Abstract
The sphingolipids are a diverse family of lipids with important roles in membrane compartmentalization, intracellular signaling, and cell-cell recognition. The central sphingolipid metabolite is ceramide, formed by the transfer of a variable length fatty acid from coenzyme A to a sphingoid base, generally sphingosine or dihydrosphingosine (sphinganine) in mammals. This reaction is catalyzed by a family of six ceramide synthases (CerS1-6). CerS activity is usually assayed using either radioactive substrates or LC-MS/MS. We describe a CerS assay with fluorescent, NBD-labeled sphinganine as substrate. The assay is readily able to detect endogenous CerS activity when using amounts of cell or tissue homogenate protein that are lower than those reported for the radioactive assay, and the Michaelis-Menten constant was essentially the same for NBD-sphinganine and unlabeled sphinganine, indicating that NBD-sphinganine is a good substrate for these enzymes. Using our assay, we confirm that the new clinical immunosuppressant FTY720 is a competitive inhibitor of CerS activity, and show that inhibition requires the compound's lipid tail and amine headgroup. In summary, we describe a fluorescent assay for CerS activity that circumvents the need to use radioactive substrates, while being more accessible and cheaper than LC-MS based assays.
Keywords: ceramides, enzymology/enzyme mechanisms, fatty acid/transferase, mass spectrometry, sphingolipids
The sphingolipids are a diverse family of lipids and glycolipids that are essential for compartmentalization of cell membranes as signaling metabolites and as membrane anchors for carbohydrate patterning of the extracellular surface (1, 2). The lipid ceramide is the central metabolite of the sphingolipid family and can be modified on its primary hydroxyl headgroup through the addition of a phosphocholine group forming sphingomyelin, a phosphate group forming ceramide 1-phosphate, or a hexose group forming glucosyl- or galactosylceramide. Sequential addition of galactose, N-acetylgalactosamine, and neuraminic acid groups to glucosylceramide gives rise to a diverse array of glycolipids termed globosides and gangliosides, which pattern the cell surface and are critically important mediators of higher order mammalian physiology (2).
In de novo sphingolipid synthesis, dihydroceramide is formed by transfer of a fatty acid chain from fatty acid-CoA to the amino group of sphinganine. This reaction is catalyzed by a family of six ceramide synthases (CerS1-6; also referred to as LASS1-6), in the endoplasmic reticulum. Dihydroceramide is then enzymatically desaturated to form ceramide. Sphingosine may also be N-acylated by CerS enzymes, directly forming ceramide through what is known as the sphingolipid salvage pathway. The fatty acids transferred to sphinganine or sphingosine vary in length from 14 to 28 carbons, are most commonly saturated or mono-unsaturated, and may be hydroxylated (1, 3). Each CerS enzyme exhibits a preference for fatty acids of different length, for example CerS1 preferentially catalyses the addition of 18-carbon (C18) fatty acids to sphinganine (4), whereas CerS2 preferentially adds long chain (C22–C26) fatty acids (5). The different acyl chain lengths of the ceramides appear to confer distinct functional roles; for example, long chain ceramides synthesized by CerS2 are essential constituents in myelin (6), C18 ceramide is an important pro-apoptotic mediator (7, 8), and very long chain ceramides synthesized by CerS3 are critically important for skin barrier function (9).
There are two methods that are regularly used for assaying ceramide synthase activity. One method uses tritiated sphinganine or sphingosine and resolution of ceramide products using TLC (10, 11). Tritiated chemicals, although safe if used properly, are dangerous if ingested. Unlike other common radiochemicals, tritium is difficult to monitor. In most countries, work with radiochemicals involves extra paperwork whereas work with fluorescent substrates is safer and involves much less bureaucracy. An alternative radioactive assay with 14C-labeled fatty acid substrate has also been described (12). Another approach is to quantify ceramide formation using LC-MS/MS (13). Although highly accurate and sensitive, this approach requires lengthy access to highly specialized equipment and specific expertise. The HPLC takes 15 min per sample. For a relatively small experiment involving, for example, eight samples in triplicate and external standards at the start and end of the run, plus regular blanks, the machine time required is at least a full working day and usually an overnight booking. This method is therefore somewhat impractical when equipment is shared or centralized and regular CerS assays are needed. It is also associated with considerable costs when access or cost-recovery fees are charged, as is commonly the case with expensive core equipment.
Herein, we describe a simple fluorescent assay for CerS activity in cultured cell or tissue homogenates, which uses commercially available sphinganine labeled with an NBD fluorescent group (NBD-sphinganine; (2S,3R)-2-amino-18-((7-nitrobenzo[c][1,2,5]oxadiazol-4-yl)amino)octadecane-1,3-diol). We show that this substrate is efficiently utilized by CerS enzymes, and that the assay can be used to screen for inhibitors of CerS activity.
