Abstract
Sulfatide is 3-O-sulfogalactosylceramide that is synthesized by two transferases (ceramide galactosyltransferase and cerebroside sulfotransferase) from ceramide and is specifically degraded by a sulfatase (arylsulfatase A). Sulfatide is a multifunctional molecule for various biological fields including the nervous system, insulin secretion, immune system, hemostasis/thrombosis, bacterial infection, and virus infection. Therefore, abnormal metabolism or expression change of sulfatide could cause various diseases. Here, we discuss the important biological roles of sulfatide in the nervous system, insulin secretion, immune system, hemostasis/thrombosis, cancer, and microbial infections including human immunodeficiency virus and influenza A virus. Our review will be helpful to achieve a comprehensive understanding of sulfatide, which serves as a fundamental target of prevention of and therapy for nervous disorders, diabetes mellitus, immunological diseases, cancer, and infectious diseases
Keywords: bacterial infection, biological function, diabetes, glycolipid, hemostasis, immune system, nervous system, sulfatide, thrombosis, viral infection
INTRODUCTION OF SULFATIDE
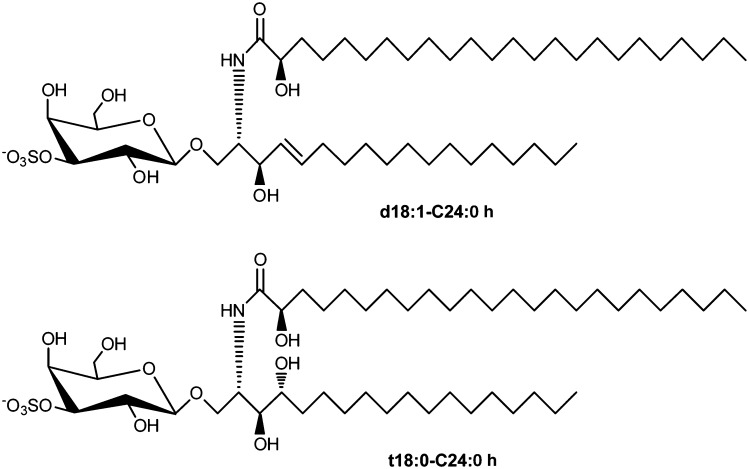
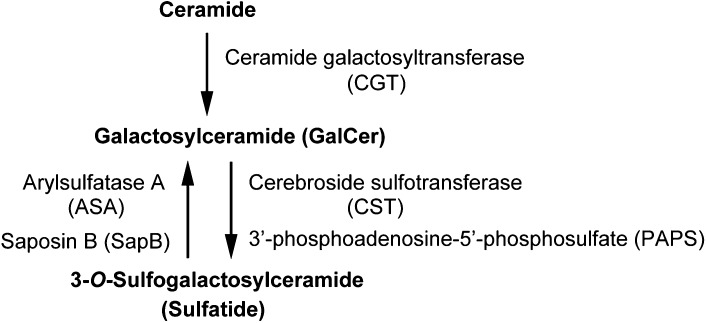
3-O-sulfogalactosylceramide is the first sulfoglycolipid isolated from the human brain and was given the name sulfatide by Thudichum in 1884 (1). The synthesis of sulfatide starts at the endoplasmic reticulum by the addition of galactose from UDP-galactose to ceramides, which is catalyzed by the UDP-galactose:ceramide galactosyltransferase (CGT; EC 2. 4. 1. 45) (2). Galactosylceramide (GalCer), which is the precursor of sulfatide, is transported to the Golgi apparatus. Then sulfatide is finally synthesized by 3-O-sulfation of the galactose residue through 3′-phosphoadenosine-5′-phosphosulfate:cerebroside sulfotransferase (CST; EC 2. 8. 2. 11) (3, 4). CST has been reported to be a homodimeric protein localized to a late Golgi apparatus (5). Sulfatide exhibits various structures, including different lengths of the acyl chain and ceramide moiety, which can be hydroxylated, as well as other sphingolipids. Some ceramide moieties have been characterized by MALDI-TOF MS from sulfatide in the rat spinal cord, lung, colon, and stomach. In detail, the major sulfatide is composed of ceramides possessing 4-sphingenine (d18:1) with C22 hydroxy FAs (C22:0 h), C23:0 h, C24:0 h, and C24:1 h and with C24 normal FAs (C24:0) and C24:1 (Fig. 1). The minor sulfatide is composed of ceramides possessing d18:1 with C16:0, C16:0 h, C18:0, C18:0 h, C20:0, C21:0, C22:1, C22:0, C21:0 h, C23:0, C26:1, and C26:0 and phytosphingosine (t18:0) with C20:0 h and C24:0 h. The FA compositions of sulfatide are dependent on specific tissues. For example, d18:1-C16:0 h exists in the rat stomach and t18:0-C24:0 h exists in the rat colon (6). In human serous papillary ovarian carcinoma tissues, sulfatide with FAs C22:0 and C24:0 at the ceramide moiety is clearly increased compared with that in normal ovarian stromal tissue (7). Seminolipid, 3-O-sulfo monogalactosylalkylacylglycerol, is also synthesized from alkylacylglycerol by CGT and CST. Seminolipid is synthesized in spermatocytes and is maintained in the subsequent germ cell stages, suggesting a role in fertilization (8). Although CST and CGT appear to recognize to some extent a wide spectrum of hydrophobic ceramide moieties, sulfatide and GalCer appear to be preferentially synthesized from ceramides with C22 and C24 FAs in rat organs and human serous papillary ovarian carcinoma. In addition, for the human glioma cell line U87 MG, overexpression of p53 results in a dramatically increased level of sulfatide possessing C18, despite no significant change in the level of sulfatide possessing C24 (9). Genes other than CST and CGT can also have an effect on ceramide composition of sulfatide. The 3-O-sulfate group of sulfatide is specifically hydrolyzed by arylsulfatase A (ASA; EC 3. 1. 6. 8) in lysosomes. ASA activity requires the help of sphingolipid activator protein-1, also called saposin B (SapB), which extracts sulfatide from membranes and thereby makes it accessible to ASA (10) (Fig. 2). An alternative sulfatase-independent pathway of sulfatide degradation has been suggested by indications in a neuroblastoma cell line for direct generation of ceramide from endocytosed sulfatide without prior desulfation (11). Sulfatide is localized mainly in the Golgi apparatus (3), cellular membrane, and lysosomes in the cytosol (12). Sulfatide is abundant in the kidney, gastrointestinal tract, islet of Langerhans, trachea, and particularly the brain, especially in many human cancer cell lines and tissues such as primary human lung adenocarcinoma tissues (13), human gastric cancer tissues (14), human renal carcinoma cell line (SMKT-R3 cell line) (15–17) and human renal carcinoma tissues (18), primary human colorectal cancer tissues (19), human ovarian malignant and benign cancer tissues (20), and human serous papillary ovarian carcinoma tissues (7). Deficiency of the lysosomal enzyme ASA (21) or mutations in the gene coding for SapB (22) leads to the accumulation of lysosomal sulfatide and development of metachromatic leukodystrophy (MLD), a demyelinating disease. Sulfatide was also shown to be associated with the nervous system, insulin secretion in the islet of Langerhans, immune system, hemostasis/thrombosis, cancer, and microbial infection.
Fig. 1.
Structure of sulfatide. For ceramide moieties of sulfatide, d18:1-C24:0 h (major sulfatide in rat organs) and t18:0-C24:0 h (minor sulfatide in rat organs) mean 4-sphingenine with C24 hydroxy FA and phytosphingosine with C24 hydroxy FA, respectively (6).
Fig. 2.
Metabolism of sulfatide synthesis and degradation.
BIOLOGICAL FUNCTIONS OF SULFATIDE
Nervous system
In the nervous system, sulfatide is abundant in the myelin sheath and comprises 4% of total myelin lipids (23). Most of the sulfatide in the nervous system is present in myelinating cells (oligodendrocytes in central nerves and Schwann cells in peripheral nerves), in which active sulfatide synthesis proceeds. Sulfatide functions as a negative regulator of oligodendrocyte differentiation (myelination processes) (24) and is involved in an increase in oligodendrocyte survival (25). For ceramide moieties of sulfatide, hydroxy FAs are uniquely distributed in gray matter of the human brain, as compared with dominant distribution of nonhydroxy FAs in white matter. Because the hydroxy FAs originate from myelinating oligodendrocytes in gray matter, sulfatide with the hydroxy FAs is suggested to influence myelin stability (26). On the other hand, the myelin formation of cultured Schwann cells appears to be initiated by sulfatide through its binding to extracellular matrixes, like tenascin-R or laminin, which binds to integrins (signaling molecules) and can stimulate c-Src/Fyn kinase (27–29). Histological analysis showed that axons of the optic nerve in CST-deficient mice were well-myelinated. However, electron microscopic analysis of myelinated axons revealed disorganized termination of the lateral loops at the node of Ranvier (30, 31). Recently, sulfatide was demonstrated to be a novel inhibitor of myelin-associated axon outgrowth (32).
