Abstract
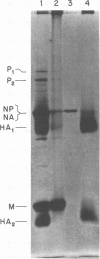
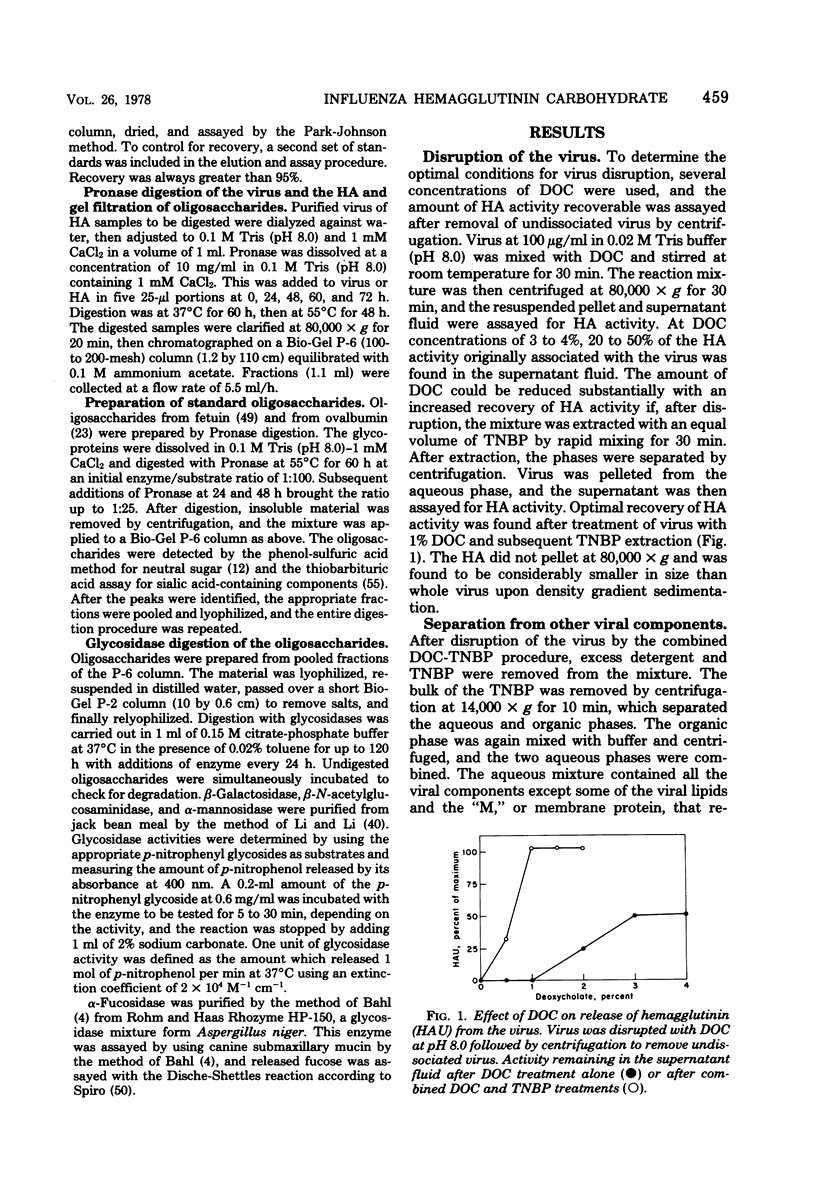
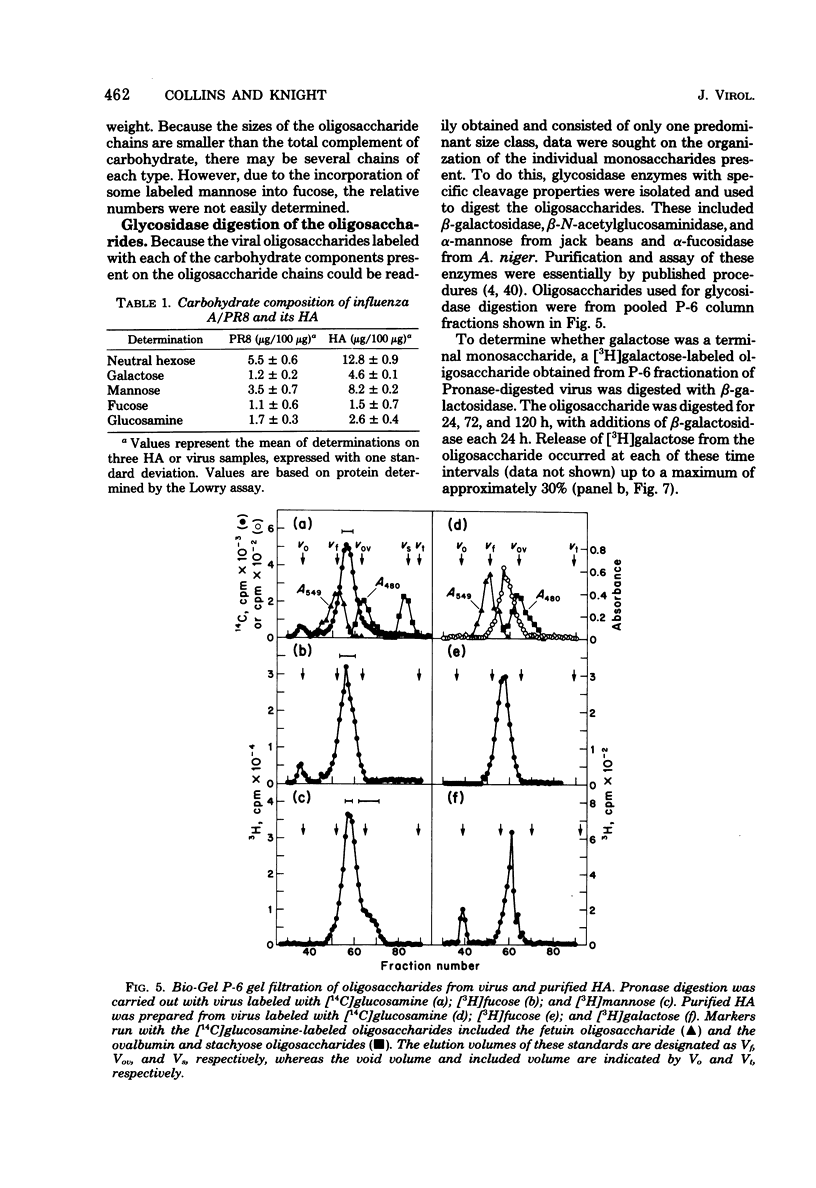
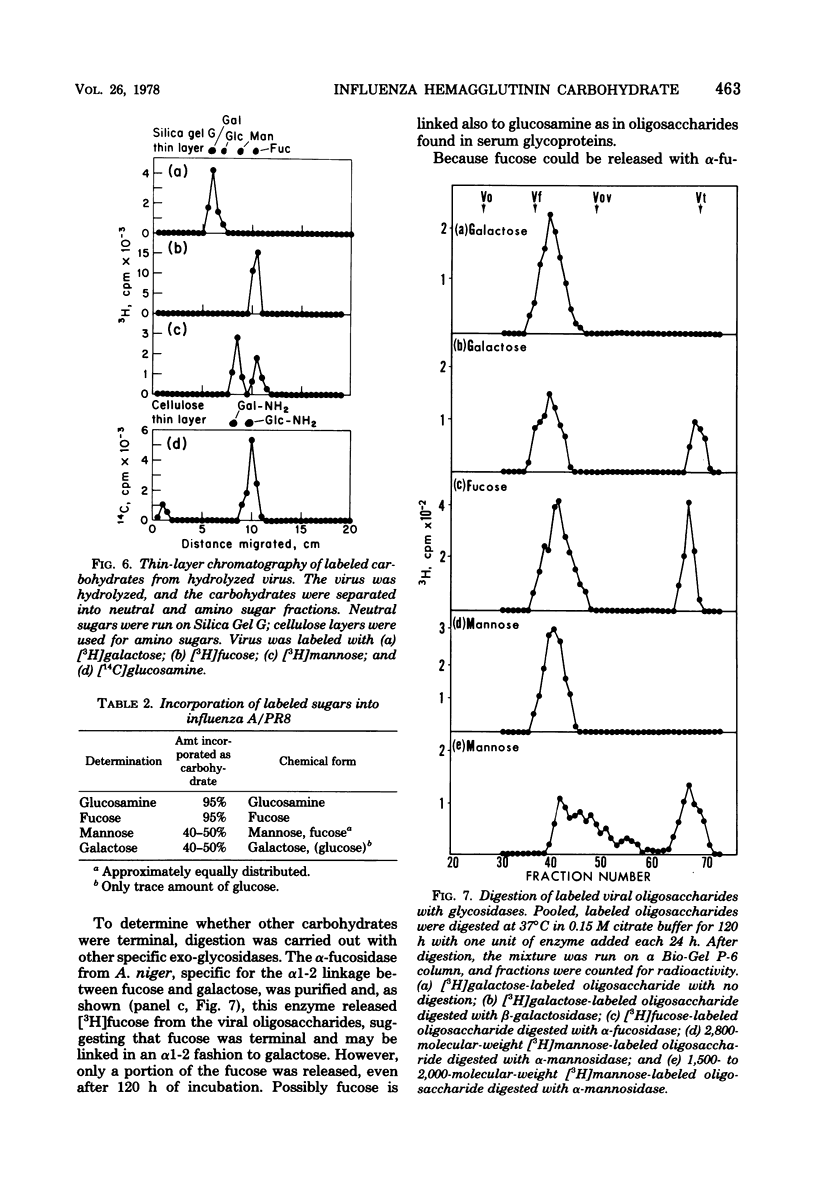
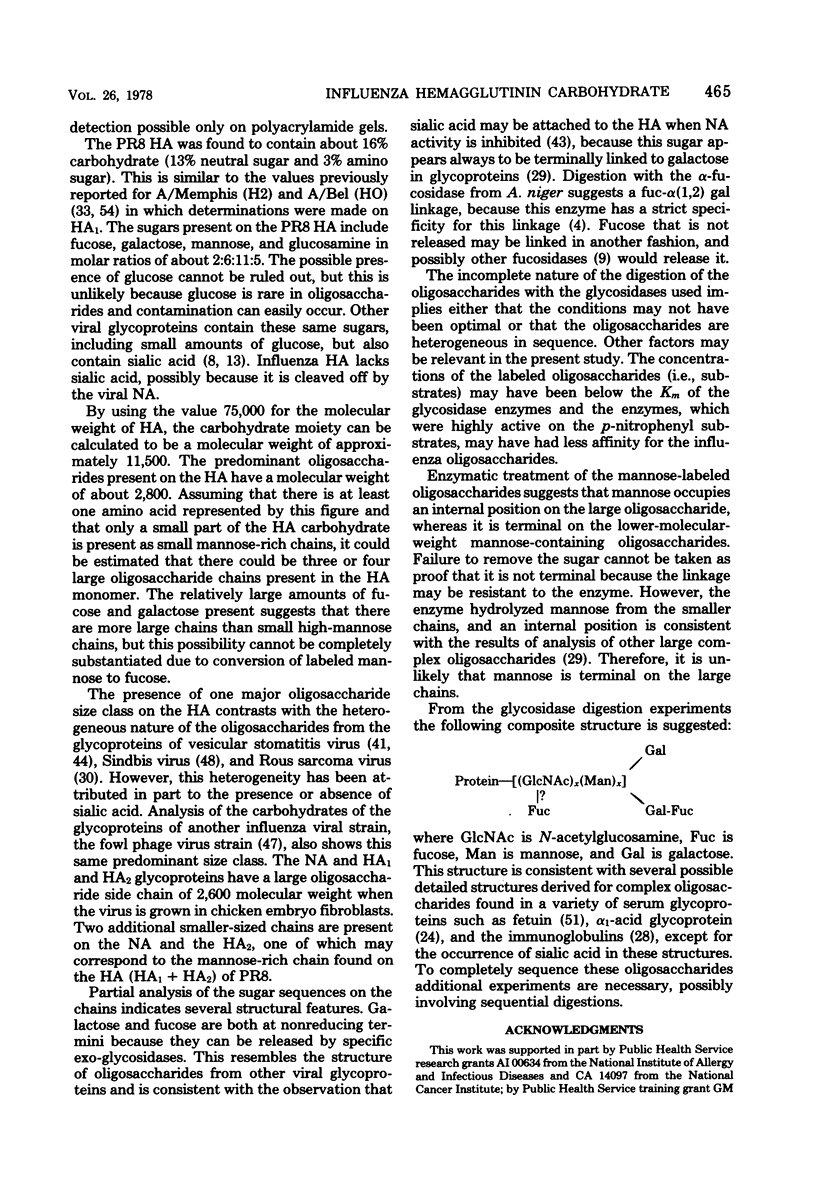
Hemagglutinin from influenza A/PR8 virus was purified after treatment of the virus with sodium deoxycholate followed by extraction with tri-n-butyl phosphate. This fully disrupted the virus while preserving hemagglutinating activity. The hemagglutinin was obtained in the form of small aggregates that could be separated from other viral components. Purified hemagglutinin was hydrolyzed to determine carbohydrate composition and digested with Pronase to analyze oligosaccharide structures. Sugars present in the hemagglutinin were galactose, mannose, fucose, and glucosamine in molar rates of about 6:11:2:5, and these comprised 16% of the hemagglutinin glycoprotein. Oligosaccharides obtained from virus included a major component of a molecular weight of 2,800, composed of glucosamine, galactose, mannose, and fucose, and a minor heterogenous component of a molecular weight of 1,500 to 2,000, containing predominantly mannose. The 2,800-molecular-weight oligosaccharide was a constituent of the hemagglutinin, and treatment of this large oligosaccharide with specific exo-glycosidases demonstrated the presence of terminal galactose and fucose and allowed the deduction of a general structure for this component.