Abstract
Psoriasis, a chronic inflammatory skin disease, has been linked to increased myocardial infarction and stroke. Functional impairment of HDL may contribute to the excess cardiovascular mortality of psoriatic patients. However, data available regarding the impact of psoriasis on HDL composition and function are limited. HDL from psoriasis patients and healthy controls was isolated by ultracentrifugation and shotgun proteomics, and biochemical methods were used to monitor changed HDL composition. We observed a significant reduction in apoA-I levels of HDL from psoriatic patients, whereas levels of apoA-II and proteins involved in acute-phase response, immune response, and endopeptidase/protease inhibition were increased. Psoriatic HDL contained reduced phospholipid and cholesterol. With regard to function, these compositional alterations impaired the ability of psoriatic HDL to promote cholesterol efflux from macrophages. Importantly, HDL-cholesterol efflux capability negatively correlated with psoriasis area and severity index. We observed that control HDL, as well as psoriatic HDL, inhibited dihydrorhodamine (DHR) oxidation to a similar extent, suggesting that the anti-oxidative activity of psoriatic HDL is not significantly altered. Our observations suggest that the compositional alterations observed in psoriatic HDL reflect a shift to a pro-inflammatory profile that impairs cholesterol efflux capacity of HDL and may provide a link between psoriasis and cardiovascular disease.
Keywords: high density lipoprotein, paraoxonase, proteome, phospholipids, cardiovascular disease, inflammation
Psoriasis is a very common disease that today is considered a chronic inflammatory immune disorder affecting more than the skin (1). Traditional cardiovascular risk factors, such as hypertension, dyslipidemia, and obesity, are more frequent in psoriatic patients (2–4). However, even after adjusting for these risk factors, psoriasis has been shown to be associated with a higher incidence of myocardial infarction, stroke, and cardiovascular mortality (3, 5, 6). In moderate to severe psoriasis, a significantly deteriorated lipid profile was observed compared with healthy controls, with higher values of low-density lipoprotein, triglycerides, and significantly decreased HDL levels (7).
Recent studies clearly demonstrated that inflammation impairs reverse cholesterol transfer in vivo (8, 9), providing evidence that inflammation impairs HDL function. Emerging evidence suggests that assessment of HDL plasma concentrations alone is insufficient and indicate that the quality, rather than the mere quantity, of HDL determines its potential beneficial effects against atherosclerosis (10). HDL is a complex lipoprotein particle with a broad variety of functions, also exerting atheroprotective activity via effects on the endothelium and by potent anti-inflammatory capabilities (11–13). Recent studies have identified HDL-associated proteins to be involved in the regulation of lipid metabolism, complement activation, growth-factor secretion, and proteolysis (14–19).
Functional impairment of HDL may contribute to the increased cardiovascular mortality experienced by psoriatic patients, but the impact of psoriasis on the composition and function of HDL has not been assessed. As qualitative alterations of HDL seem to be linked with increased cardiovascular complications, we hypothesized that HDL from psoriatic patients displays altered protein cargo and lipid composition, thereby rendering HDL dysfunctional.
In this explorative study, quantitative shotgun proteomic profiling and biochemical analysis were used to investigate whether the systemic inflammatory state in psoriasis modifies HDL composition and function.
METHODS
Characteristics of study subjects and blood collection
Blood was sampled from patients with moderate to severe chronic plaque-type psoriasis and healthy volunteers after obtaining written informed consent, according to a protocol approved by the Institutional Review Board of the Medical University of Graz (No. 21-523 ex. 09/10). Blood was collected in Vacuette serum tubes (Greiner, Kremsmünster, Austria). The clinical characteristics of control subjects (n = 15) and psoriatic patients (n = 15) are given in Table 1. Plasma lipid profiles of psoriasis patients displayed characteristics of metabolic dyslipidemia, with increased plasma triglycerides and total cholesterol similar to that reported in previous studies (7, 20). The patients had a mean psoriasis area and severity index (PASI) of 10.4, indicating a moderate to severe disease status.
TABLE 1.
Clinical characteristics of study subjects
| Characteristic | Control | Psoriasis |
| N | 15 | 15 |
| Age (yr) | 37.2 ± 7.4 | 41.8 ± 12.6 |
| Male/female | 7/8 | 11/4 |
| PASI | — | 11.4 (10.3–16.6) |
| CRP (mg/dl) | 0.2 (0–1) | 2.7 (1.2–5.6) |
| Total cholesterol (mg/dl) | 174 (157–203) | 215 (166–227) |
| Triglycerides (mg/dl) | 87 (54–126) | 131 (74–206) |
| HDL-cholesterol (mg/dl) | 49 (42–67) | 38 (32–55) |
| LDL-cholesterol (mg/dl) | 106 (92–122) | 119 (82–162) |
Results are given as medians with the interquartile range.
Isolation of HDL
Serum density was adjusted with potassium bromide (Sigma, Vienna, Austria) to 1.24 g/ml, and a two-step density gradient was generated in centrifuge tubes (16 × 76 mm, Beckman) by layering the density-adjusted plasma (1.24 g/ml) underneath a NaCl density solution (1.006 g/ml) as described (15, 21). Tubes were sealed and centrifuged at 90,000 rpm for 4 h in a 90Ti fixed angle rotor (Beckman Instruments, Krefeld, Germany). After centrifugation, the HDL-containing band was collected, desalted via PD10 columns (GE Healthcare, Vienna, Austria), and immediately used for experiments or stored at −70°C.