MATERIALS AND METHODS
Materials
NBD-sphinganine, nervonoyl (C24:1)-CoA, sphinganine, C16:0 dihydroceramide, and C24:1 dihydroceramide were purchased from Avanti Polar Lipids (Alabaster, AL). Palmitoyl (C16:0) and stearoyl (C18:0)-CoA, and Fumonisin B1 were from Sigma Aldrich (Castle Hill, NSW, Australia). Silica Gel 60 TLC plates were from Merck (Kilsyth, VIC, Australia). All cell culture media reagents were from Invitrogen (Mulgrave, VIC, Australia). Human CerS2 and CerS5 cDNAs were purchased as full-length TrueClone cDNAs from OriGene (Rockville, MD) in vector pCMV-XL6 FTY720 was purchased from Cayman Chemical (Ann Arbor, MI). AAL(S) was synthesized following the procedure reported by Hinterding et al. (14). The synthesis of AAL(S) analogs HDTF020, HDTG022, and HDTG046 will be described in detail in a separate publication.
Cell culture and transfection
HEK293 cells were grown in DMEM medium supplemented with 10% FBS, 2 mM L-glutamine, and penicillin/streptomycin. Cells were transfected in DMEM containing 2% FBS with polyethylenimine (PEI) reagent (Sigma Aldrich) (15). Each 175 cm2 flask of cells was transfected with 50 μg plasmid cDNA and 75 μl of a 1 mg/ml stock of PEI. DNA and PEI were premixed for 10 min before adding to the cells, and cells were harvested 24 h after transfection.
Preparation of cell or tissue homogenates
Preparation of cell or tissue homogenates was performed essentially as described by Lahiri et al (10). Two 175 cm2 flasks of cells were scraped into 20 ml PBS using a cell scraper, then pelleted and resuspended in 1 ml lysis buffer containing 20 mM Hepes, pH 7.4, 25 mM KCl, 2 mM MgCl2, 250 mM Sucrose, and complete protease inhibitor cocktail (Roche). The cells were broken on ice with 50 to 100 strokes of a small glass Dounce homogenizer (checking cells under a light microscope to ensure that they were broken). The suspension was then centrifuged at 1,000 g for 10 min to clear unbroken cells and nuclei. The supernatant (crude cell homogenate) was dispensed into aliquots and stored at −80°C. The protein concentration was determined by bicinchoninic acid (BCA) assay relative to BSA standards. To obtain crude tissue homogenates, 50–100 mg fresh mouse tissues were homogenized in 2 ml lysis buffer as detailed above.
Fluorescent CerS assay
The assay was performed essentially as described (10) except using NBD-sphinganine in place of unlabeled sphinganine spiked with tritiated sphinganine. Reaction buffer was 20 mM Hepes, pH 7.4, 25 mM KCl, 2 mM MgCl2, 0.5 mM DTT, 0.1% (w/v) fatty acid-free BSA, 10 μM NBD-sphinganine, and 50 μM fatty acid-CoA. For many assays, sucrose (250 mM) was included in the reaction buffer to maintain consistency in reaction conditions across different samples (i.e., due to different volumes of homogenate in different reactions). Our results indicated that the inclusion of sucrose did not adversely affect the reaction rate. The 100 μl reactions were started with the addition of 50 μg homogenate protein and incubated with shaking at 35°C for 30–120 min. Reactions were stopped with the addition of 250 μl chloroform/methanol (2:1). The tubes were vortexed thoroughly, centrifuged at 21,000 g for 1 min to separate the phases, and the lower organic phase was transferred to a 5 ml glass tube. The aqueous phase was reextracted as above and the two organic extracts were combined then dried down in a SpeedVac SC210 (ThermoFisher Scientific), resuspended in 100 μl methanol with vortexing, spotted (2 × 2 μl) onto aluminum-backed Silica Gel 60 TLC plates, and resolved in chloroform/methanol/water (8:1:0.1, v/v/v) (16). Fluorescence was detected directly on the TLC plates using a LAS Mini 4000 imaging system and fluorescent pixel intensity was quantified using the associated ImageQuant vendor software (GE Healthcare, Rydalmere, NSW, Australia). NBD-sphinganine standards of different concentrations were spotted onto the TLC plate before imaging, in order to construct a standard curve for fluorescence vs pmoles compound.
To assess the effect of FTY720, AAL(S), and Fumonisin B1 on enzyme kinetics, the cell homogenate was preincubated for 5 min with each inhibitor, then added to an equal volume of reaction buffer containing the substrates to start the reactions, such that the final inhibitor concentration was 20 μM.
NBD-dihydroceramide purification and verification by high-resolution MS
Reactions (100 μl) containing 20 μM NBD-sphinganine, 100 μM C16:0- or C24:1-CoA, and 50 μg CerS5- or CerS2-expressing cell extract were incubated for 4 h at 35°C. The total reaction product was extracted and resolved on TLC as described above. Fluorescent products, which were clearly visible under laboratory lights due to their abundance, were cut out of the TLC and washed off the silica with 200 μl chloroform/methanol (2:1), into glass test tubes. The extracts were dried down and reconstituted in 0.5 ml methanol, then analyzed on an Orbitrap LTQ XL mass spectrometer (Thermo Fisher Scientific, North Ryde, NSW, Australia) using a nanospray (nano-electrospray) ionization source. The instrument was calibrated with a standard calibration solution (as outlined in the instrument manual) on the day of analysis. The instrument conditions were optimized for sensitivity on each compound of interest using LC tune software. The analysis was carried out in positive ion mode using the orbitrap FTMS analyzer at a resolution of 100,000 fwhm (full width at half maximum). Samples (5 μl) were drawn up into a glass needle and inserted into the nanospray source. Ions generated were measured over the mass range 150–2000 Da. Data was acquired in full scan mode over 60 s and analyzed using the Qual Browser feature in Xcaliber 2.1 (Thermo Fisher Scientific).