Myelin and lymphocyte protein (MAL) is a highly hydrophobic molecule and a tetraspan lipid raft-associated protein. Association of MAL with sulfatide has been shown in cultured oligodendrocytes, myelin, and plasma membranes from the rat spinal cord. MAL might be involved in the vesicular transport of myelin proteins and lipids, particularly sulfatide, to the myelin membrane (33, 34). The incorporation of MAL during myelin maturation would organize and stabilize glycosphingolipids in microdomains and thus seal the myelin membrane for small molecules and promote regular curvature of compact myelin. In addition, such clusters of glycosphingolipids might contribute to myelin membrane adhesion by interactions with calcium ions serving as a bridge (35). MAL might also support a signaling or surface recognition function of sulfatide and/or other molecules.
Sulfatide also appears to function in myelin maintenance and to affect glial-axon signaling as exemplified by indications of uncompacted myelin and vacuolar degeneration in adult CST-deficient mice. The spinal cords of CST-deficient mice exhibited modest demyelination (36). In a previous study, demyelination was not observed in the optic nerve of CST-deficient mice (30, 31). CST-deficient mice may display region-specific myelin instability. The disturbed glial-axon contact leads to improper localization and abnormal maintenance of sodium and potassium ion channel clusters, although sulfatide does not appear to be essential for initial cluster formation of sodium ion channels (30).
Small amounts of sulfatide have been detected in neurons and astrocytes (37, 38). Cultured astrocytes are capable of synthesizing small amounts of sulfatide (39, 40). Low expression levels of both CGT and CST are detectable in various neurons. Relatively large amounts of C18:0-sulfatide are present in neurons. In contrast, myelin is enriched in very-long-chain FA C22/C24-sulfatide (41, 42). Alternatively, it has been suggested that sulfatide could be imported into neurons by apoE receptor-mediated endocytosis of sulfatide-containing apoE proteins (43).
Thus, because sulfatide in the nervous system can potentially affect functional properties of membrane proteins such as ion channels, ion pumps, receptors, and transporters, abnormal expression levels of sulfatide are linked to neurological symptoms. Accumulation of sulfatide with FA C18:0 in neurons causes lethal audiogenic seizures in mice (44). MLD develops due to deficiency of ASA activity (or SapB) that results in intralysosomal storage of sulfatide in the nervous system. This leads to progressive demyelination (45). Large quantities of sulfatides can be found in the urine of MLD patients (46). There was no difference in quantitative levels of ceramide compositions (d18:1-C22:1, sphingadienine d18:2-C22:0, d18:1-C22:0 h, d18:1-C24:0, d18:1-C24:0 h, d18:2-C23:0 h, and d18:2-C24:0 h) of sulfatide in the urine samples from patients with two forms of clinical manifestation: motor form (homozygous for the ASA mutation P426L) and psychotic form (heterozygous for the ASA mutation I179S) (47). Parkinson's disease patients exhibit levels of sulfatide elevated by 30–40% in the superior frontal and cerebellar gray matter (48). However, a recent study has demonstrated that sulfatide from lipid rafts of human brain gray matter is reduced by 30% in incidental Parkinson's disease manifested as Lewy body pathology in the brain stem without motor symptoms (but not in classic Parkinson's disease manifested as a complex motor disorder) (49). Loss of sulfatide in Alzheimer's disease is most severe in the cerebral gray matter (>90% reduction) compared with about 50% in the white matter. For ceramide moieties of sulfatide, compositional distributions (C18:0, C22:0, C23:0, C24:1, C24:0, C25:1, and C26:1 of hydroxy or nonhydroxy FAs) were unchanged in the Alzheimer's disease brain (26). Because CST activity in the brain is normal in Alzheimer's disease, it has been suggested that accelerated degradation of sulfatide leads to ceramide production (50). LDL receptor-related protein 1 (LPR1), a highly expressed protein in neurons of the central nervous system, plays critical roles in lipoprotein metabolism, synaptic transmission, and clearance of amyloid-β peptide associated with Alzheimer's disease. LPR1 deletion in mice also leads to a global defect in brain lipid metabolism characterized by decreased levels of cholesterol, sulfatide, and GalCer. These lipid deficits correlate with eventual neurodegeneration (51). ApoE potentially controls import levels of sulfatide to cells in the brain. The human apoE ϵ4 allele is a genetic risk factor for Alzheimer's disease (52), and apoE-deficient mice exhibit significantly reduced levels of sulfatide in the brain (39). Deficiency of apoE expression may correlate with Alzheimer's disease through sulfatide reduction in the brain. Herpes simplex virus-1 (HSV-1) infection in the brain is related to Alzheimer's disease susceptibility by binding of the virus to heparan sulfate proteoglycans, α2-macroglobulin, nectin receptors, blood-borne lipoproteins, and apoE (53, 54). Although the mechanism by which HSV-1 infection correlates with Alzheimer's disease is not clear, virus binding to apoE might result in reduction of sulfatide in the brain (Table 1).
TABLE 1.
Biological functions of sulfatide in the nervous system
| Functions of sulfatide | |
| A negative regulator of oligodendrocytes differentiation in the central nervous system (24) | |
| An initiation factor of the myelin formation of Schwann cells in the peripheral nervous system (27–29) | |
| An inhibitor of myelin-associated axon outgrowth (32) | |
| Association with MAL (clustering of sphingoglycolipids or transporters) (33, 34) | |
| Myelin maintenance (26, 36) | |
| Glial-axon signaling (localization and maintenance of sodium and potassium ion channel clusters) (30, 36) | |
| Involvement of sulfatide in nervous diseases | |
| Demyelination by accumulation of lysosomal sulfatide in MLD (45) | |
| Elevated levels of sulfatide in the superior frontal and cerebellar gray matter of Parkinson's disease patients (48) | |
| Reduced levels of sulfatide in lipid rafts of human brain gray matter in incidental Parkinson's disease (49) | |
| Severe loss of sulfatide in the cerebral gray matter and the white matter of Alzheimer's disease patients (through LPR1 deletion and apoE-mediated mechanism?) (39, 50–54) | |
| Lethal audiogenic seizures caused by accumulation of sulfatide in mouse neurons (44) | |
Kidney
Many kidney-derived cell lines contain sulfatide. The function of sulfatide in the kidney has been investigated using CGT- or CST-deficient mice. No abnormalities were found in tissue weight, morphology, and histology of kidneys in CGT-deficient mice. In CGT-deficient mice, several parameters, including blood urea nitrogen, creatinine, sodium ion, potassium ion, chloride ion, urinary osmolality, and β-N-acetyl-d-glucosaminidase excretion were within normal ranges. There were no significant differences in kidney function among CGT genotypes (55). Similarly, CST-deficient mice did not show any major morphological or functional defects in the kidney (8). Although sulfatide is not thought to be essential for normal kidney function, the role of sulfatide in kidney function might be partially compensated by increased expression of other sulfated glycolipids and sulfated cholesterol in sulfatide-deficient mice.
In protein-overload nephropathy mice, the level of sulfatide in serum decreases as the disease progresses. Acute kidney dysfunction lowers the level of sulfatide in serumw through downregulation of CST gene expression in lipoprotein-producing organs such as the liver (56, 57). Reduction of serum sulfatide level in patients with end-stage renal disease was detected prior to induction of hemodialysis therapy (58). Kidney function itself also seems to be associated with regulation of sulfatide level in serum and lipoprotein-producing organs.
L-selectin has been identified as a lymphoid homing receptor. Exogenously added sulfatide considerably inhibited monocyte infiltration after unilateral ureteral obstruction (UUO) treatment (59). L-selectin ligand activity was not detected in CST-deficient mice with or without UUO treatment, whereas the distributions of L-selectin ligand activity and sulfatide were relocated from distal tubules to the endothelium of peritubular capillaries, where monocytes infiltrated, after UUO treatment in wild-type mice (60). Sulfatide is a major L-selectin ligand in the kidney, and binding between L-selectin and sulfatide plays an essential role in monocyte infiltration into the kidney interstitium.
MAL is expressed at the apical surface of the kidney and stomach in the rat and mouse. Sulfatide is abundant in apical membranes of distal kidney tubuli and the glandular stomach epithelium. MAL forms complexes with glycosphingolipids, including sulfatide in kidney membranes, and might contribute to stabilization and apical sorting of glycosphingolipid-enriched microdomains (61, 62) (Table 2).
TABLE 2.
Biological functions of sulfatide in the kidney
| Functions of sulfatide | |
| Not essential for morphology or physiological function of the kidney (8, 55) | |
| A major l-selectin ligand essential for monocyte infiltration into the kidney interstitium (59, 60) | |
| Complex formation with MAL (associated with stabilization and apical sorting of sphingoglycolipid-enriched microdomains?) (61, 62) | |
Diabetes mellitus
The C16:0 isoform of sulfatide is predominantly found in secretory granules and at the surface membrane of β cells in the islet of Langerhans and rat β TC3 cells (63). Sulfatide expression has not been detected in rat α TC1-9 cells. Incubation of β TC3 cells with the C16:0 isoform of sulfatide (but not with the C24:0 isoform) inhibited glucose-induced insulin secretion by reducing ATP-sensitive potassium channel sensitivity (64). On the other hand, lack of the C16:0 isoform of sulfatide was also found in the pancreas of the type II diabetic mouse model, the db/db mouse (65). Sulfatide is required for normal insulin secretion through activation of ATP-sensitive potassium ion channels and stimulation of calcium ion-dependent exocytosis (66). The C16:0 isoform of sulfatide dramatically improves insulin crystal preservation, whereas the C24:0 isoform has almost no effect (65). Sulfatide facilitates the instant monomerization of insulin during its secretion from the β cells of the pancreas. Sulfatide may also promote proinsulin folding and seems to serve as a molecular chaperone for insulin. This is one of the first examples of a nonprotein chaperone (67).