Determination of plasma and HDL-lipid composition
Levels of total cholesterol, nonesterified cholesterol, triglycerides, choline-containing phospholipids (Diasys, Holzheim, Germany), sphingomyelin (Cayman Europe, Tallinn, Estonia), and lysophosphatidylcholine (LPC) (Cosmo Bio Co. LTD, Tokyo, Japan) were measured enzymatically (15, 22). Sphingomyelin values were subtracted from total choline-containing phospholipids to quantify phosphatidylcholine. LDL cholesterol was calculated according to the Friedewald equation using HDL-cholesterol values measured in the supernatant of the phosphotungstic acid/MgCl2 precipitation.
Apolipoprotein determination by immunoturbidimetry
ApoA-I, apoA-II, apoB, apoC-II, apoC-III and apoE (Greiner, Flacht, Germany) were determined by immunoturbidimetry. All lipoprotein analyses were performed on an Olympus AU640 analyzer (Olypmpus Diagnostika, Hamburg, Germany).
Cholesterol efflux capability of HDL
Cholesterol efflux assay was performed as described previously (15). Briefly, RAW264.7 macrophages (7 × 106 cells/well), maintained in DMEM with 10% fetal bovine serum (FBS), were plated in 48-well plates. Cells were labeled for 24 h with [3H]cholesterol (1 μCi/ml) in medium containing 5% FBS, 50 µg/ml aggregated LDL, and the liver X receptor (LXR) agonist TO-901317 (2 µmol/l). After labeling, cells were washed and equilibrated in serum-free media containing 0.2% BSA for 2 h. To determine [3H]cholesterol efflux, cells were incubated with 50 µg/ml HDL for 3 h at 37°C.
Paraoxonase activity assay
Ca2+-dependent arylesterase activity was determined with a photometric assay using phenylacetate as the substrate (23). HDL (2 µg protein) or apoB-depleted serum (3 µl) was added to 200 µl buffer containing 100 mmol/l Tris, 2 mmol/l CaCl2 (pH 8.0), and phenylacetate (1 mmol/l). The rate of hydrolysis of phenylacetate was monitored by the increase of absorbance at 270 nm, and readings were taken every 30 s at room temperature to generate a kinetic plot. The slope from the kinetic chart was used to determine ΔAb270nm / min. Enzymatic activity was calculated with the Beer-Lambert Law from the molar extinction coefficient of 1,310 L × mol−1 × cm−1 for phenylacetate.
Lp-PLA2 activity assay
Lp-PLA2 activity was measured with a commercially available photometric assay (Cayman Europe, Talinn, Estonia) using 2-thio PAF as substrate.
Determination of the anti-oxidative capacity of HDL
The anti-oxidative activity of HDL was determined as previously described with modifications (24). Briefly, dihydrorhodamine (DHR) was suspended in DMSO to a 50 mmol/l stock, which was diluted in HEPES (20 mmol/L HEPES, 150 mmol/l NaCl, pH 7.4) containing 1 mmol/l 2,2’-azobis-2-methyl-propanimidamide-dihydrochloride (AAPH) to a 50 µmol/l working reagent. In a 384-well, 7.5 µg HDL protein was placed, 15 µl of DHR working reagent was added, and the volume was completed to 100 µl with HEPES buffer. The increase in fluorescence due to the oxidation of DHR was measured every 2 min for 1 h at 538 nm. The increase in fluorescence per minute was determined for samples containing only DHR and for samples containing DHR and individual HDL samples from healthy controls or psoriasis patients.
LC-MS/MS analysis
Proteomic profiling of HDL was performed as previously described (15). HDL was digested with trypsin, and the resulting peptides were separated using a nano-HPLC. The samples were ionized in the nanospray source equipped with nanospray tips and analyzed in a LTQ-FT mass spectrometer (Thermo Scientific, Waltham, MA). The standard deviation of spectral counts was below 10% between duplicates. Spectral counts were recorded and used for data analysis by searching the human SwissProt public database downloaded on May 4, 2011, with Spectrum Mill Rev. A.03.03.084 SR4 (Agilent, Vienna, Austria). Detailed search criteria were as follows: trypsin; maximum missed cleavage sites = 2; fixed modification = carbamidomethylation at cysteine; variable modification = oxidized methionine; precursor mass tolerance = ±0.05 Da; and product mass tolerance = ±0.7 Da. Protein hits were subjected to automatic validation by Spectrum mill as follows. For precursor charge of 2, score threshold = 6.0; percent scored peak intensity (%SPI) threshold = 60.0; fwd-rev score threshold = 2.0; and rank 1–2 score threshold = 2.0. For precursor charge of 1, score threshold = 6.0; %SPI threshold = 70.0; fwd-rev score threshold = 2.0; and rank 1–2 score threshold = 2.0. For precursor charge of 3, score threshold = 8.0; %SPI threshold = 70.0; fwd-rev score threshold = 2.0; and rank 1–2 score threshold = 2.0.
Statistical analysis
Differences in plasma and HDL parameters between control subjects and psoriatic patients were analyzed using 2-tailed Mann-Whitney U-test. Changes in the HDL proteome were evaluated from spectral counts of automatically validated proteins (i.e., the number of MS/MS spectra assigned to a protein). To assess for data normality, the Shapiro-Wilk test (at the level of 10%) was used. Because proteomic data markedly violate the assumption of normality, the Mann-Whitney U-test was employed for analysis of differences.
All correlations between compositional and functional data were determined with the use of Pearson product-moment estimates. Significance was accepted at *P < 0.05 and **P < 0.01. Statistical analyses were performed with PASW Statistics version 18.