CerS assay using LC-MS/MS
To measure ceramide formation using LC-MS/MS, the assay was performed essentially as described above except that sphinganine was used as the substrate in place of NBD-sphinganine. Note that for these experiments measuring Km, the sphinganine concentration varied from 0.625 to 40 μM, while all other conditions were kept constant. Each sample was spiked with 40 μl of 0.5 μM C17:0 ceramide as an internal standard for recovery and mass spectrometry immediately prior to stopping the reactions with chloroform/methanol. Reactions were then extracted and dried as described above and resuspended in 200 μl HPLC mobile phase consisting of 20%:80% Mobile phase A (2 mM ammonium formate/0.2% formic acid in water): Mobile phase B (1 mM ammonium formate/0.2% formic acid in methanol). Lipids were resolved on a 3 × 150 mm Agilent XDB-C8 column (5 μM pore size) at a flow rate of 0.5 ml/min using a 15 min gradient starting at 20:80 A:B, increasing to 100% B after 1 min, holding at 100% B for 13 min, then reequilibrating to 20:80 A:B for 1 min. Lipids were detected on a Thermo Quantum Access triple quadrupole mass spectrometer operating in positive ion mode. Precursor and product ions used to identify reaction products were as follows: C17:0 ceramide, m/z 552.6 → 264.1; C16:0 dihydroceramide, m/z 540.5 → 266.1; C24:1 dihydroceramide, m/z 650.7 → 266.1. Linear calibration curves were constructed using synthetic C16:0 and C24:1 dihydroceramide external standards over the range 0.032–100 pmol on-column. The ratio of external to internal standard peak area was used to construct calibration curves and determine the amount of dihydroceramide product in experimental samples (13).
Statistical analysis and curve fitting
All curves were fit using GraphPad PRISM software, which directly calculates Km and Vmax from the Michaelis-Menten curves. Coefficient of Variation (%CV) was calculated as the standard deviation divided by the mean, expressed as a percentage.
RESULTS
Suitability of NBD-sphinganine as a substrate for CerS assays
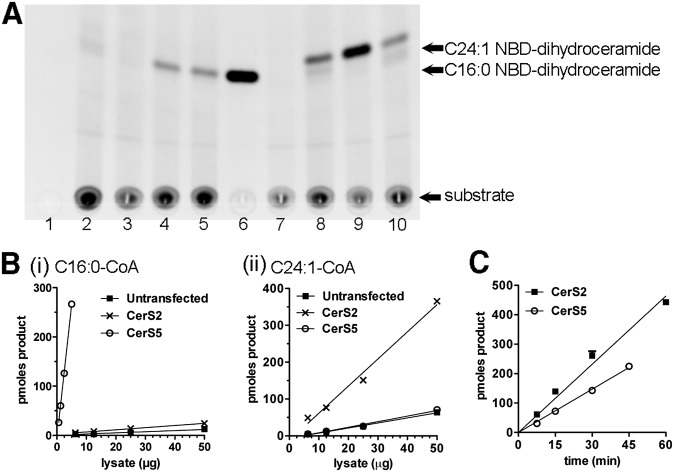
We first determined whether NBD-sphinganine would be tolerated as a substrate for CerS enzymes. Homogenates were prepared from untransfected HEK293 cells or cells expressing human CerS2 or CerS5, and CerS activity was determined using C16:0- or C24:1-CoA as the fatty acid substrate. No product was formed in the absence of cell lysate (Fig. 1A, lanes 3 and 7) or NBD-sphinganine (lane 1) and only a faint smear was visible in the absence of added fatty acid-CoA substrate (lane 2). The amount of product formed was dependent on the particular CerS enzyme expressed (Fig. 1A, B), and linear with respect to lysate protein concentration (Fig. 1B). Our results are in agreement with previous studies showing that CerS5 preferentially utilizes shorter-chain fatty acid substrates (C14–C18), whereas CerS2 preferentially utilizes long-chain substrates (C22–C26) (11); and results indicating that overexpressing CerS5 in HEK293 cells produces a much higher specific activity than CerS2 (10). Product formation was linear with respect to time under conditions in which no more than 30% of the NBD-sphinganine substrate (i.e., 300 pmoles) was converted to product (Fig. 1C); above this level availability of substrate becomes rate-limiting for product formation, as illustrated for the CerS2-driven reaction in Fig. 1C.
Fig. 1.