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADA G. L., GOTTSCHALK A. The component sugars of the influenza-virus particle. Biochem J. 1956 Apr;62(4):686–689. doi: 10.1042/bj0620686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aymard-Henry M., Coleman M. T., Dowdle W. R., Laver W. G., Schild G. C., Webster R. G. Influenzavirus neuraminidase and neuraminidase-inhibition test procedures. Bull World Health Organ. 1973;48(2):199–202. [PMC free article] [PubMed] [Google Scholar]
- Bachmayer H. Selective solubilization of hemagglutinin and neuraminidase from influenza viruses. Intervirology. 1975;5(5):260–272. doi: 10.1159/000149923. [DOI] [PubMed] [Google Scholar]
- Bahl O. P. Glycosidases of aspergillus niger. II. Purification and general properties of 1,2-alpha-L-fucosidase. J Biol Chem. 1970 Jan 25;245(2):299–304. [PubMed] [Google Scholar]
- Bucher D. J., Li S. S., Kehoe J. M., Kilbourne E. D. Chromatographic isolation of the hemagglutinin polypeptides from influenza virus vaccine and determination of their amino-terminal sequences. Proc Natl Acad Sci U S A. 1976 Jan;73(1):238–242. doi: 10.1073/pnas.73.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D. J., Keegstra K. Purification and composition of the proteins from Sindbis virus grown in chick and BHK cells. J Virol. 1976 Dec;20(3):676–686. doi: 10.1128/jvi.20.3.676-686.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen R. B., Pierce J. G. Purification and properties of an alpha-L-fucosidase from rat epididymis. J Biol Chem. 1972 Jan 10;247(1):23–32. [PubMed] [Google Scholar]
- Clamp J. R., Bhatti T., Chambers R. E. The determination of carbohydrate in biological materials by gas-liquid chromatography. Methods Biochem Anal. 1971;19:229–344. doi: 10.1002/9780470110386.ch3. [DOI] [PubMed] [Google Scholar]