RESULTS
Psoriasis is associated with altered HDL composition
We hypothesized that HDL from psoriatic patients might display altered protein cargo and lipid composition. Therefore, we isolated HDL from psoriatic patients (n = 15) and healthy individuals (n = 15) by ultracentrifugation. To identify HDL-associated proteins, we performed proteomic analysis of purified HDL using LC-MS/MS. Table 1 describes the clinical characteristics of the subjects studied. We identified 38 HDL-associated proteins in control subjects and 55 HDL-associated proteins in psoriatic subjects. Identified HDL-associated proteins were grouped into functional categories (Table 2); statistical analysis identified several proteins to be significantly altered (Table 2). The analysis revealed that apoA-I and apoM were significantly reduced, whereas several acute-phase proteins, including SAA, prothrombin, and α-1-acid glycoprotein 1, were increased. Moreover, we observed an increased content of apoA-II, α-1-antitrypsin, and IgA-1 on HDL isolated from psoriasis patients.
TABLE 2.
Identification of HDL-associated proteins
| % HDL-derived Peptides (n = 15) |
|||||
| Protein | Control |
Psoriasis | P | ||
| Lipid metabolism | |||||
| ApoA-I | 59.66 | (15/15) | 51.55 | (15/15) | <0.001 |
| ApoA-II | 4.47 | (15/15) | 5.20 | (15/15) | 0.045 |
| ApoC-III | 4.42 | (15/15) | 4.21 | (15/15) | 0.600 |
| ApoC-I | 3.45 | (15/15) | 3.03 | (15/15) | 0.107 |
| ApoE | 3.12 | (15/15) | 3.21 | (15/15) | 0.803 |
| ApoB-100 | 1.84 | (15/15) | 3.17 | (14/15) | 0.461 |
| ApoD | 1.39 | (15/15) | 1.36 | (15/15) | 0.735 |
| ApoM | 0.91 | (15/15) | 0.65 | (15/15) | 0.007 |
| ApoLI | 0.69 | (15/15) | 0.58 | (15/15) | 0.285 |
| ApoC-II | 0.63 | (15/15) | 0.61 | (15/15) | 0.720 |
| ApoA-IV | 0.62 | (15/15) | 0.88 | (15/15) | 0.122 |
| ApoC-IV | 0.29 | (13/15) | 0.26 | (14/15) | 0.579 |
| ApoF | 0.29 | (15/15) | 0.18 | (15/15) | 0.008 |
| ApoH | 0.00 | — | 0.02 | (02/15) | 0.487 |
| Enzymes | |||||
| PON 1 | 2.33 | (15/15) | 2.51 | (15/15) | 0.509 |
| Plasminogen | 0.00 | — | 0.01 | (01/15) | 0.326 |
| Acute phase response | |||||
| SAA | 0.63 | (15/15) | 1.20 | (15/15) | 0.009 |
| Prothrombin | 0.45 | (15/15) | 0.78 | (15/15) | 0.006 |
| α-2-HS-glycoprotein | 0.12 | (08/15) | 0.29 | (13/15) | 0.044 |
| α-1-Acid glycoprotein 1 | 0.10 | (09/15) | 0.27 | (14/15) | <0.001 |
| Complement activation/inhibition | |||||
| Complement C3 | 0.38 | (15/15) | 0.72 | (15/15) | 0.041 |
| Complement C4-B | 0.08 | (07/15) | 0.13 | (13/15) | 0.195 |
| Clusterin | 0.06 | (06/15) | 0.07 | (08/15) | 0.557 |
| Complement H | 0.00 | — | 0.02 | (01/15) | 0.326 |
| Binding proteins | |||||
| Albumin | 8.08 | (15/15) | 10.27 | (15/15) | 0.033 |
| Serotransferrin | 0.27 | (13/15) | 0.60 | (15/15) | 0.045 |
| Transthyretin | 0.21 | (09/15) | 0.39 | (12/15) | 0.123 |
| Vitamin D-BP | 0.07 | (05/15) | 0.20 | (12/15) | 0.024 |
| Retinol-BP 4 | 0.03 | (03/15) | 0.14 | (11/15) | 0.027 |
| Protease inhibitors | |||||
| α-1-antitrypsin | 0.48 | (15/15) | 0.95 | (15/15) | 0.001 |
| AMBP | 0.04 | (04/15) | 0.16 | (07/15) | 0.066 |
| α-2-Macroglobulin | 0.00 | — | 0.15 | (02/15) | 0.306 |
| Kininogen-1 | 0.00 | — | 0.03 | (03/15) | 0.144 |
| α-1-Antichymotrypsin | 0.00 | — | 0.01 | (02/15) | 0.154 |
| Protease C1 inhibitor | 0.00 | — | 0.01 | (02/15) | 0.154 |
| Immune response | |||||
| IgK | 0.42 | (14/15) | 0.41 | (15/15) | 0.919 |
| IgG-1 | 0.41 | (12/15) | 0.84 | (15/15) | 0.034 |
| IgA-1 | 0.04 | (03/15) | 0.18 | (11/15) | 0.023 |
| IgL-2 | 0.03 | (02/15) | 0.19 | (08/15) | 0.064 |
| IgK chain V-III | 0.00 | — | 0.12 | (07/15) | 0.014 |
| Ig heavy chain V-III | 0.00 | — | 0.05 | (04/15) | 0.067 |
| Other | |||||
| SAA4 | 2.38 | (15/15) | 1.75 | (15/15) | 0.005 |
| Platelet basic protein | 0.49 | (15/15) | 0.71 | (15/15) | 0.032 |
| Platelet factor 4 | 0.39 | (13/15) | 0.48 | (15/15) | 0.238 |
| Haptoglobin | 0.11 | (06/15) | 0.51 | (11/15) | 0.033 |
| Hemopexin | 0.07 | (05/15) | 0.17 | (11/15) | 0.101 |
| Hemoglobin β | 0.00 | — | 0.10 | (08/15) | 0.008 |
| α-1B-Glycoprotein | 0.00 | — | 0.04 | (06/15) | 0.005 |
| PEDF | 0.00 | — | 0.03 | (05/15) | 0.122 |
| Fibrinogen α | 0.00 | — | 0.03 | (03/15) | 0.144 |
| Hemoglobin α | 0.00 | — | 0.02 | (01/15) | 0.326 |
| APMAP | 0.00 | — | 0.02 | (02/15) | 0.178 |
| PSAP-B | 0.00 | — | 0.01 | (02/15) | 0.153 |
HDL was isolated from 15 control subjects and 15 psoriatic patients by ultracentrifugation. The HDL proteome was analyzed by LC-MS/MS, and data were analyzed by searching the human NCBI database with Mascot 2.2 (MatrixScience). Values represent the percentage of peptides of the total peptide count per analyzed subject. The average peptide count was 923 ± 160 peptides (n = 30). The number of subjects with the protein identified is noted in parentheses. Statistical significance was calculated with the Mann-Whitney U-test. AMBP, α-1-microglobulin/bikunin precursor; APMAP, adipocyte plasma membrane-associated protein; PEDF, pigment epithelium-derived factor; PSAP-B, pulmonary surfactant-associated protein B; Retinol-BP 4, retinol-binding protein 4; Vitamin D-BP, vitamin D-binding protein.