Synthesis of C16:0 or C24:1 NBD-dihydroceramide catalyzed by endogenous or overexpressed ceramide synthases. A: TLC image illustrating that CerS reaction proceeds only in the presence of cell lysate and fatty acid-CoA. Homogenates of untransfected (lanes 1, 2, 4, and 8), CerS2- (lanes 5 and 9), or CerS5-expressing (lanes 6 and 10) HEK293 cells (25 μg/reaction) were incubated for 30 min with 10 μM NBD-sphinganine and 50 μM C16:0-CoA (lanes 3–6) or C24:1-CoA (lanes 7–10). Lane 1: no NBD-sphinganine control; Lane 2: No fatty acid-CoA control; Lanes 3 and 7: no homogenate control (i.e., no enzyme). B: Product formed as a function of lysate concentration, using (i) C16:0-CoA or (ii) C24:1-CoA as the fatty acid substrate. Reactions in B(i) were run for 30 min while those in (ii) were run for 60 min. C: Product formation as a function of time, using CerS5-expressing cell homogenate (5 μg/reaction) and C16:0-CoA (50 μM), or CerS2-expressing homogenate (50 μg/reaction) and C24:1-CoA (50 μM). Data points are mean and standard error of triplicate assays and representative of two or more independent experiments.
The identity of the products formed was confirmed using a high-resolution Orbitrap mass spectrometer: C16:0 and C24:1 NBD-dihydroceramide were prepared using the respective fatty acid-CoA substrates and lysates of HEK293 cells overexpressing CerS5 (C16:0) or CerS2 (C24:1), and purified from TLC plates as described in Materials and Methods. Precise m/z values of 740.5277 (M + Na; expected m/z 740.5302; mass accuracy 3.38 ppm) and 850.6369 (M + Na; expected m/z 850.6398; mass accuracy 3.47 ppm) were obtained for C16:0 and C24:1 NBD-dihydroceramide, respectively, indicating that the expected products had been formed. As indicated in Fig. 2, (right-most lane) C16:0 NBD-dihydroceramide migrates more slowly on TLC than C24:1 NBD-dihydroceramide, using the choloroform/methanol/water solvent (8:1:0.1) system (16).
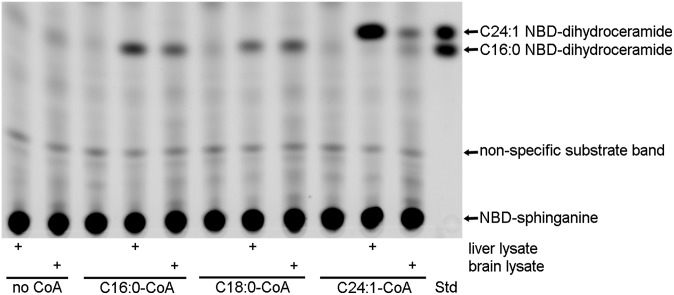
Fig. 2.
Synthesis of C16:0, C18:0 and C24:1 NBD-dihydroceramide catalyzed by endogenous tissue ceramide synthases. CerS reactions (120 min) were run in the presence of 10 μM NBD-sphinganine, 50 μM fatty acid-CoA (as indicated on the figure), and 50 μg mouse liver or cerebellum homogenate. The first two lanes on the left were from reactions with tissue homogenate but no fatty acid-CoA. The right-most lane (Std) contains a mixture of C16:0 and C24:1 NBD-dihydroceramide standards prepared as described in text.
It was also possible to detect CerS activity toward C16:0, C18:0, and C24:1-CoA substrates in tissue homogenates (Fig. 2). Using equivalent amounts of protein, the liver homogenate contained much more C24:1 CerS activity, and slightly higher C16:0 CerS activity, whereas C18:0 CerS activity was similar, if not slightly higher in cerebellum. This pattern is in agreement with the published observation that CerS2 mRNA is expressed at a much higher level in the liver (5, 11). However, CerS1 expression is highest in the brain, and we might therefore have expected significantly higher C18:0 CerS activity in cerebellum. This was not observed, possibly indicative of the balance between CerS1 and other enzymes that possess C18 CerS activity, specifically CerS4 and CerS5; or posttranscriptional regulation of CerS1. It is also possible that abundant lipids in the crude brain homogenate interfere somewhat with the CerS assay.
Limit of detection
Using our assay, it is possible to detect 0.5 pmoles of reaction product on the TLC plate, yielding %CV values for triplicate reactions in the range 2%–10% in multiple different experiments. When the amount of NBD-ceramide on the TLC plate dropped below 0.25 pmoles, the %CV increased appreciably (>20%). Note that this limit of detection was determined by running reactions and resolving the product on TLC (as distinct from determining the limit of detection using a synthetic standard), and is therefore a reflection of the limit of resolution for the assay with cell lysate.