Anti-sulfatide antibodies in serum are frequently present at diagnosis and before the onset of diabetes (68). Anti-sulfatide antibodies associated with type I diabetes inhibit insulin secretion and exocytosis from β cells (66). Treatment of nonobese diabetic (NOD) mice with sulfatide reduced the incidence of diabetes to 35%, as compared with 85% for the control animals (69). The T cell-dependent immune system is implicated in the development of type I diabetes, and it is therefore of interest that β cells produce a peptide-based hormone that is potentially immunogenic and an autoantigen in type 1 diabetes. Sulfatide has been shown to possess anti-inflammatory properties. The C16:0 isoform of sulfatide inhibits the production of IL-1, IL-6, IL-10, TNF-α, and the chemokines MIP-1α and IL-8 (70–72). Because sulfatide has a selectin-mediated anti-inflammatory effect, it inhibits insulitis and prevents diabetes in NOD mice by blockage of L-selectin (73, 74). Sulfatide also has the ability to reduce caspase-3/7-dependent apoptosis in insulin-inducing cells by blocking pro-apoptotic effects of IL-1β, IFN-1β, and TNF-α (75). These findings suggest involvement of sulfatide in the pathogenesis of type 1 diabetes.
Sulfatide might also be involved in type 2 diabetes. Sulfatide inhibits secretion of TNF-α. Insulin resistance is associated with low serum levels of sulfatide and elevated production of TNF-α in type 2 diabetic patients (76, 77). At the β-cell level, sulfatide might suppress type 2 diabetes through activation of potassium channels, mainly attributable to the C16:0 isoform of sulfatide (66). Single-nucleotide polymorphisms in the gene encoding the CST enzyme show lower insulin resistance or confer increased risk of type 2 diabetes (78) (Table 3).
TABLE 3.
Biological functions of sulfatide in the islet of Langerhans
| Functions of sulfatide | |
| Insulin secretion through activation of ATP-sensitive potassium ion channels and stimulation of calcium ion-dependent exocytosis (63, 66) | |
| Instant monomerization of insulin during its secretion (67) | |
| Proinsulin folding as a molecular chaperone for insulin (67) | |
| Involvement of sulfatide or anti-sulfatide antibodies in type 1 diabetes | |
| Elevated levels of anti-sulfatide antibodies in serum (66, 68) | |
| Inhibitory effect of anti-sulfatide antibodies on insulin secretion and exocytosis from β cells (66) | |
| Prevention of insulitis and diabetes in NOD mice treated with sulfatide by blockage of L-selectin (69, 73, 74) | |
| Anti-inflammatory effect of sulfatide on cytokine production (70–72) | |
| Anti-apoptotic effect of sulfatide in insulin-producing cells (75) | |
| Involvement of sulfatide in type 2 diabetes | |
| Mitigation of insulin resistance by inhibition of TNF-α secretion (76, 77) | |
| Insulin secretion from β cells through activation of potassium channels? (66) | |
| Modifications of insulin resistance by single-nucleotide polymorphisms in the CST (78) | |
Immune system
T cells recognize lipid antigens bound through CD1 molecules, which are nonpolymorphic, major histocompatibility complex (MHC) class I-like. Various antigen-presenting cells, such as dendritic cells, macrophages, subsets of B cells, thymocytes, hepatocytes, and tumor cells, express CD1 molecules, which can be classified into five subtypes (a–e). CD1 (a–d) molecules can bind to sulfatide. While CD1a, -b, and -c are involved in presenting (mostly microbial) lipid antigens to conventional T cells, CD1d presents lipids, glycolipids, and lipoproteins, which can be of self or foreign origin, to natural killer T (NKT) cells. CD1a, -b, and -c are able to load sulfatide on the cell surface without proteasome processing as a lipid antigen and prime T helper type 1 and T helper type 2 responses (79). CD1a uniquely localizes to the early endocytic recycling compartment, consistent with the localization of sulfatide. The intracellular trafficking route of CD1a is essential for efficient presentation of lipid antigens such as sulfatide that traffic through the early endocytic and recycling pathways (80). CD1d-restricted NKT cells can be categorized into two groups. Type 1 NKT cells can recognize αGalCer/CD1d-tetramers. Major type 2 NKT cells can recognize sulfatide/CD1d-tetramers and are activated by multiple tissue-specific isoforms (various ceramide moieties) of sulfatide (81). Sulfatide-reactive type 2 NKT cells isolated from unprimed animals have an oligoclonal T cell receptor repertoire with predominant usage of Vα3/Vα1-Jα7/Jα9 and Vβ8.1/Vβ3.1-Jβ2.7 gene segments that exhibit features of both antigen-specific conventional T cells and innate-like cells (82). Sulfatide-reactive type 2 NKT cells are able to regulate type 1 NKT cells by modulating the function of dendritic cells, resulting in protection from autoimmune disease (83, 84) and ischemic reperfusion injury (85, 86).
Selectins are an important family of adhesion molecules involved in capturing circulating leukocytes by inducing “rolling” along the endothelium. E-selectin is expressed only on endothelial cells activated by proinflammatory stimuli of cytokines such as IL-1 and TNF-α. P-selectin is stored in α-granules of normal platelets and in granules (called Weibel-Palade bodies) of normal endothelial cells. Once the platelets and endothelial cells are activated by proinflammatory stimuli of cytokines such as IL-1 and TNF-α or by inflammatory mediators such as histamine and thrombin, P-selectin is quickly expressed on the surface. L-selectin is present on the surface of leukocytes and interacts with its ligands on endothelial cells, resulting in subsequent migration (called homing) of leukocytes to inflammation sites (87). Sulfatide is a native ligand for L- and P-selectin, but not for E-selectin. Sulfatide is also abundantly expressed in many types of cancer and tumor. Metabolic inhibition of sulfation by incubation of mouse colon carcinoma MC-38 cells with sodium chlorate or enzymatic desulfation by treatment of the cells with arylsulfatase results in decreased P-selectin binding and leads to attenuation of metastasis in mouse lungs. Abundant sulfatide on the surface of cancer cells possibly serves as a native ligand for P-selectin, contributing to facilitation of metastasis (88, 89). Binding of L-selectin to sulfatide triggers upregulation of the expression of the chemokine coreceptor CXCR4 surface expression on surfaces of human leukocytes such as CD4+ T cells. Interaction of chemokine ligand 12 (CXCL12) with CXCR4 induces chemostasis for leukocytes (90). Sulfatide is thought to contribute to migration (homing) of leukocytes to the injured endothelium and to cancer cells that abundantly express sulfatide.
Chemokines are a family of small chemostatic cytokines that provide directional cues for leukocyte trafficking. The chemokines MCP-1/CCL2, IL-8/CXCL8, SDF-1α/CXCL12, MIP-1α/CCL3, and MIP-1β/CCL4 bind selectively to sulfatide with the site overlapping the glycosaminoglycan-binding site of MCP-1/CCL2. They are involved in human immunodeficiency virus (HIV)-1 infection, hematopoiesis, angiogenesis, embryonic development, tumor metastasis, and graft rejection (90, 91). Sulfatide might function as a receptor of these chemokines.
Sulfatide can bind to scavenger receptors on macrophages. This binding enhances uptake of sulfatide-painted apoptotic cells by macrophages and results in enhanced TGF-β1 generation, IL-6 secretion, and P-selectin expression (92). For antigen-presenting cells (human CD1d-transfected monocytic leukemia THP-1 cells), addition of the C16:0 isoform of sulfatide reduces and the C18:0 or the C24:1 isoform increases expression of indoleamine 2,3-dioxygenase 1 (DOX1), an enzyme catalyzing the initial and rate-limiting step in the catabolism of tryptophan along the kynurenine pathway, which represents a fine-tuned mechanism for regulating several immune responses (93). Various biological functions of sulfatide are thought to be dependent on ceramide moieties of sulfatide (Table 4).
TABLE 4.