To validate the proteomic result, we performed immunoturbidimetry analysis of major apolipoproteins (apoA-I, apoA-II, apoC-II, apoC-III, and apoE), demonstrating that the proteomic data are valid (supplementary Table I).
In addition, we analyzed the lipid moiety of HDL for its major lipid classes. HDL derived from psoriatic patients displayed a significantly decreased content of total cholesterol, phosphatidylcholine, and sphingomyelin (Table 3). LPC content was not significantly altered.
TABLE 3.
HDL-lipid composition
| Component | Control | Psoriasis |
| Total cholesterol (µg/mg protein) | 278 (235–305) | 225 (200–255)a |
| Cholesterylester (µg/mg protein) | 200 (173–225) | 175 (153–198)a |
| Free cholesterol (µg/mg protein) | 78 (55–83) | 45 (43–58)a |
| Triglycerides (µg/mg protein) | 52 (33–67) | 63 (42–101) |
| Phosphatidylcholine (µg/mg protein) | 445 (417–480) | 395 (350–433)a |
| Sphingomyelin (µg/mg protein) | 53 (46–62) | 40 (35–47)a |
| LPC (nmol/mg protein) | 9.5 (7.4–10.8) | 8.6 (6.8–13.0) |
Results are given as medians with the interquartile range.
P< 0.01 (Mann-Whitney U-test).
Psoriatic HDL shows reduced cholesterol efflux capacity from macrophages
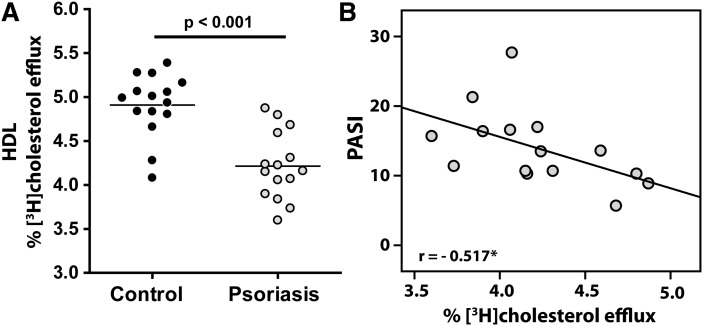
Cholesterol efflux capacity of apoB-depleted serum shows a strong inverse correlation with the likelihood of coronary artery disease (10). Strikingly, HDL from psoriatic patients was significantly less efficient in promoting cholesterol efflux from macrophages compared with controls (Fig. 1A). Importantly, PASI negatively correlated with cholesterol efflux capacity of isolated HDL (Fig. 1B), whereas serum C-reactive protein (CRP) values and gender did not correlate with HDL-cholesterol efflux capacity (supplementary Table II).
Fig. 1.
Psoriasis impairs cholesterol efflux capability of HDL. (A) HDL isolated from 15 control subjects and 15 psoriatic patients was examined for its ability to induce [3H]cholesterol efflux from RAW264.7 macrophages. [3H]cholesterol-labeled cells were incubated with 50 µg/ml HDL for 3 h. Cholesterol efflux is expressed as radioactivity in the supernatant relative to total radioactivity (in supernatant and cells). Values shown represent means of two individual experiments performed in duplicate. (B) Correlation between PASI and [3H]cholesterol efflux induced by HDL. The Pearson correlation coefficient is noted; *P < 0.05.