Comparison of NBD-sphinganine to unlabeled sphinganine
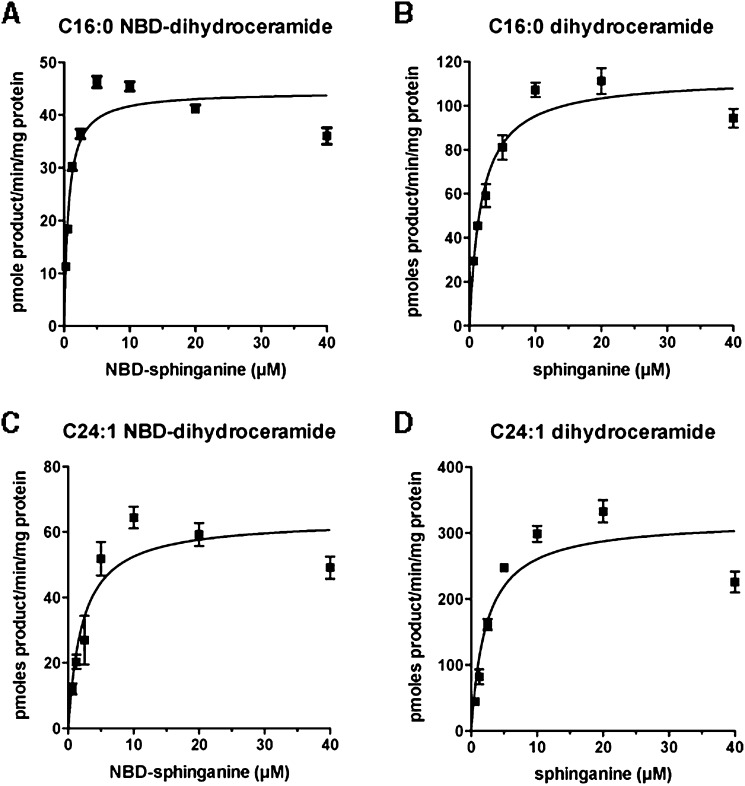
To determine how NBD-sphinganine compares to the natural substrate in terms of enzyme affinity, the Km for NBD-sphinganine, with product detected using TLC and a fluorescence imager, was compared with that obtained with unlabeled sphinganine, in which the product was detected using LC-MS/MS. In three independent experiments with C16:0-CoA as the fatty acid substrate, a Km of 1.91 ± 0.84 μM (mean ± SE) was measured for NBD-sphinganine. This is very similar to the average Km of 1.16 ± 0.36 μM measured for sphinganine using the LC-MS assay. As C24:1 CoA is utilized by different CerS enzymes compared with C16:0 CoA, we also measured the affinity of CerS enzymes for NBD-sphinganine using C24:1 CoA as the fatty acid substrate: an average Km of 3.61 ± 1.86 μM was obtained with NBD-sphinganine, and 3.05 ± 0.81 μM with sphinganine. A representative experiment for each condition is shown in Fig. 3. These results indicate that NBD-sphinganine is a good substrate for ceramide synthases, with very similar affinity to the natural substrate.
Fig. 3.
NBD-sphinganine is equivalent to natural sphinganine as a substrate for endogenous ceramide synthases. Untransfected HEK293 cell extracts were incubated with 100 μM C16:0-CoA (A and B) or C24:1-CoA (C and D) and varying concentrations of NBD-sphinganine (A and C) or sphinganine (B and D). Product formed was quantified by TLC with a fluorescent imager (A and C) or LC-MS/MS (B and D). Data points are the mean and standard error of triplicate reactions, and Km values derived results from three such assays are given in-text.
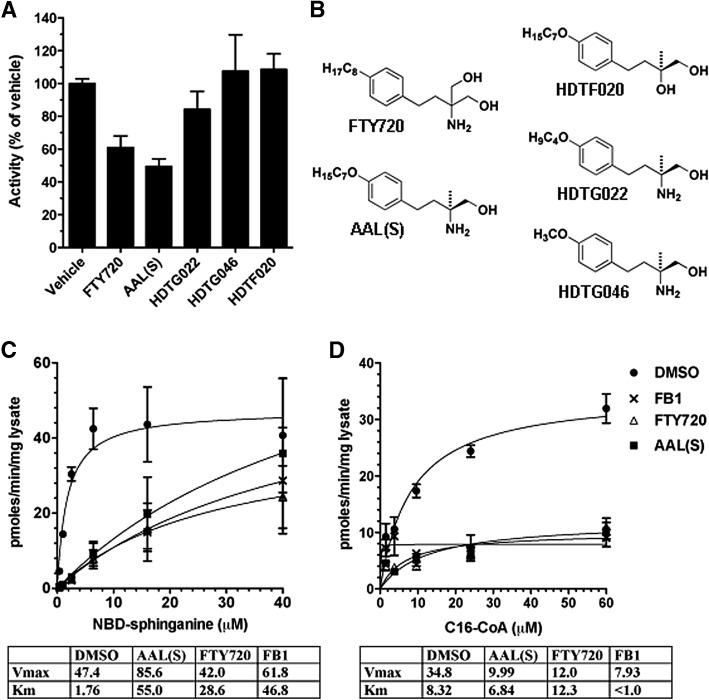
Inhibition of CerS activity by FTY720: structural requirements for inhibition
Two publications from 2009 demonstrated inhibition of CerS activity by the new therapeutic FTY720, recently approved for treatment of relapsing multiple sclerosis. FTY720 was reported to inhibit endogenous C16, C18, and C24 CerS activity of several human cell lines (17) as well as primary human aortic endothelial cells (13). We first tested whether the nonphosphorylatable, chiral deoxy FTY720 analog AAL(S) (18, 19) inhibited C16:0 CerS activity in cell homogenates. C16:0 CerS activity was inhibited with slightly greater potency by AAL(S) when compared with FTY720 (Fig. 4A). The mode of inhibition for both FTY720 and AAL(S) was clearly competitive with respect to NBD-sphinganine (Fig. 4C), as the Km for NBD-sphinganine increased from 1.76 μM in the vehicle control to 28.6 μM (FTY720) or 55.0 μM [AAL(S)], whereas the Vmax did not decrease. In accordance with competition for the sphinganine binding site, inhibition was noncompetitive with respect to C16:0-CoA (Fig. 4D), with no significant change in the Km but a reduction in the Vmax from 34.8 product/min/mg protein in the vehicle control to 12.0 and 9.99 pmoles product/min/mg protein, respectively, for FTY720 and AAL(S). We further investigated whether two key structural features of FTY720/AAL(S) are required for CerS inhibition: the acyl lipid tail and the amine group (Fig. 4B). Both the amine group (eliminated in HDTF020) and the lipid tail (eliminated in HDTG046) were essential for inhibition of C16:0 dihydroceramide synthesis, and reducing the lipid tail by only three carbons (HDTG022) reduced the extent of inhibition at 10 μM from 50% to only 16% inhibition, relative to the vehicle control (Fig. 4A).