Biological functions of sulfatide in the immune system
| Functions of sulfatide in lipid recognition of T cells to antigen-presenting cells | |
| Priming of T helper type 1 and type 2 responses through sulfatide loading to CD1 (a–c) molecules (79, 80) | |
| Recognition of sulfatide/CD1d-tetramers by major type 2 NKT cells (81) | |
| Protection from autoimmune disease and ischemic reperfusion injury by sulfatide-reactive type 2 NKT cells (82–86) | |
| Functions of sulfatide as a selectin ligand | |
| A native ligand for L- and P-selectin but not for E-selectin (87) | |
| Cancer metastasis involving P-selectin (88, 89) | |
| Upregulation of CXCR4 surface expression of leukocytes through L-selectin (recruitment of leukocytes to injured endothelium?) (90) | |
| Functions of sulfatide as a chemokine receptor | |
| Selective binding to chemokines (involved in HIV-1 infection, hematopoiesis, angiogenesis, embryonic development, tumor metastasis, and graft rejection?) (90, 91) | |
| Functions of sulfatide in macrophages | |
| Uptake of apoptotic cells by macrophages (92) | |
Hemostasis/thrombosis
Sulfatide has strong anticoagulant activity that prolongs bleeding time, probably due to both its binding activity to fibrinogen, inhibiting the conversion to fibrin, and its direct inhibitory effect on thrombin activity, when simply injected into animals (94). On the other hand, sulfatide also enhances thrombosis, possibly through participation of blood coagulation factor XII, when injected into mice with blood vessel walls that are heavily damaged or when continuously infused into mice through plastic cannulae. Annexin V is known to inhibit the binding of coagulant factors Xa and Va to phosphatidylserine on the exposed surfaces of activated platelets. The binding of annexin V to sulfatide accelerates coagulation (95). The interaction between sulfatide and P-selectin, both of which are expressed on platelets, is important for stable platelet adhesion and aggregation during hemostasis and thrombosis (96). Therefore, sulfatide is thought to have both activities for coagulation and anticoagulation. Sulfatide in serum lipoproteins has been suggested to affect the pathogenesis of cardiovascular disease. This disease might be caused by the development of arterial thrombosis through platelet aggregation and platelet-leukocyte interactions associated with both sulfatide and P-selectin on platelets (97, 98). Disabled-2 (Dab2) is a negative regulator of platelet aggregation and is released to the extracellular surface upon platelet activation. Dab2 at the platelet surface coexists in two states, a sulfatide-bound state and an integrin receptor-bound state. The multi-target ability of Dab2 may play a regulatory role in platelet-leukocyte adhesion and aggregation events (99, 100) (Table 5).
TABLE 5.
Biological functions of sulfatide in hemostasis/thrombosis
| Anticoagulant activity of sulfatide | |
| Binding to fibrinogen (inhibition of conversion to fibrin) (94) | |
| Direct inhibitory effect on thrombin activity (94) | |
| Coagulant activity of sulfatide | |
| Thrombosis possibly through blood coagulation factor XII (95) | |
| Coagulation by binding to annexin V (95) | |
| Stable platelet adhesion and aggregation through P-selectin (96) | |
| Pathogenesis of cardiovascular disease caused by sulfatide in serum lipoproteins? (97, 98) | |
| Sulfatide binding of Dab2 at the platelet surface (99, 100) | |
Cancer and tumor
Sulfatide is abundant in the kidney, gastrointestinal tract, islet of Langerhans, trachea, and particularly the brain, especially in many human cancer cell lines and tissues such as primary human lung adenocarcinoma tissues (13), human gastric cancer tissues (14), human renal carcinoma cell line (SMKT-R3 cell line) (15–17), and human renal carcinoma tissues (18), primary human colorectal cancer tissues (19), human ovarian malignant and benign cancer tissues (20), and human serous papillary ovarian carcinoma tissues (7). Elevated expression of sulfatide is commonly found in many human cancer cell lines and tissues and may possibly be used as a biomarker of some cancer cells. However, primary human lung squamous cell carcinoma tissues and undifferentiated small cell carcinoma tissues have much lower levels of sulfatide than the levels in primary human lung adenocarcinoma tissues (13). For human ovarian cancers, sulfatide content in malignant cancers is significantly higher than in benign cancers (20). For human gastric cancer tissues, it has also been reported that expression levels of sulfatide were low (101) and that expression levels of CST mRNA were highly variable among several patients (102). For human hepatocellular carcinoma, CST activity in serum of patients is elevated compared with that in healthy subjects (103), although the CST level of hepatocarcinoma tissues is similar to that in normal controls (104). For human renal cell carcinoma cell lines, marked increases of CST mRNA and CST activity were observed in six cell lines (SMKT-R1, SMKT-R2, SMKT-R3, SMKT-R4, TOS-1, and TOS-2), whereas ACHN cells showed only a slight increase, as compared with normal cells (105). Wilms’ tumor (nephroblastoma) shows no detection of sulfatide (106). Thus, elevated expression of sulfatide is not necessarily common in all types of cancer. In addition, because lipids can be exchanged between the cellular membrane and serum-containing medium in cells cultured in vitro, it may be necessary to confirm whether elevated levels of sulaftide are an artifact in cultured cancer cell lines.
Mechanisms of the elevation of sulfatide expression have been investigated in human renal cell lines. For human renal cancer cell lines including SMKT-R3, CST activity is elevated through a signaling pathway involving protein kinase C (16, 105), tyrosine kinases (105, 107), and Ras (108), by the action of cytokines such as epidermal growth factor (109), TNF-α (17), and hepatocyte growth factor (110), resulting in the accumulation of sulfatide. Metabolic inhibition of sulfation by incubation of mouse colon carcinoma MC-38 cells with sodium chlorate or enzymatic desulfation by treatment of the cells with arylsulfatase results in decreased P-selectin binding and leads to attenuation of metastasis in mouse lungs. Abundant sulfatide on the surfaces of cancer cells possibly serves as a native ligand for P-selectin, contributing to facilitation of metastasis (88, 89) (Table 4). The relationship between the initiation and promotion mechanism of cancer and elevated expression of sulfatide remains unknown.
ROLE OF SULFATIDE IN BACTERIAL INFECTION
Adhesion of bacteria to the mucosal surface is an initial important step for infection. Sulfatide is known to bind to many bacteria, including Actinobacillus pleuropneumoniae (111), Bordetella pertussis (112), Campylobacter jejuni (DNA binding protein from starved cells; Dps) (113, 114), 987P-fimbriated enterotoxigenic Escherichia coli (116), enterotoxigenic Escherichia coli TOP10 strain (coli surface antigen 6) (116), Haemophilus ducreyi (117), Haemophilus influenzae (118), Helicobacter pylori (119), Lactobacillus reuteri (JCM1081 and TM105 strains) (120), Moraxella catarrhalis (121), Mycoplasma hominis (122), Mycoplasma hyopneumoniae (123), Mycoplasma pneumonia (124), and Pseudomonas aeruginosa (125). Sulfatide is one of the glycolipid receptors that serve to adhere these bacteria to the mucosal surface. Borrelia burgdorferi Sensu Lato 297, a virulent strain, shows binding to GalCer but not to sulfatide (126).
A. pleuropneumoniae and M. hyopneumoniae are swine respiratory pathogens. B. pertussis, H. influenzae, M. catarrhalis, M. pneumonia, and P. aeruginosa are human pharyngeal or respiratory pathogens. Sulfatide is present in the human and swine tracheas (123, 124). These bacteria are thought to use sulfatide to adhere directly to the host respiratory tract. Hsp70 is exposed on the outer surface of H. influenzae and contributes to the ability of H. influenzae to bind to sulfatide (118). Interaction of the N-terminal domain of Hsp70 with sulfatide also results in the inhibition of Hsp70 ATPase activity, suggesting that Hsp70/sulfatide binding might modulate chaperone function (127). Sulfatide mediates attachment of P. aeruginosa strain 361 to human pharyngeal epithelial cells, whereas P. aeruginosa mucoid strain 8830 does not bind to sulfatide (128). Recognition by sulfatide of P. aeruginosa is dependent on the strain. All strains of B. pertussis bind to asialo GM1, whereas only virulent strains bind to sulfatide (112).
987P-fimbriated enterotoxigenic E. coli, enterotoxigenic E. coli TOP10 strain, H. pylori, and L. reuteri adhere to the mucosal surface of the host gastrointestinal tract, where sulfatide is endogenously expressed and is exogenously loaded (129). Sulfatide recognition of H. pylori has been investigated in detail. H. pylori, the human causative agent of active chronic gastritis, can bind to some sialo- and asialo-glycoconjugates containing sulfatide. Treatment of H. pylori at low pH induces specific binding to sulfatide. It is possible that increased levels of surface hsp70 on H. pylori following low-pH treatment mediate the increased sulfatide recognition (130). L. reuteri JCM1081 and TM105 strains bind to sulfatide and gangliotetraosylceramide (asialo-GM1), both of which are recognized by H. pylori. L. reuteri strains sharing glycolipid specificity with H. pylori help to prevent infection in an early stage of colonization of H. pylori (120). H. ducreyi and M. hominis are pathogens of the human genital tract, present in sulfatide (131). The interaction between H. ducreyi and sulfatide is mediated by a 58.5 kDa heat shock protein of its organism (117).
Malaria (Plasmodia) sporozoites pass from the salivary gland of an infected Anopheles mosquito into the host blood stream during feeding, followed by invasion into host hepatocytes. Plasmodium berghei sporozoites bind specifically to sulfatide through circumsporozoite proteins, which densely coat malaria sporozoites (132). Sulfatide is present on the hepatocyte surface membrane (133). Malaria sporozoites may use sulfatide for invasion into host hepatocytes.