- Etchison J. R., Holland J. J. Carbohydrate composition of the membrane glycoprotein of vesicular stomatitis virus. Virology. 1974 Jul;60(1):217–229. doi: 10.1016/0042-6822(74)90379-1. [DOI] [PubMed] [Google Scholar]
- FROMMHAGEN L. H., KNIGHT C. A., FREEMAN N. K. The ribonucleic acid, lipid, and polysaccharide constituents of influenza virus preparations. Virology. 1959 Jun;8(2):176–197. doi: 10.1016/0042-6822(59)90003-0. [DOI] [PubMed] [Google Scholar]
- Gandhi S. S., Stanley P., Taylor J. M., White D. O. Inhibition of influenza viral glycoprotein synthesis by sugars. Microbios. 1972 Jan;5(17):41–50. [PubMed] [Google Scholar]
- HARBOE A. THE NORMAL ALLANTOIC ANTIGEN WHICH NEUTRALIZES THE INFLUENZA VIRUS HI-ANTIBODY TO HOST MATERIAL. Acta Pathol Microbiol Scand. 1963;57:488–492. doi: 10.1111/j.1699-0463.1963.tb05116.x. [DOI] [PubMed] [Google Scholar]
- Hayman M. J., Skehel J. J., Crumpton M. J. Purification of virus glycoproteins by affinity chromatography using Lens culinaris phytohaemagglutinin. FEBS Lett. 1973 Jan 15;29(2):185–188. doi: 10.1016/0014-5793(73)80557-5. [DOI] [PubMed] [Google Scholar]
- Higginbotham J. D., Schöyen R., Mortensson-Egnund K., How M. J., Harboe A. Antibody-combining oligosaccharides from a chick allantoic glycopeptide sulphate associated with influenza virus haemagglutinin. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(3):349–356. doi: 10.1111/j.1699-0463.1971.tb00072.x. [DOI] [PubMed] [Google Scholar]