Altered HDL composition is linked to impaired cholesterol efflux capacity
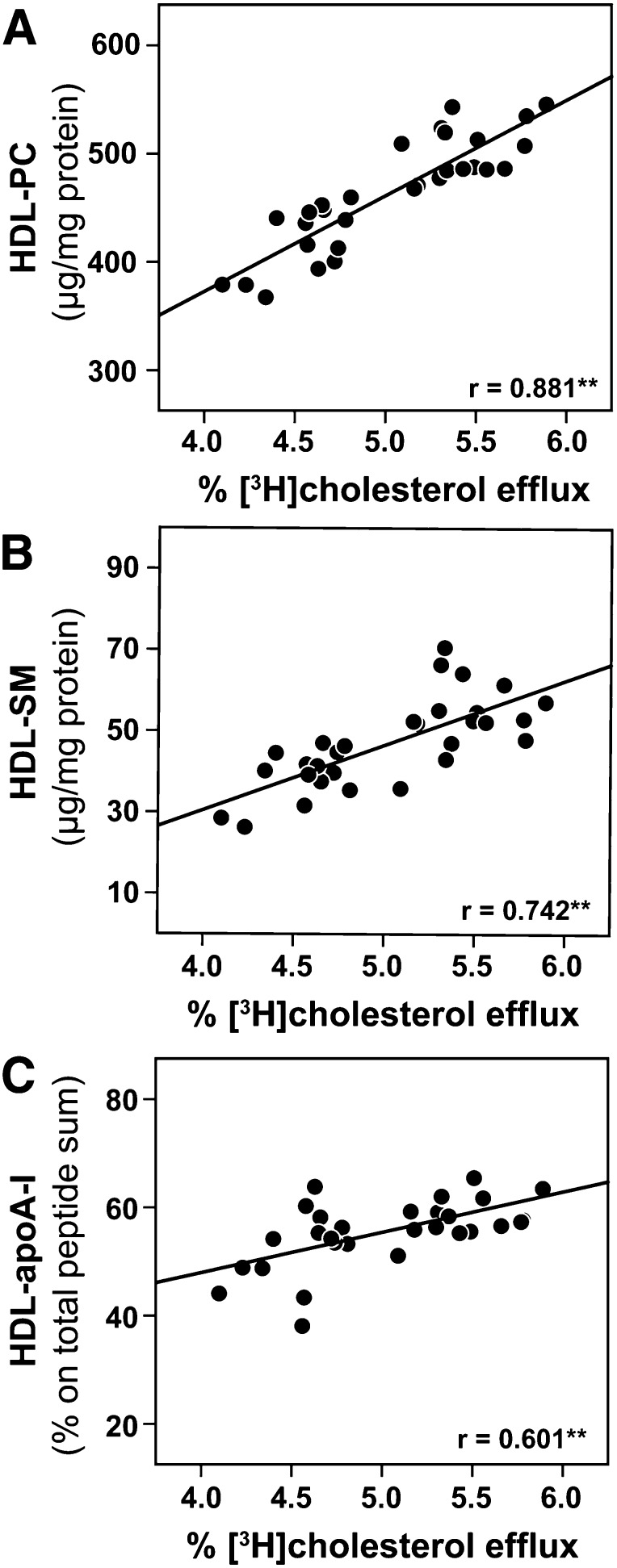
To assess which compositional alterations are involved in the impairment of cholesterol efflux capacity, we performed correlation analysis between cholesterol efflux capability of HDL and compositional data. We observed that HDL-associated phosphatidylcholine (Fig. 2A), HDL-associated sphingomyelin (Fig. 2B) and HDL-associated apoA-I (Fig. 2C) were the strongest positive predictors of HDL-cholesterol efflux capacity. Interestingly, apoA-II, of which the overall amount was increased in psoriatic patients, was not associated with cholesterol efflux capability (supplementary Table III).
Fig. 2.
HDL-phosphatidylcholine and HDL-associated apoA-I correlate with the cholesterol efflux capability of HDL. Correlation between HDL-mediated [3H]cholesterol efflux from macrophages and the HDL content of (A) phosphatidylcholine (HDL-PC), (B) sphingomyelin (HDL-SM), and (C) apoA-I. Pearson's correlation coefficients are noted for each plot; **P < 0.01.
A full list of correlations of cholesterol efflux capacity with all HDL-associated proteins can be found in supplementary Table III.
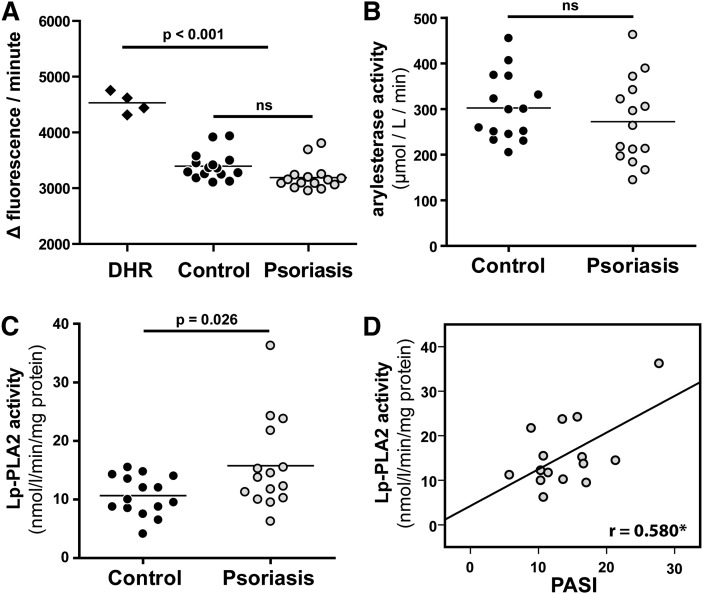
Capability of psoriatic HDL to inhibit dihydrorhodamine oxidation (anti-oxidative activity) is not altered
Besides the important role in lipid metabolism, HDL exhibits unique anti-oxidative activity. Therefore, we assessed the intrinsic ability of HDL to be oxidized by measuring increasing fluorescence due to dihydrorhodamine 123 oxidation over time (24). Interestingly, we observed that control HDL and HDL from psoriatic patients inhibited DHR oxidation to a similar extent (Fig. 3A), indicating that the ability of psoriatic HDL to inhibit DHR oxidation is not altered. Previous data suggested that HDL-associated paraoxonase (PON) and/or lipoprotein associated phospholipase A2 (Lp-PLA2) contribute to the anti-oxidant activity of HDL (25, 26). In good agreement with proteomic data (Table 2), we observed that PON activity was not altered (Fig. 3B), whereas Lp-PLA2 activity was significantly increased in psoriatic HDL (Fig. 3C) and correlated with disease severity (Fig. 3D). Similar results were obtained when PON and Lp-PLA2 activities were assessed in apoB-depleted serum of controls and psoriatic patients (supplementary Fig. I).
Fig. 3.