STb, a 48 amino acid heat-stable enterotoxin b, is secreted by enterotoxigenic E. coli strains and is responsible for diarrheal diseases in many animals including humans. STb binds strongly to sulfatide present in the pig jejunum mucosal surface, but not to GalCer and asialo gangliosides. The gangliosides GM3, GM2, and GD1b exhibit binding levels of at least 40% relative to sulfatide. It has been suggested that sialic acid residues are also involved in the binding process of STb (134). The interaction between sulfatide and STb is time and temperature dependent and low affinity, and it is not affected by pH (135). Because laminin, a sulfatide binding protein, or sulfatase pretreatment of ligated rat intestinal loops significantly inhibits the in vivo action of STb, sulfatide represents a functional STb receptor (134).
The activity of phospholipase C from Clostridium perfringens is increased by the presence of sulfatide in the dilauroylphosphatidylcholine monolayer but is inhibited by gangliosides such as GM1, GD1a, and GT1b. Similarly, the activity of phospholipase A2 in the porcine pancreas is increased by the presence of sulfatide in the dilauroylphosphatidic acid monolayer but is inhibited by GM1, GD1a, and GT1b (136).
Mycobacterium tuberculosis is the causative agent of human tuberculosis. Sulfated glycolipids of the cell wall of M. tuberculosis are implicated in virulence. It has been proposed that sulfated glycolipids may be involved in intracellular survival of virulent M. tuberculosis by their interaction with phagosomes and prevention of lysosomal fusion. The principal sulfated lipid of M. tuberculosis has been identified as 2,3,6,6′-tetraacyl-α,α′-d-trehalose-2′-sulfate. However, the gene Rv1317 in M. tuberculosis encodes a novel glycolipid sulfotransferase with activity toward GalCer. Sulfatide may be a component of the cell wall of M. tuberculosis and may be involved in virulence (137). On the other hand, it has also been shown that sulfatide from bacteria promotes phagocytosis and phagosome-lysosome fusion of Sphingomonas paucimobilis by human peripheral polymorphonuclear leukocytes (138).
Dps protein from C. jejuni induces rapid paranodal myelin detachment and down-modulation of nodal sodium channels by binding to sulfatide on the myelin and the nodes of Ranvier. This may be involved in Guillain-Barre syndrome (GBS) associated with C. jejuni enteritis (113, 114) (Table 6).
TABLE 6.
Biological functions of sulfatide in bacterial infections
| Bacteria capable of adherence to sulfatide | |
| Respiratory bacteria | |
| Actinobacillus pleuropneumoniae (111) | |
| Bordetella pertussis (112) | |
| Haemophilus influenzae (118) | |
| Moraxella catarrhalis (121) | |
| Mycoplasma hyopneumoniae (123) | |
| Mycoplasma pneumoniae (124) | |
| Pseudomonas aeruginosa (strain 361) (125) | |
| Gastrointestinal bacteria | |
| 987P-fimbriated enterotoxigenic Escherichia coli (115) | |
| Enterotoxigenic Escherichia coli TOP10 strain (116) | |
| Helicobacter pylori (119) | |
| Lactobacillus reuteri (JCM1081 and TM105 strains) (120) | |
| Genital bacteria | |
| Haemophilus ducreyi (117) | |
| Mycoplasma hominis (122) | |
| Bacterial surface molecules for sulfatide binding | |
| Hsp70 on H. influenzae and H. pylori (118, 130) | |
| 58.5 kDa heat shock protein on H. ducreyi (117) | |
| Protozoa capable of adherence to sulfatide | |
| Specific binding of P. berghei sporozoites through circumsporozoite proteins (132) | |
| A receptor for invasion to host hepatocytes? (133) | |
| Involvement of sulfatide in STb from enterotoxigenic E. coli stains | |
| A functional STb receptor in the intestinal tract (134, 135) | |
| Enhancing effect of sulfatide on enzyme activity | |
| Activity of phospholipase C from C. perfringens (136) | |
| Activity of phospholipase A2 from porcine pancreas (136) | |
| Functions of sulfatide in the cell wall of bacteria | |
| Virulence of M. tuberculosis? (137) | |
| Promotion of phagocytosis and phagosome-lysosome fusion of S. paucimobilis by human peripheral polymorphonuclear leukocytes (138) | |
| GBS associated with bacterial infection | |
| Sulfatide binding of Dps protein from C. jejuni? (113, 114) | |
ROLE OF SULFATIDE IN VIRUS INFECTION AND REPLICATION
HIV
The involvement of sulfatide in virus infection has been mostly investigated for HIV-1. Trimerized gp120-gp41 envelope glycoprotein complexes on HIV-1 interact with viral receptor CD4 molecules, inducing a conformational change in gp120 that allows for its subsequent interaction with a chemokine coreceptor: CXCR4 for T-cell-tropic and CCR5 for macrophage-tropic. The interaction of gp120 with a chemokine coreceptor promotes the exposure and subsequent insertion of the hydrophobic gp41 fusion peptide into the host cell membrane for virus entry into susceptible cells. Surface glycoprotein gp120 on HIV-1 binds not only to CD4 and CXCR4 or CCR5 but also to glycolipids, including sulfatide, GalCer, lactosylceramide (LacCer), GM3, and GD3. The third variable region “V3 loop” of HIV-1 gp120 interacts with these glycolipids but not with CD4 (139). HIV-1 can also infect CD4- cells of neural and intestinal tissues. In CD4-negative cells of neural and intestinal tissues susceptible to HIV-1 infection, GalCer and sulfatide seem to function as alternate virus binding receptors (140). The role of sulfatide in HIV-1 infection would be not only to serve as secondary attachment sites but also to participate actively in transmembrane signaling. For example, sulfatide can trigger an increase in cytosolic calcium ions and enhancement of mRNA expression of TNF-α and IL-8 in human neutrophils (141) and activation of tyrosine kinase in CD4− glial cells (142).
Sulfatide does not play a significant role in HIV infection of CD4+ monocyte-derived macrophages or monocytic U937 cells (143). The binding of gp120 V3 loop to LacCer, GM3, and GD3 on CD4+ lymphocytes has been suggested to play a role in the fusion process between the viral envelope and the cellular plasma membrane (139). For RD cells (CD4−/GalCer−/CXCR4+ human rhabdomyosarcoma cell line), direct interaction between gp120 and CXCR4 is thought to be a very low probability. For Caco-2/Cl2 (CD4−/GalCer−/CXCR4− human intestinal cell line), the additon of sulfatide into the cellular membrane confers HIV-1 binding but not entry. For HT-29 cells (CD4−/GalCer+/CXCR4+ human intestinal cell line), the incorporation of sulfatide into cells leads to increased HIV-1 binding and decreased initial infection. The cell surface of most HT-29 cells exhibits both GalCer and CXCR4. The interactions with both receptors are required for virus entry into cells (144). For U373-MG cells and SK-N-MC cells (two CD4− cell lines derived from the nervous system), treatment with antibodies against GalCer inhibits viral entry and infection (145). gp120 can also penetrate into a monomolecular film of GalCer but not into a monomolecular film of sulfatide or into a mixed monolayer of GalCer and sulfatide. These results indicate that GalCer plays a role as a functional receptor for HIV-1 and that sulfatide competes with GalCer for gp120 binding. The binding of recombinant gp120 to sulfatide exhibits the strongest activity of tested glycolipids, including GalCer (146). The attachment of HIV-1 to the surface membrane of Sulfatide+/GalCer+ cells may proceed through predominant binding to sulfatide. Sulfatide-attached HIV-1 cannot be delivered to CXCR4 due to the instability of the gp120-sulfatide complex, leading to an insufficiency for initiation of the fusion process. For the CD4-independent pathway in HIV-1 infection, GalCer acts as a functional receptor, but sulfatide can efficiently prevent infection by mediating strong gp120 binding and its inability to initiate the fusion process (147). On the other hand, HIV-1 can infect SK-N-MC cells (148) and primary oligodendrocytes (149) (CD4−/GalCer+/sulfatide+/CXCR4+ cell lines). Of these CD4−/CXCR4+ cells, a subset of cells with rich sulfatide and poor GalCer may not be subjected to HIV-1 infection. The strong binding of HIV-1 to sulfatide has been applied to the development of an anti-HIV reagent. For example, polysulfated galactose-derivatized dendrimers have been reported to be binding antagonists of HIV-1 infection (146).
Myelin degeneration is commonly observed in the central nervous system of HIV-1-infected individuals, especially in patients with HIV-1-associated dementia. It has been shown that mean sulfatide concentrations are still elevated in cerebrospinal fluid (CSF) from the majority of asymptomatic HIV-1-infected patients, compared with those in HIV-1-negative controls, but those were significantly increased in acquired immunodeficiency syndrome (AIDS) patients with or without opportunistic infections or lymphoma in the central nervous system. The increased sulfatide concentrations did not differ significantly between patients with and without dementia. The sulfatide levels are associated with blood-brain barrier function but not with intrathecal immunoglobulin production (150). Elevated titers of anti-sulfatide antibodies are also observed in serum from HIV-1-infected individuals with or without central nervous system complications. However, anti-sulfatide antibodies are not detectable in CSF. Therefore, anti-sulfatide antibodies are not a major component in the pathogenesis of the central nervous system myelin damage in HIV-1 infection (151). The elevated level of anti-sulfatide antibodies in AIDS patients can cause demyelination by binding to the surface of Schwann cells or to the myelin sheath and activating the complement cascade. Patients in the advanced stage of AIDS occasionally develop GBS, which is an acute autoimmune polyneuropathy affecting the peripheral nervous system. The anti-sulfatide autoimmune antibodies may play a role in the pathogenesis of peripheral nervous injuries such as GBS (152). Elevated levels of their autoimmune antibodies in HIV-1-infected patients may be involved in injury of the peripheral nervous system rather than that of the central nervous system (Table 7).