Fig. 4.
Inhibition of endogenous C16 dihydroceramide synthesis by FTY720. A: Effect of FTY720 analogs at a concentration of 10 μM on C16:0 CerS activity of untransfected HEK293 lysates. Lysate in reaction buffer was preincubated for 5 min with compounds before addition of 50 μM C16:0-CoA and 10 μM NBD-sphinganine to start the reactions. Product formed was normalized to vehicle control. B: Structures of FTY720 analogs tested. C: Effect of FTY720 (△), AAL(S) (▪), FB1 (×), or DMSO vehicle control (•) on endogenous CerS activity of HEK293 homgenates, in the presence of 50 μM C16:0-CoA and varying concentrations of NBD-sphinganine. D: Effect of inhibitors on C16:0 CerS activity in the presence of 10 μM NBD-sphinganine and varying concentrations of C16:0-CoA.
C16:0 CerS activity in our assay was also inhibited by Fumonisin B1 (FB1), a well established CerS inhibitor (Figs. 4C, D) (20). Inhibition was competitive with respect to NBD-sphinganine (Km increased from 1.76 to 46.8 μM), but unlike FTY720 and AAL(S), inhibition with FB1 showed the pattern of uncompetitive inhibition with respect to C16-CoA (both Vmax and Km decreased).
DISCUSSION
The present study has revealed that NBD-sphinganine may be used in place of sphinganine as a substrate for CerS activity assays. The assay works well with endogenous or overexpressed CerS enzymes, and cell or tissue homogenates. Based on measurements of the Michaelis-Menten constant, the fluorescently-labeled substrate appears to show an equivalent enzyme affinity to the natural substrate, sphinganine. Km values reported herein (between 1 and 2 μM for C16:0 CerS, and between 3 and 4 μM for C24:1 CerS activity) are very similar to those reported in recent literature where a similar assay was employed (10, 12). As discussed by Lahiri et al. (10), older results using different assay conditions (including detergent) yielded much higher Km values. Berdyshev et al. (13) reported a Km of 68 nM for CerS2, but that is the only report ascribing a sub-micromolar Km to the CerS enzymes. CerS activity was subject to substrate inhibition at 40 μM sphinganine or NBD-sphinganine, the highest concentration tested (Fig. 3). Substrate inhibition is observed for many metabolic enzymes and often occurs at physiologically relevant concentrations (21). However, it is difficult to determine if substrate inhibition of CerS enzymes is physiologically relevant because of the difficulty in estimating the local sphinganine concentration in the endoplasmic reticulum membrane. Theoretically, substrate inhibition could be a mechanism limiting the synthesis of ceramides in the presence of abundant sphinganine.
The assay was further verified by demonstrating that C16:0 CerS activity is inhibited by FB1. We observed competitive inhibition with respect to NBD-sphinganine, in agreement with Merrill et al. (20). Inhibition with respect to C16:0-CoA was uncompetitive in our assay (Fig. 4D), whereas Merrill et al. reported mixed inhibition with respect to C18:0-CoA. Importantly, FB1 is a sphinganine analog, and both our results and theirs indicate that competition with the sphinganine substrate is the primary mechanism (20). Inhibition of endogenous C16:0 CerS activity by FTY720 and its related nonphosphorylatable analog AAL(S) was also competitive with respect to NBD-sphinganine, in agreement with the findings of Berdyshev et al. (13) but differing from the uncompetitive model proposed by Lahiri et al. (17). In further support of the mode of inhibition involving competition for the sphinganine binding site, C16:0 CerS activity was not inhibited by FTY720/AAL(S) analogs lacking either the lipid “tail” or the amine headgroup, which are characteristic features of sphinganine.