Anti-oxidative capacity of HDL form psoriatic patients is not impaired. (A) The anti-oxidative activity of HDL was determined by inhibition of AAPH-initiated oxidation of DHR. Incubation of DHR in the presence of HDL from healthy subjects (control) or psoriasis patients (Psoriasis) led to a reduction in the oxidation of DHR. Results represent measurements of two independent experiments. (B) Arylesterase activity of HDL-associated PON was measured using phenylacetate as substrate. (C) Lipoprotein-associated phospholipase A2 (Lp-PLA2) activity of HDL was measured using 2-thio PAF as substrate. The arylesterase and Lp-PLA2 activities of HDL were calculated from the slopes of the kinetic chart of three independent experiments. (D) Correlation between PASI and Lp-PLA2 activities of control and psoriatic HDL. The Pearson correlation coefficient is noted; *P < 0.05.
DISCUSSION
The data presented here describe for the first time marked alterations in the composition and function of psoriatic HDL and provide evidence that psoriatic HDL takes on a pro-inflammatory profile. We observed a significantly impaired capacity of psoriatic HDL to mobilize cholesterol from macrophages, the critical first step of reverse cholesterol transport. Importantly, cholesterol efflux capability negatively correlated with the severity of psoriasis. Our findings raise the possibility that dysfunctional HDL contributes to accelerated atherosclerosis in psoriatic patients.
Shotgun proteomic profiling and biochemical analyses were applied to investigate psoriasis-induced alterations in the HDL proteome and lipid composition. Our present data suggest that psoriasis shifts the HDL composition into a pro-inflammatory profile.
Psoriatic HDL contained less phospholipids and cholesterol and markedly reduced levels of apoA-I, whereas apoA-II and several acute-phase proteins, such as SAA, α-1-antitrypsin, prothrombin, and α-1-acid-glycoprotein 1, were significantly increased. Moreover, proteins involved in complement activation, such as complement C3 and hemoglobin and its scavenger protein haptoglobin, were enriched in psoriatic HDL. Interestingly, recent studies reported significantly increased complement C3, hemoglobin, haptoglobin, and hemopexin in HDL of coronary heart disease patients, which lost its anti-inflammatory capacity by accumulation of pro-inflammatory SAA1 (28, 29).
Although cholesterol efflux from macrophages represents only a small fraction of the overall flux through reverse cholesterol transport, it is probably the most relevant factor regarding atheroprotection (10). In the present study, we observed that many of the proteins that are enriched in psoriatic HDL (with the exception of apoA-II) negatively correlated with the cholesterol efflux capability of HDL. In addition to the changes in the protein composition of HDL, a decrease in the phospholipid and cholesterol content of HDL was observed, which strikingly signifies that psoriasis induced alterations in the lipid com position of HDL.
Our results suggest that a loss of apoA-I, phosphatidylcholine, and sphingomyelin content of psoriatic HDL is a key determinant of the low cholesterol efflux capability. This assumption is based on the highly significant correlation between apoA-I/phosphatidylcholine content and the cholesterol efflux capability of HDL. Sphingomyelin is known to bind cholesterol with high affinity, suggesting that the sphingomyelin content of HDL contributes to cholesterol association with HDL, as previously suggested (30).
In accordance with our findings, previous studies suggested that antiatherogenic activities of HDL are inversely correlated with systemic inflammation in rheumatoid arthritis patients (31–33). Moreover, a recent study has provided evidence that autoantibodies against apoA-I contribute to reduced HDL levels in systemic lupus erythema tosus patients, independently of hepatic HDL biogenesis. This finding indicates that premature clearance of apoA-I/HDL immune complexes may contribute to compositional alterations and low apoA-I/HDL levels (34). As psoriatic HDL contains increased levels of (auto)antibodies, immune complex formation may result in accelerated clearance and may render HDL dysfunctional.
Notably, we observed that platelet basic protein content was significantly increased in psoriatic HDL compared with control HDL. This may be of particular interest, as there is evidence for in vivo platelet activation in psoriatic patients contributing to the development of thrombotic events (35).
Another important finding of our study was that Lp-PLA2 activity, also known as platelet-activating factor acetylhydrolase (PAF-AH), is significantly increased in psoriatic HDL. Previous studies have implicated that the potent phospholipid mediator PAF (1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine) plays a role in the pathogenesis of psoriasis (36). Plasma levels of PAF were found to be increased in patients with psoriasis, and a significant decrease in PAF levels was observed with clinical improvement after treatment (37). Therefore, increased HDL-associated Lp-PLA2 activity in psoriatic patients may more effectively inactivate PAF and contribute to clinical improvement. On the other hand, a recent meta-analysis reported that Lp-PLA2 levels are positively associated with an increased risk of developing coronary artery disease (38); hence, the role of HDL-associated Lp-PLA2 in diseases remains unclear and needs further investigation.
In summary, the results of our study indicate that compositional alterations observed in psoriatic HDL reflect a pro-inflammatory profile. Consequently, such compositional alterations may improve our ability to monitor therapeutic responses and may provide a novel basis for the identification of psoriatic patients at increased risk of cardiovascular disease. The abnormal efflux capacity of HDL in psoriatic patients may provide a link between the association of psoriasis and cardiovascular disease; however, larger studies are needed to validate these findings.
Supplementary Material
Acknowledgments
The authors thank Sabine Dirnberger and Isabella Bambach for technical support.
Footnotes
Abbreviations:
- CRP
- C-reactive protein
- DHR
- dihydrorhodamine
- Lp-PLA2
- lipoprotein-associated phospholipase A2
- LPC
- lysophosphatidylcholine
- PASI
- psoriasis area and severity index
- PON
- paraoxonase
This work was supported by the Austrian Science Fund FWF Grants P21004-B02, P22976-B18 (to G.M.), and P-22521-B18 (to A.H.). M.H. and S.C. were supported by the molecular medicine PhD program of the Medical University of Graz.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one figure and three tables.