TABLE 7.
Biological functions of sulfatide in virus infection
| HIV-1 infection | |
| Strong binding with the V3 loop of HIV-1 gp120 (139) | |
| Binding receptor in CD4- cells of neural and intestinal tissues (140, 142, 143, 149) | |
| Active participation in transmembrane signaling for HIV-1 infection? (141, 142) | |
| Ability to initiate the fusion process of HIV-1 by mediating binding to GalCer but not to sulfatide (sulfatide not being a functional receptor) (144-147) | |
| Elevated levels of sulfatide in CSF and anti-sulfatide antibodies in serum of HIV-1-infected patients (150, 151) | |
| Pathogenesis of HIV-1-associated dementia and GBS? (152) | |
| HCV infection | |
| Pathogenesis of HCV-associated MC? (154) | |
| Contribution to HCV replication? (155) | |
| IAV infection | |
| Enhancement of IAV replication by increasing progeny virus particle formation through promotion of nuclear export of newly synthesized vRNP to the cytoplasm (158) | |
| Vaccinia virus | |
| Binding receptor for virus attachment to the cell surface? (161) | |
Recently, there has been an interesting report about the use of sulfatide in HIV therapy. Sulfatide administration in SCID-Hu animals, coimplanted with human fetal liver and thymus, inhibits HIV-1 replication and retains hematopoeisis that is lost during HIV-1 infection. The inhibitory effect of sulfatide administration on HIV-1 replication is more efficient than the effect of treatment with the nucleoside analog reverse transcriptase inhibitor azidothymidine. Sulfatide itself has no association with anemia or bone marrow suppression, which are severe side effects of highly active anti-retroviral therapy. In the future, sulfatide or sulfatide derivatives may become useful tools of HIV-1 therapy (153).
Hepatitis C virus
Elevated titers of both anti-GM1 and anti-sulfatide antibodies were observed in plasma of several patients with hepatitis C virus (HCV)-associated IgMκ/IgG mixed cryoglobulinemia (MC). MC is an immunological disorder characterized by immune complex-mediated systemic vasculitis involving small vessels, which may present with renal, cutaneous, rheumatologic, and/or neurological manifestations. A close relationship between cryoglobulinemia and chronic HCV infection has been suggested, but the pathogenetic role of HCV infection in MC has not been elucidated (154). Host sphingolipid synthesis is known to be essential for HCV replication (155). Therefore, sulfatide might be involved in HCV replication (Table 7).
Influenza A virus
Because sialic acid plays an essential role as the virus receptor in the influenza A virus (IAV) life cycle, IAV binds to sialo-glycoconjugates on the host cell surface via the viral envelope glycoprotein, hemagglutinin (HA). Another envelope glycoprotein, neuraminidase (NA), exhibits sialidase activity for cleaving sialic acid residues and facilitates release of progeny viruses from the cell surface membrane. Sulfatide has been ubiquitously detected in various epithelial cell lines and animal tissues that permit sufficient IAV replication.
We had found that IAV strongly binds to sulfatide, which has no sialic acid (156), and that sulfatide inhibits IAV sialidase activity under an acidic condition but not under a neutral condition (157). We have tried to elucidate the role of sulfatide in the IAV infection cycle. Recently, we investigated the function of sulfatide in the IAV infection cycle by knockdown of sulfatide expression in Madin-Darby canine kidney (MDCK) cells, which are known to adequately support IAV replication, and by genetic upregulation of sulfatide expression in COS-7 cells, which lack sulfatide expression and sufficient IAV replication (158).
COS cells are a sulfatide-defective cell line due to defective CGT activity (159). It has been suggested that sulfatide accumulation leads to a feedback inhibition of GalCer synthesis (41). We therefore transfected COS-7 cells with both CST and CGT genes from MDCK cells and established two cell clones that stably express sulfatide. Infection of these sulfatide-enriched cells with IAV resulted in obvious enhancement of IAV multiple replications by 500–3,000 times in progeny virus titers in comparison with the parent cells, which showed little replication of IAV. In contrast, sulfatide-enriched cells showed slight reduction in the initial infection in comparison with that of the parent cells. Furthermore, we also generated sulfatide-knockdown cells from MDCK cells by transfection with the ASA gene and by RNA interference targeting CST mRNA. The sulfatide-knockdown cells showed a reduction in progeny virus titers generated by IAV replication and a slight increase in initial infection. Taken together, sulfatide expression in mammalian cells greatly enhances IAV multiple replications. We previously established a mouse anti-sulfatide monoclonal antibody (MAb; named GS-5) (59). Treatment with GS-5 for 4–24 h after IAV infection induced a great reduction in IAV multiple replications, but GS-5 treatment before and at the same time as IAV infection had no effect on viral initial infection and replication. In contrast, an anti-glycosphingolipid, Gb3Cer MAb (named TU-1), exhibited no inhibition of IAV replication under similar conditions. Sulfatide expression and GS-5 treatment in host cells also had the same effect on other strains independent of viral subtype (H1N1, H3N2, or H5N3) and host (human or avian). These results suggest that sulfatide on the cell surface is involved in IAV replication.
Nucleoprotein (NP) protein of IAV forms viral ribonucleoprotein (vRNP) complexes together with the viral polymerase (PB1, PB2, and PA), and viral genome RNA segments in the nucleus and the vRNP complexes are then exported to the cytoplasm. On the other hand, two surface spike glycoproteins, HA and NA, are transported to the cellular surface membrane via an exocytic pathway. In sulfatide-enriched cells at 7 h after infection, most of the newly synthesized viral NP had translocated from the nucleus to the cytoplasm, but in the parent cells, most of the newly synthesized viral NP remained in the nucleus. In both sulfatide-enriched and parent cells, most of the newly synthesized HA and NA was localized to the cell surface. In sulfatide-enriched cells that were treated with GS-5 but not with TU-1 after IAV infection, most of the newly synthesized viral NP remained in the nucleus despite normal localization of newly synthesized viral HA. The same effect was observed by treatment of virus-infected cells with anti-H3 HA MAb (named 2E10), which blocks the binding of IAV and sulfatide. In contrast, anti-H3 HA MAb (named 1F8), which does not block the binding of IAV to sulfatide, and anti-N2 NA MAb (named SI-4), as well as TU-1, did not suppress nuclear export of the viral NP. These results indicate that association of sulfatide with HA delivered to the cell surface induces translocation of the newly synthesized viral NP from the nucleus to the cytoplasm. Administration of GS-5 also protected mice against lethal challenge with mouse-pathogenic IAV and markedly reduced progeny virus titers in the lungs of the mice. This finding suggests that an inhibitor of binding between HA and sulfatide would be a novel reagent that inhibits viral particle formation and virus replication.
Recombinant HA protein of IAV (subtype H5N3) is produced by a baculovirus protein expression system. The recombinant HA protein can bind not only to sialoglycolipid but also to sulfatide. This is a useful tool for elucidation of the binding domain of HA with sulfatide and for development of new anti-IAV reagents (160) (Table 7).
Vaccinia virus
Vaccinia virus, a surrogate of variola virus that causes smallpox, can bind to sulfatide through A27 and L5 viral membrane proteins. Sulfatide inhibits virus attachment to the cell surface and prevents mortality in a lethal mouse model of infection with the vaccinia virus Western-Reserve strain. These results suggest that sulfatide plays a role as one of the receptors for vaccinia virus (161) (Table 7).
PERSPECTIVES
Sulfatide is a highly multifunctional glycolipid. Abnormal metabolism of sulfatide can be associated with development of many diseases, including MLD, diabetes, and autoimmune diseases. Additionally, many bacteria and some viruses utilize sulfatide for processes of their infection and replication. Elucidation of the biological functions of sulfatide will reveal mechanisms underlying the development of these diseases and infection processes, leading to the development of drugs for treatment of sulfatide-associated diseases. However, sulfatide is a highly multifunctional glycolipid involved in the nervous system, diabetes, immune system, hemostasis/thrombosis, bacterial infection, and virus infection, as described in this review. Therefore, if sulfatide in cells and tissues is blocked or downregulated by a direct binding agent, genetic technique, or pharmacodynamic agent affecting sulfatide metabolism, it is very likely that various side effects associated with sulfatide will occur. For anti-bacterial and anti-viral reagents, direct and specific binding inhibitors against the sulfatide binding site on bacterial or viral adhesion proteins are thought to be more suitable for practical use to prevent the predicted side effects attributed to sulfatide in the host.