- Kaluza G., Scholtissek C., Rott R. Inhibition of the multiplication of enveloped RNA-viruses by glucosamine and 2-deoxy-D-glucose. J Gen Virol. 1972 Mar;14(3):251–259. doi: 10.1099/0022-1317-14-3-251. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Scholtissek C., Rott R. Inhibition of glycoprotein biosynthesis of influenza virus by D-glucosamine and 2-deoxy-D-glucose. Virology. 1972 Sep;49(3):723–734. doi: 10.1016/0042-6822(72)90529-6. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Keller J., Baenziger J., Kornfeld S. The structure of the glycopeptide of human gamma G myeloma proteins. J Biol Chem. 1971 May 25;246(10):3259–3268. [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Comparative aspects of glycoprotein structure. Annu Rev Biochem. 1976;45:217–237. doi: 10.1146/annurev.bi.45.070176.001245. [DOI] [PubMed] [Google Scholar]
- LAVER W. G. STRUCTURAL STUDIES ON THE PROTEIN SUBUNITS FROM THREE STRAINS OF INFLUENZA VIRUS. J Mol Biol. 1964 Jul;9:109–124. doi: 10.1016/s0022-2836(64)80094-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Valentine R. C. Morphology of the isolated hemagglutinin and neuraminidase subunits of influenza virus. Virology. 1969 May;38(1):105–119. doi: 10.1016/0042-6822(69)90132-9. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Webster R. G. Preparation and immunogenicity of an influenza virus hemagglutinin and neuraminidase subunit vaccine. Virology. 1976 Feb;69(2):511–522. doi: 10.1016/0042-6822(76)90481-5. [DOI] [PubMed] [Google Scholar]
