen HS, Bloomberg JJ. Critical features of training that facilitate adaptive generalization of over ground locomotion. Gait Posture. 2009;29:242–248. doi: 10.1016/j.gaitpost.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shumway-Cook A, Woollacott M. Motor Control Theory and Practical Applications. Williams & Wilkins; Baltimore: 1995. [Google Scholar]
- 18.Snijders T, Bosker R. Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling. Sage Publications; Thousand Oaks, CA: 1999. [Google Scholar]
- 19.Vereeck L, Wuyts F, Truijen S, Van de Heyning PH. Clinical assessment of balance: normative data, and gender and age effects. Int J Audiol. 2008;47:67–75. doi: 10.1080/14992020701689688. [DOI] [PubMed] [Google Scholar]
- 20.Whitney SL, Herdman SJ. Physical therapy assessment of vestibular hypofunction. In: Herdman SJ, editor. Vestibular Rehabilitation. 3. FA Davis; Philadelphia: 2007. pp. 272–308. [Google Scholar]