For IAV, highly pathogenic avian IAV (H5N1) has often been transmitted from poultry to humans since 1997. Swine-origin IAV (H1N1) occurred suddenly, and H1N1 infection has been rapidly spreading worldwide among humans since 2009. The development of antiviral drugs will probably play a critical role in a future influenza pandemic. NA inhibitors such as zanamivir and oseltamivir are currently being used for protection against and treatment of influenza. However, the emergence of drug-resistant viruses has become a serious problem. Most current human IAVs have also become resistant to a viral M2 ion channel inhibitor (amantadine) since 2006 and to oseltamivir since 2008. Our studies show that sulfatide promotes translocation of the newly synthesized IAV RNP complexes from the nucleus to the cytoplasm by association with HA delivered to the cell surface. Administration of anti-sulfatide MAb resulted in strong inhibition of IAV replication, even after virus infection, and protected mice against lethal challenge with IAV. Inhibitors of the binding of IAV and sulfatide have potential as novel and efficient antiviral drugs that can be used against conventional drug-resistant viruses or for patients with serious symptoms caused by advanced infection. For treatment of IAV infection, inhibitors specifically and directly targeting the sulfatide binding site on viral HA might be useful for preventing the predicted side effects and might confer more powerful antiviral activity due to inhibition of progeny virus formation than NA inhibitors that inhibit the release process but not the virus formation process.
Metabolism of common gangliosides is very complicated due to sequential reaction of various transferases. On the other hand, metabolism of sulfatide is very simple in comparison to that of most gangliosides (Fig. 2). In fact, abnormal expression and suppression of sulfatide can be remedied by gene operations or by inhibitors against CST, CGT, and ASA. In terms of glycolipid-associated diseases, gene therapies or pharmacodynamic agents targeting sulfatide metabolism could be used for sulfatide-associated diseases if side effects could be prevented. Results of future studies on sulfatide should lead to a detailed fundamental understanding of illnesses arising from an abnormal nervous system (MLD resulting from sulfatide accumulation in lysosomes due to deficient activity of ASA, possible association of Parkinson's disease with either elevated or reduced levels of sulfatide, possibility of Alzheimer's disease associated with reduction of sulfatide due to promotion of sulfatide degradation or to loss of sulfatide transporter, the possibility of abnormal formations and functions of myelination, sodium and potassium channels, and transporters resulting from a decrease or loss of sulfatide expression), autoimmune diseases, diabetes, infarction, bacterial or viral infectious diseases, and other incompletely understood diseases. Further progression of fundamental study and drug development for sulfatide-associated diseases will require cooperation of many researchers belonging to various fields, including lipid-biology, glyco-biology, hematology, endocrinology, neurology, immunology, microbiology, and virology.
Footnotes
Abbreviations:
- AIDS
- acquired immunodeficiency syndrome
- ASA
- arylsulfatase A
- CGT
- UDP-galactose:ceramide galactosyltransferase
- CSF
- cerebrospinal fluid
- CST
- cerebroside sulfotransferase
- Dab2
- Disabled-2
- Dps
- DNA binding protein from starved cells
- GalCer
- galactosylceramide
- GBS
- Guillain-Barre syndrome
- HA
- hemagglutinin
- HCV
- hepatitis C virus
- HIV
- human immunodeficiency virus
- HSV-1
- herpes simplex virus-1
- IAV
- influenza A virus
- LacCer
- lactosylceramide
- LPR1
- LDL receptor-related protein 1
- MAb
- monoclonal antibody
- MAL
- myelin and lymphocyte protein
- MC
- mixed cryoglobulinemia
- MDCK
- Madin-Darby canine kidney
- MHC
- major histocompatibility complex
- MLD
- metachromatic leukodystrophy
- NA
- neuraminidase
- NKT
- natural killer T
- NOD
- nonobese diabetic
- NP
- nucleoprotein
- SapB
- saposin B
- STb
- 48-amino-acid heat-stable enterotoxin b
- UUO
- unilateral ureteral obstruction
- vRNP
- viral ribonucleoprotein
This work was supported by the Global COE Program from the Japan Society for the Promotion of Science and by MEXT/JSPS KAKENHI Grant Number (C; 23590549), the Grant from Mizutani Foundation for Glycoscience, the Grant-in-Aid from the Tokyo Biochemical Research Foundation, the Grant-in-Aid from the Hamamatsu Scientific Research Foundation, the Sasakawa Scientific Research Grant from The Japan Science Society and Adaptable and Seamless Technology Transfer Program (A-step) through Target-driven R&D, JST.
REFERENCES
- 1.Eckhardt M. 2008. The role and metabolism of sulfatide in the nervous system. Mol. Neurobiol. 37: 93–103 [DOI] [PubMed] [Google Scholar]
- 2.Bosio A., Binczek E., Le Beau M. M., Fernald A. A., Stoffel W. 1996. The human gene CGT encoding the UDP-galactose ceramide galactosyl transferase (cerebroside synthase): cloning, characterization, and assignment to human chromosome 4, band q26. Genomics. 34: 69–75 [DOI] [PubMed] [Google Scholar]
- 3.Honke K., Tsuda M., Hirahara Y., Ishii A., Makita A., Wada Y. 1997. Molecular cloning and expression of cDNA encoding human 3′-phosphoadenylylsulfate:galactosylceramide 3′-sulfotransferase. J. Biol. Chem. 272: 4864–4868 [DOI] [PubMed] [Google Scholar]
- 4.Hirahara Y., Tsuda M., Wada Y., Honke K. 2000. cDNA cloning, genomic cloning, and tissue-specific regulation of mouse cerebroside sulfotransferase. Eur. J. Biochem. 267: 1909–1917 [DOI] [PubMed] [Google Scholar]
- 5.Yaghootfam A., Sorkalla T., Häberlein H., Gieselmann V., Kappler J., Eckhardt M. 2007. Cerebroside sulfotransferase forms homodimers in living cells. Biochemistry. 46: 9260–9269 [DOI] [PubMed] [Google Scholar]
- 6.Kyogashima M., Tamiya-Koizumi K., Ehara T., Li G., Hu R., Hara A., Aoyama T., Kannagi R. 2006. Rapid demonstration of diversity of sulfatide molecular species from biological materials by MALDI-TOF MS. Glycobiology. 16: 719–728 [DOI] [PubMed] [Google Scholar]
- 7.Liu Y., Chen Y., Momin A., Shaner R., Wang E., Bowen N. J., Matyunina L. V., Walker L. D., McDonald J. F., Sullards M. C., et al. 2010. Elevation of sulfatides in ovarian cancer: an integrated transcriptomic and lipidomic analysis including tissue-imaging mass spectrometry. Mol. Cancer. 9: 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Honke K., Hirahara Y., Dupree J., Suzuki K., Popko B., Fukushima K., Fukushima J., Nagasawa T., Yoshida N., Wada Y., et al. 2002. Paranodal junction formation and spermatogenesis require sulfoglycolipids. Proc. Natl. Acad. Sci. USA. 99: 4227–4232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He H., Conrad C. A., Nilsson C. L., Ji Y., Schaub T. M., Marshall A. G., Emmett M. R. 2007. Method for lipidomic analysis: p53 expression modulation of sulfatide, ganglioside, and phospholipid composition of U87 MG glioblastoma cells. Anal. Chem. 79: 8423–8430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kolter T., Sandhoff K. 2005. Principles of lysosomal membrane digestion: stimulation of sphingolipid degradation by sphingolipid activator proteins and anionic lysosomal lipids. Annu. Rev. Cell Dev. Biol. 21: 81–103 [DOI] [PubMed] [Google Scholar]
- 11.Zeng Y., Cheng H., Jiang X., Han X. 2008. Endosomes and lysosomes play distinct roles in sulfatide-induced neuroblastoma apoptosis: potential mechanisms contributing to abnormal sulfatide metabolism in related neuronal diseases. Biochem. J. 410: 81–92 [DOI] [PubMed] [Google Scholar]
- 12.Burkart T., Caimi L., Siegrist H. P., Herschkowitz N. N., Wiesmann U. N. 1982. Vesicular transport of sulfatide in the myelinating mouse brain. Functional association with lysosomes? J. Biol. Chem. 257: 3151–3156 [PubMed] [Google Scholar]
- 13.Yoda Y., Gasa S., Makita A., Fujioka Y., Kikuchi Y., Hashimoto M. 1979. Glycolipids in human lung carcinoma of histologically different types. J. Natl. Cancer Inst. 63: 1153–1160 [PubMed] [Google Scholar]