Using 20–50 μg total lysate protein, we could reliably quantify endogenous CerS activity in HEK293 cell lysates, mouse liver, or brain homogenates. This compares favorably with published results using tritiated sphinganine (10). Measurement of endogenous CerS activity in rat liver microsomes has been reported to require hundreds of micrograms of microsomal protein per assay, which is more than an order of magnitude more starting material than the 50 μg liver homogenate used in our assay (22). In a Methods paper employing 14C-labeled C16-CoA, 75 μg microsomal membrane protein was used, therefore requiring much more starting material than is needed for our assay (12). Picomole quantities of reaction product were detected on the TLC plates with very low experimental replicate variability (CV < 10%). One picomole corresponds to only 0.1% of the standard 1 nmole substrate added to each reaction, providing a large dynamic range for detecting variation in activity among different biological samples.
The fluorescent NBD-sphinganine substrate is commercially available and costs approximately the same as the tritiated substrate per assay, assuming that 1 nmole NBD-sphinganine in a 100 μl reaction is used for the fluorescent version and 0.2–0.25 μCi tritiated sphinganine per reaction is used for the radioactive assay, as published (10, 11). Although per-assay costs are similar, it is much cheaper to implement the fluorescent assay because smaller amounts of labeled substrate can be purchased compared with the minimum amount of tritiated substrate that can be purchased. Costs can be further reduced by running 50 μl reactions, making the fluorescent assay more cost effective. The LC-MS/MS based assay is very sensitive, with a sub-pM limit of detection, but will generally cost much more when equipment access or cost recovery fees must be paid. An important advantage with the fluorescent assay format is that the fluorescent signal on the TLC plate can be quantified immediately using densitometry. To detect tritiated ceramide, one must either scrape the ceramide bands from the TLC into vials and quantify the radioactivity by scintillation counting or expose the TLC plate to film or a phosphorimager screen. Due to its lower energy emission in comparison to other radioisotopes, it can take several days for sufficient signal to develop. The radioactive assay format also requires that radioactive material be used in a fume hood (due to the use of chloroform), which can create logistical difficulties in countries where the use of radioactive substances is tightly regulated. A disadvantage with the fluorescent format is that the signal can fade under laboratory lights if adequate precautions are not taken to protect the samples from light. TLC-based methods are able to distinguish long chain (C14–C18) from very long chain (C22–C26) ceramide and dihydroceramide but cannot clearly distinguish C16:0 from C18:0 or C24:0 from C24:1 dihydroceramde, for example (16, 23). Although this is a drawback with any TLC-based approach, the appearance of a product band in our assay is dependent on provision of a particular fatty acid-CoA substrate (Figs. 1A and 2). Individual dihydroceramide species can be readily distinguished using LC-MS/MS, which is an advantage of this approach.
In summary, we present a fluorescent alternative to tritium or LC-MS/MS for quantifying ceramide synthase activity. It is anticipated that this fluorescent assay format will increase the accessibility of CerS assays throughout the scientific community.
Footnotes
Abbreviations:
- CerS
- ceramide synthase
- dihydroceramide
- dhCer
- NBD-sphinganine
- (2S,3R)-2-amino-18-((7-nitrobenzo[c][1,2,5]oxadiazol-4-yl)amino)octadecane-1,3-diol
- PEI
- polyethylenimine
This research was supported by Cancer Institute NSW Fellowship Grant ECF/1-03 (A.S.D), a grant from Cure Cancer Australia and Cancer Council NSW (A.S.D), NHMRC Project Grant APP1024966 (A.S.D), an Australian Postgraduate Award (H.D.T), and a Cancer Therapeutics CRC PhD Top-up Scholarship (H.D.T).
REFERENCES
- 1.Hannun Y. A., Obeid L. M. 2008. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 9: 139–150 [DOI] [PubMed] [Google Scholar]
- 2.Lopez P. H., Schnaar R. L. 2009. Gangliosides in cell recognition and membrane protein regulation. Curr. Opin. Struct. Biol. 19: 549–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christie W. W. The Lipid Library. Available from: http://www.lipidlibrary.aocs.org. Accessed February 15, 2012.