- Lehnhardt W. F., Winzler R. J. Determination of neutral sugars in glycoproteins by gas-liquid chromatography. J Chromatogr. 1968 May 7;34(4):471–479. doi: 10.1016/0021-9673(68)80091-3. [DOI] [PubMed] [Google Scholar]
- Moyer S. A., Tsang J. M., Atkinson P. H., Summers D. F. Oligosaccharide moieties of the glycoprotein of vesicular stomatitis virus. J Virol. 1976 Apr;18(1):167–175. doi: 10.1128/jvi.18.1.167-175.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. S., Etchison J. R., Summers D. F. Glycosylation sites of vesicular stomatitis virus glycoprotein. J Virol. 1976 Sep;19(3):871–878. doi: 10.1128/jvi.19.3.871-878.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rott R., Becht H., Klenk H. D., Scholtissek C. Interactions of concanavalin A with the membrane of infleunza virus infected cells and with envelope components of the virus particle. Z Naturforsch B. 1972 Mar;27(3):227–233. doi: 10.1515/znb-1972-0303. [DOI] [PubMed] [Google Scholar]
- SPIRO R. G. Studies on fetuin, a glycoprotein of fetal serum. II. Nature of the carbohydrate units. J Biol Chem. 1962 Feb;237:382–388. [PubMed] [Google Scholar]
- Sefton B. M., Keegstra K. Glycoproteins of Sindbis virus: priliminary characterization of the oligosaccharides. J Virol. 1974 Sep;14(3):522–530. doi: 10.1128/jvi.14.3.522-530.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P., Crook N. E., Streader L. G., Davidson B. E. The polypeptides of influenza virus. 8. Large-scale purification of the hemagglutinin. Virology. 1973 Dec;56(2):640–645. doi: 10.1016/0042-6822(73)90066-4. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]