REFERENCES
- 1.Flammer A. J., Ruschitzka F. Psoriasis and atherosclerosis: two plaques, one syndrome? Eur. Heart J. doi: 10.1093/eurheartj/ehr425. Epub ahead of print. November 21, 2011; doi:10.1093/eurheartj/ehr425. [DOI] [PubMed] [Google Scholar]
- 2.Neimann A. L., Shin D. B., Wang X., Margolis D. J., Troxel A. B., Gelfand J. M. 2006. Prevalence of cardiovascular risk factors in patients with psoriasis. J. Am. Acad. Dermatol. 55: 829–835 [DOI] [PubMed] [Google Scholar]
- 3.Kaplan M. J. 2008. Cardiometabolic risk in psoriasis: differential effects of biologic agents. Vasc. Health Risk Manag. 4: 1229–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerdes S., Mrowietz U. 2009. Impact of comorbidities on the management of psoriasis. Curr. Probl. Dermatol. 38: 21–36 [DOI] [PubMed] [Google Scholar]
- 5.Mehta N. N., Azfar R. S., Shin D. B., Neimann A. L., Troxel A. B., Gelfand J. M. 2010. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur. Heart J. 31: 1000–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gelfand J. M., Neimann A. L., Shin D. B., Wang X., Margolis D. J., Troxel A. B. 2006. Risk of myocardial infarction in patients with psoriasis. JAMA. 296: 1735–1741 [DOI] [PubMed] [Google Scholar]
- 7.Rocha-Pereira P., Santos-Silva A., Rebelo I., Figueiredo A., Quintanilha A., Teixeira F. 2001. Dislipidemia and oxidative stress in mild and in severe psoriasis as a risk for cardiovascular disease. Clin. Chim. Acta. 303: 33–39 [DOI] [PubMed] [Google Scholar]
- 8.McGillicuddy F. C., de la Llera M. M., Hinkle C. C., Joshi M. R., Chiquoine E. H., Billheimer J. T., Rothblat G. H., Reilly M. P. 2009. Inflammation impairs reverse cholesterol transport in vivo. Circulation. 119: 1135–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Annema W., Nijstad N., Tolle M., de Boer J. F., Buijs R. V., Heeringa P., van der Giet M., Tietge U. J. 2010. Myeloperoxidase and serum amyloid A contribute to impaired in vivo reverse cholesterol transport during the acute phase response but not group IIA secretory phospholipase A(2). J. Lipid Res. 51: 743–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khera A. V., Cuchel M., de la Llera-Moya M., Rodrigues A., Burke M. F., Jafri K., French B. C., Phillips J. A., Mucksavage M. L., Wilensky R. L., et al. 2011. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N. Engl. J. Med. 364: 127–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Besler C., Heinrich K., Rohrer L., Doerries C., Riwanto M., Shih D. M., Chroni A., Yonekawa K., Stein S., Schaefer N., et al. 2011. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J. Clin. Invest. 121: 2693–2708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Lenten B. J., Hama S. Y., de Beer F. C., Stafforini D. M., McIntyre T. M., Prescott S. M., La Du B. N., Fogelman A. M., Navab M. 1995. Anti-inflammatory HDL becomes pro-inflammatory during the acute phase response. Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J. Clin. Invest. 96: 2758–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barter P. J., Nicholls S., Rye K. A., Anantharamaiah G. M., Navab M., Fogelman A. M. 2004. Antiinflammatory properties of HDL. Circ. Res. 95: 764–772 [DOI] [PubMed] [Google Scholar]
- 14.Alwaili K., Bailey D., Awan Z., Bailey S. D., Ruel I., Hafiane A., Krimbou L., Laboissiere S., Genest J. 2012. The HDL proteome in acute coronary syndromes shifts to an inflammatory profile. Biochim. Biophys. Acta. 1821: 405–515 [DOI] [PubMed] [Google Scholar]
- 15.Holzer M., Birner-Gruenberger R., Stojakovic T., El-Gamal D., Binder V., Wadsack C., Heinemann A., Marsche G. 2011. Uremia alters HDL composition and function. J. Am. Soc. Nephrol. 22: 1631–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davidsson P., Hulthe J., Fagerberg B., Camejo G. 2010. Proteomics of apolipoproteins and associated proteins from plasma high-density lipoproteins. Arterioscler. Thromb. Vasc. Biol. 30: 156–163 [DOI] [PubMed] [Google Scholar]
- 17.Levels J. H., Bleijlevens B., Rezaee F., Aerts J. M., Meijers J. C. 2007. SELDI-TOF mass spectrometry of high-density lipoprotein. Proteome Sci. 5: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davidson W. S., Silva R. A., Chantepie S., Lagor W. R., Chapman M. J., Kontush A. 2009. Proteomic analysis of defined HDL subpopulations reveals particle-specific protein clusters: relevance to antioxidative function. Arterioscler. Thromb. Vasc. Biol. 29: 870–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaisar T., Pennathur S., Green P. S., Gharib S. A., Hoofnagle A. N., Cheung M. C., Byun J., Vuletic S., Kassim S., Singh P., et al. 2007. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J. Clin. Invest. 117: 746–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mallbris L., Granath F., Hamsten A., Stahle M. 2006. Psoriasis is associated with lipid abnormalities at the onset of skin disease. J. Am. Acad. Dermatol. 54: 614–621 [DOI] [PubMed] [Google Scholar]