- 4.Venkataraman K., Riebeling C., Bodennec J., Riezman H., Allegood J. C., Sullards M. C., Merrill A. H., Jr., Futerman A. H. 2002. Upstream of growth and differentiation factor 1 (uog1), a mammalian homolog of the yeast longevity assurance gene 1 (LAG1), regulates N-stearoyl-sphinganine (C18-(dihydro)ceramide) synthesis in a fumonisin B1-independent manner in mammalian cells. J. Biol. Chem. 277: 35642–35649 [DOI] [PubMed] [Google Scholar]
- 5.Laviad E. L., Albee L., Pankova-Kholmyansky I., Epstein S., Park H., Merrill A. H., Jr., Futerman A. H. 2008. Characterization of ceramide synthase 2: tissue distribution, substrate specificity, and inhibition by sphingosine 1-phosphate. J. Biol. Chem. 283: 5677–5684 [DOI] [PubMed] [Google Scholar]
- 6.Ben-David O., Pewzner-Jung Y., Brenner O., Laviad E. L., Kogot-Levin A., Weissberg I., Biton I. E., Pienik R., Wang E., Kelly S., et al. 2011. Encephalopathy caused by ablation of very long acyl chain ceramide synthesis may be largely due to reduced galactosylceramide levels. J. Biol. Chem. 286: 30022–30033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koybasi S., Senkal C. E., Sundararaj K., Spassieva S., Bielawski J., Osta W., Day T. A., Jiang J. C., Jazwinski S. M., Hannun Y. A., et al. 2004. Defects in cell growth regulation by C18:0-ceramide and longevity assurance gene 1 in human head and neck squamous cell carcinomas. J. Biol. Chem. 279: 44311–44319 [DOI] [PubMed] [Google Scholar]
- 8.Senkal C. E., Ponnusamy S., Bielawski J., Hannun Y. A., Ogretmen B. 2010. Antiapoptotic roles of ceramide-synthase-6-generated C16-ceramide via selective regulation of the ATF6/CHOP arm of ER-stress-response pathways. FASEB J. 24: 296–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jennemann R., Rabionet M., Gorgas K., Epstein S., Dalpke A., Rothermel U., Bayerle A., van der Hoeven F., Imgrund S., Kirsch J., et al. 2012. Loss of ceramide synthase 3 causes lethal skin barrier disruption. Hum. Mol. Genet. 21: 586–608 [DOI] [PubMed] [Google Scholar]
- 10.Lahiri S., Lee H., Mesicek J., Fuks Z., Haimovitz-Friedman A., Kolesnick R. N., Futerman A. H. 2007. Kinetic characterization of mammalian ceramide synthases: determination of K(m) values towards sphinganine. FEBS Lett. 581: 5289–5294 [DOI] [PubMed] [Google Scholar]
- 11.Mizutani Y., Kihara A., Igarashi Y. 2005. Mammalian Lass6 and its related family members regulate synthesis of specific ceramides. Biochem. J. 390: 263–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bose R., Kolesnick R. 2000. Measurement of ceramide synthase activity. Methods Enzymol. 322: 378–382 [DOI] [PubMed] [Google Scholar]
- 13.Berdyshev E. V., Gorshkova I., Skobeleva A., Bittman R., Lu X., Dudek S. M., Mirzapoiazova T., Garcia J. G., Natarajan V. 2009. FTY720 inhibits ceramide synthases and up-regulates dihydrosphingosine 1-phosphate formation in human lung endothelial cells. J. Biol. Chem. 284: 5467–5477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hinterding K., Albert R., Cottens S. 2002. First asymmetric synthesis of chiral analogues of the novel immunosuppressant FTY720. Tetrahedron Lett. 43: 8095–8097 [Google Scholar]
- 15.Aricescu A. R., Lu W., Jones E. Y. 2006. A time- and cost-efficient system for high-level protein production in mammalian cells. Acta Crystallogr. D Biol. Crystallogr. 62: 1243–1250 [DOI] [PubMed] [Google Scholar]
- 16.van Echten-Deckert G. 2000. Sphingolipid extraction and analysis by thin-layer chromatography. Methods Enzymol. 312: 64–79 [DOI] [PubMed] [Google Scholar]
- 17.Lahiri S., Park H., Laviad E. L., Lu X., Bittman R., Futerman A. H. 2009. Ceramide synthesis is modulated by the sphingosine analog FTY720 via a mixture of uncompetitive and noncompetitive inhibition in an Acyl-CoA chain length-dependent manner. J. Biol. Chem. 284: 16090–16098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Don A. S., Martinez-Lamenca C., Webb W. R., Proia R. L., Roberts E., Rosen H. 2007. Essential requirement for sphingosine kinase 2 in a sphingolipid apoptosis pathway activated by FTY720 analogues. J. Biol. Chem. 282: 15833–15842 [DOI] [PubMed] [Google Scholar]
- 19.Brinkmann V., Davis M. D., Heise C. E., Albert R., Cottens S., Hof R., Bruns C., Prieschl E., Baumruker T., Hiestand P., et al. 2002. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J. Biol. Chem. 277: 21453–21457 [DOI] [PubMed] [Google Scholar]
- 20.Merrill A. H., Jr., van Echten G., Wang E., Sandhoff K. 1993. Fumonisin B1 inhibits sphingosine (sphinganine) N-acyltransferase and de novo sphingolipid biosynthesis in cultured neurons in situ. J. Biol. Chem. 268: 27299–27306 [PubMed] [Google Scholar]
- 21.Reed M. C., Lieb A., Nijhout H. F. 2010. The biological significance of substrate inhibition: a mechanism with diverse functions. Bioessays. 32: 422–429 [DOI] [PubMed] [Google Scholar]
- 22.Hirschberg K., Rodger J., Futerman A. H. 1993. The long-chain sphingoid base of sphingolipids is acylated at the cytosolic surface of the endoplasmic reticulum in rat liver. Biochem. J. 290: 751–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barrier L., Ingrand S., Fauconneau B., Page G. 2010. Gender-dependent accumulation of ceramides in the cerebral cortex of the APP(SL)/PS1Ki mouse model of Alzheimer's disease. Neurobiol. Aging. 31: 1843–1853 [DOI] [PubMed] [Google Scholar]