- 21.Holzer M., Gauster M., Pfeifer T., Wadsack C., Fauler G., Stiegler P., Koefeler H., Beubler E., Schuligoi R., Heinemann A., et al. 2011. Protein carbamylation renders high-density lipoprotein dysfunctional. Antioxid. Redox Signal. 14: 2337–2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Gamal D., Holzer M., Gauster M., Schicho R., Binder V., Konya V., Wadsack C., Schuligoi R., Heinemann A., Marsche G. 2012. Cyanate is a novel inducer of endothelial icam-1 expression. Antioxid. Redox Signal. 16: 129–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holzer M., Zangger K., El-Gamal D., Binder V., Curcic S., Konya V., Schuligoi R., Heinemann A., Marsche G. Myeloperoxidase-derived chlorinating species induce protein carbamylation through decomposition of thiocyanate and urea: novel pathways generating dysfunctional high-density lipoprotein. Antioxid. Redox Signal. Epub ahead of print. May 8, 2012; doi:10.1089/ars.2011.4403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelesidis T., Currier J. S., Huynh D., Meriwether D., Charles-Schoeman C., Reddy S. T., Fogelman A. M., Navab M., Yang O. O. 2011. A biochemical fluorometric method for assessing the oxidative properties of HDL. J. Lipid Res. 52: 2341–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aviram M., Rosenblat M., Bisgaier C. L., Newton R. S., Primo-Parmo S. L., La Du B. N. 1998. Paraoxonase inhibits high-density lipoprotein oxidation and preserves its functions. A possible peroxidative role for paraoxonase. J. Clin. Invest. 101: 1581–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marathe G. K., Zimmerman G. A., McIntyre T. M. 2003. Platelet-activating factor acetylhydrolase, and not paraoxonase-1, is the oxidized phospholipid hydrolase of high density lipoprotein particles. J. Biol. Chem. 278: 3937–3947 [DOI] [PubMed] [Google Scholar]
- 27.Watanabe J., Grijalva V., Hama S., Barbour K., Berger F. G., Navab M., Fogelman A. M., Reddy S. T. 2009. Hemoglobin and its scavenger protein haptoglobin associate with apoA-1-containing particles and influence the inflammatory properties and function of high density lipoprotein. J. Biol. Chem. 284: 18292–18301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tölle M., Huang T., Schuchardt M., Jankowski V., Prufer N., Jankowski J., Tietge U. J., Zidek W., van der Giet M. 2012. High-density lipoprotein loses its anti-inflammatory capacity by accumulation of pro-inflammatory-serum amyloid A. Cardiovasc. Res. 94: 154–162 [DOI] [PubMed] [Google Scholar]
- 29.Weichhart T., Kopecky C., Kubicek M., Haidinger M., Doller D., Katholnig K., Suarna C., Eller P., Tolle M., Gerner C., et al. 2012. Serum amyloid A in uremic HDL promotes inflammation. J. Am. Soc. Nephrol. 23: 934–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fournier N., Paul J. L., Atger V., Cogny A., Soni T., Llera-Moya M., Rothblat G., Moatti N. 1997. HDL phospholipid content and composition as a major factor determining cholesterol efflux capacity from Fu5AH cells to human serum. Arterioscler. Thromb. Vasc. Biol. 17: 2685–2691 [DOI] [PubMed] [Google Scholar]
- 31.Charles-Schoeman C., Lee Y. Y., Grijalva V., Amjadi S., Fitzgerald J., Ranganath V. K., Taylor M., McMahon M., Paulus H. E., Reddy S. T. Cholesterol efflux by high density lipoproteins is impaired in patients with active rheumatoid arthritis. Ann. Rheum. Dis. Epub ahead of print. January 20, 2012; doi:10.1136/annrheumdis-2011-200493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watanabe J., Charles-Schoeman C., Miao Y., Elashoff D., Lee Y. Y., Katselis G., Lee T. D., Reddy S. T. 2012. Proteomic profiling following immunoaffinity capture of HDL: association of acute phase proteins and complement factors with pro-inflammatory HDL in rheumatoid arthritis. Arthritis Rheum. 64: 1828–1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Charles-Schoeman C., Watanabe J., Lee Y. Y., Furst D. E., Amjadi S., Elashoff D., Park G., McMahon M., Paulus H. E., Fogelman A. M., et al. 2009. Abnormal function of high-density lipoprotein is associated with poor disease control and an altered protein cargo in rheumatoid arthritis. Arthritis Rheum. 60: 2870–2879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Srivastava R., Yu S., Parks B. W., Black L. L., Kabarowski J. H. 2011. Autoimmune-mediated reduction of high-density lipoprotein-cholesterol and paraoxonase 1 activity in systemic lupus erythematosus-prone gld mice. Arthritis Rheum. 63: 201–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davidovici B. B., Sattar N., Prinz J. C., Puig L., Emery P., Barker J. N., van de Kerkhof P., Stahle M., Nestle F. O., Girolomoni G., et al. 2010. Psoriasis and systemic inflammatory diseases: potential mechanistic links between skin disease and co-morbid conditions. J. Invest. Dermatol. 130: 1785–1796 [DOI] [PubMed] [Google Scholar]
- 36.Mallet A. I., Cunningham F. M. 1985. Structural identification of platelet activating factor in psoriatic scale. Biochem. Biophys. Res. Commun. 126: 192–198 [DOI] [PubMed] [Google Scholar]
- 37.Izaki S., Yamamoto T., Goto Y., Ishimaru S., Yudate F., Kitamura K., Matsuzaki M. 1996. Platelet-activating factor and arachidonic acid metabolites in psoriatic inflammation. Br. J. Dermatol. 134: 1060–1064 [PubMed] [Google Scholar]
- 38.Thompson A., Gao P., Orfei L., Watson S., Di A. E., Kaptoge S., Ballantyne C., Cannon C. P., Criqui M., Cushman M., et al. 2010. Lipoprotein-associated phospholipase A(2) and risk of coronary disease, stroke, and mortality: collaborative analysis of 32 prospective studies. Lancet. 375: 1536–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.