- 14.Hattori H., Uemura K., Taketomi T. 1981. The presence of blood group A-active glycolipids in cancer tissues from blood group O patients. Biochim. Biophys. Acta. 666: 361–369 [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi T., Honke K., Kamio K., Sakakibara N., Gasa S., Miyao N., Tsukamoto T., Ishizuka I., Miyazaki T., Makita A. 1993. Sulfolipids and glycolipid sulfotransferase activities in human renal cell carcinoma cells. Br. J. Cancer. 67: 76–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi T., Honke K., Gasa S., Sugiura M., Miyazaki T., Ishizuka I., Makita A. 1993. Involvement of protein kinase C in the regulation of glycolipid sulfotransferase activity levels in renal cell carcinoma cells. Cancer Res. 53: 2484–2489 [PubMed] [Google Scholar]
- 17.Kobayashi T., Honke K., Gasa S., Imai S., Tanaka J., Miyazaki T., Makita A. 1993. Regulation of activity levels of glycolipid sulfotransferases by transforming growth factor alpha in renal cell carcinoma cells. Cancer Res. 53: 5638–5642 [PubMed] [Google Scholar]
- 18.Sakakibara N., Gasa S., Kamio K., Makita A., Koyanagi T. 1989. Association of elevated sulfatides and sulfotransferase activities with human renal cell carcinoma. Cancer Res. 49: 335–339 [PubMed] [Google Scholar]
- 19.Morichika H., Hamanaka Y., Tai T., Ishizuka I. 1996. Sulfatides as a predictive factor of lymph node metastasis in patients with colorectal adenocarcinoma. Cancer. 78: 43–47 [DOI] [PubMed] [Google Scholar]
- 20.Makhlouf A. M., Fathalla M. M., Zakhary M. A., Makarem M. H. 2004. Sulfatides in ovarian tumors: clinicopathological correlates. Int. J. Gynecol. Cancer. 14: 89–93 [DOI] [PubMed] [Google Scholar]
- 21.Sevin C., Aubourg P., Cartier N. 2007. Enzyme, cell and gene-based therapies for metachromatic leukodystrophy. J. Inherit. Metab. Dis. 30: 175–183 [DOI] [PubMed] [Google Scholar]
- 22.Rafi M. A., Amini S., Zhang X. L., Wenger D. A. 1992. Correction of sulfatide metabolism after transfer of prosaposin cDNA to cultured cells from a patient with SAP-1 deficiency. Am. J. Hum. Genet. 50: 1252–1258 [PMC free article] [PubMed] [Google Scholar]
- 23.Ishizuka I. 1997. Chemistry and functional distribution of sulfoglycolipids. Prog. Lipid Res. 36: 245–319 [DOI] [PubMed] [Google Scholar]
- 24.Hirahara Y., Bansal R., Honke K., Ikenaka K., Wada Y. 2004. Sulfatide is a negative regulator of oligodendrocyte differentiation: development in sulfatide-null mice. Glia. 45: 269–277 [DOI] [PubMed] [Google Scholar]
- 25.Shroff S. M., Pomicter A. D., Chow W. N., Fox M. A., Colello R. J., Henderson S. C., Dupree J. L. 2009. Adult CST-null mice maintain an increased number of oligodendrocytes. J. Neurosci. Res. 87: 3403–3414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuki D., Sugiura Y., Zaima N., Akatsu H., Hashizume Y., Yamamoto T., Fujiwara M., Sugiyama K., Setou M. 2011. Hydroxylated and non-hydroxylated sulfatide are distinctly distributed in the human cerebral cortex. Neuroscience. 193: 44–53 [DOI] [PubMed] [Google Scholar]
- 27.Pesheva P., Gloor S., Schachner M., Probstmeier R. 1997. Tenascin-R is an intrinsic autocrine factor for oligodendrocyte differentiation and promotes cell adhesion by a sulfatide-mediated mechanism. J. Neurosci. 17: 4642–4651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts D. D., Rao C. N., Magnani J. L., Spitalnik S. L., Liotta L. A., Ginsburg V. 1985. Laminin binds specifically to sulfated glycolipids. Proc. Natl. Acad. Sci. USA. 82: 1306–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li S., Liquari P., McKee K. K., Harrison D., Patel R., Lee S., Yurchenco P. D. 2005. Laminin-sulfatide binding initiates basement membrane assembly and enables receptor signaling in Schwann cells and fibroblasts. J. Cell Biol. 169: 179–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishibashi T., Dupree J. L., Ikenaka K., Hirahara Y., Honke K., Peles E., Popko B., Suzuki K., Nishino H., Baba H. 2002. A myelin galactolipid, sulfatide, is essential for maintenance of ion channels on myelinated axon but not essential for initial cluster formation. J. Neurosci. 22: 6507–6514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Honke K., Zhang Y., Cheng X., Kotani N., Taniguchi N. 2004. Biological roles of sulfoglycolipids and pathophysiology of their deficiency. Glycoconj. J. 21: 59–62 [DOI] [PubMed] [Google Scholar]
- 32.Winzeler A. M., Mandemakers W. J., Sun M. Z., Stafford M., Phillips C. T., Barres B. A. 2011. The lipid sulfatide is a novel myelin-associated inhibitor of CNS axon outgrowth. J. Neurosci. 31: 6481–6492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frank M., van der Haar M. E., Schaeren-Wiemers N., Schwab M. E. 1998. rMAL is a glycosphingolipid-associated protein of myelin and apical membranes of epithelial cells in kidney and stomach. J. Neurosci. 18: 4901–4913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim T., Fiedler K., Madison D. L., Krueger W. H., Pfeiffer S. E. 1995. Cloning and characterization of MVP17: a developmentally regulated myelin protein in oligodendrocytes. J. Neurosci. Res. 42: 413–422 [DOI] [PubMed] [Google Scholar]
- 35.Coetzee T., Suzuki K., Popko B. 1998. New perspectives on the function of myelin galactolipids. Trends Neurosci. 21: 126–130 [DOI] [PubMed] [Google Scholar]
- 36.Marcus J., Honigbaum S., Shroff S., Honke K., Rosenbluth J., Dupree J. L. 2006. Sulfatide is essential for the maintenance of CNS myelin and axon structure. Glia. 53: 372–381 [DOI] [PubMed] [Google Scholar]
- 37.Berntson Z., Hansson E., Rönnbäck L., Fredman P. 1998. Intracellular sulfatide expression in a subpopulation of astrocytes in primary cultures. J. Neurosci. Res. 52: 559–568 [DOI] [PubMed] [Google Scholar]
- 38.Pernber Z., Molander-Melin M., Berthold C. H., Hansson E., Fredman P. 2002. Expression of the myelin and oligodendrocyte progenitor marker sulfatide in neurons and astrocytes of adult rat brain. J. Neurosci. Res. 69: 86–93 [DOI] [PubMed] [Google Scholar]
- 39.Han X., Cheng H., Fryer J. D., Fagan A. M., Holtzman D. M. 2003. Novel role for apolipoprotein E in the central nervous system. Modulation of sulfatide content. J. Biol. Chem. 278: 8043–8051 [DOI] [PubMed] [Google Scholar]
- 40.Isaac G., Pernber Z., Gieselmann V., Hansson E., Bergquist J., Månsson J. E. 2006. Sulfatide with short fatty acid dominates in astrocytes and neurons. FEBS J. 273: 1782–1790 [DOI] [PubMed] [Google Scholar]
- 41.Schaeren-Wiemers N., van der Bijl P., Schwab M. E. 1995. The UDP-galactose:ceramide galactosyltransferase: expression pattern in oligodendrocytes and Schwann cells during myelination and substrate preference for hydroxyceramide. J. Neurochem. 65: 2267–2278 [DOI] [PubMed] [Google Scholar]
- 42.Eckhardt M., Hedayati K. K., Pitsch J., Lüllmann-Rauch R., Beck H., Fewou S. N., Gieselmann V. 2007. Sulfatide storage in neurons causes hyperexcitability and axonal degeneration in a mouse model of metachromatic leukodystrophy. J. Neurosci. 27: 9009–9021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han X. 2007. Potential mechanisms contributing to sulfatide depletion at the earliest clinically recognizable stage of Alzheimer's disease: a tale of shotgun lipidomics. J. Neurochem. 103 (Suppl.): 171–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Zyl R., Gieselmann V., Eckhardt M. 2010. Elevated sulfatide levels in neurons cause lethal audiogenic seizures in mice. J. Neurochem. 112: 282–295 [DOI] [PubMed] [Google Scholar]
- 45.Sabourdy F., Kedjouar B., Sorli S. C., Colié S., Milhas D., Salma Y., Levade T. 2008. Functions of sphingolipid metabolism in mammals—lessons from genetic defects. Biochim. Biophys. Acta. 1781: 145–183 [DOI] [PubMed] [Google Scholar]
- 46.Cui Y., Colsch B., Afonso C., Baumann N., Tabet J. C., Mallet J. M., Zhang Y. 2008. Synthetic sulfogalactosylceramide (sulfatide) and its use for the mass spectrometric quantitative urinary determination in metachromatic leukodystrophies. Glycoconj. J. 25: 147–155 [DOI] [PubMed] [Google Scholar]
- 47.Colsch B., Afonso C., Turpin J. C., Portoukalian J., Tabet J. C., Baumann N. 2008. Sulfogalactosylceramides in motor and psycho-cognitive adult metachromatic leukodystrophy: relations between clinical, biochemical analysis and molecular aspects. Biochim. Biophys. Acta. 1780: 434–440 [DOI] [PubMed] [Google Scholar]
- 48.Cheng H., Xu J., McKeel D. W., Han X. 2003. Specificity and potential mechanism of sulfatide deficiency in Alzheimer's disease: an electrospray ionization mass spectrometric study. Cell Mol. Biol. (Noisy-le-grand). 49: 809–818 [PubMed] [Google Scholar]
- 49.Fabelo N., Martín V., Santpere G., Marín R., Torrent L., Ferrer I., Díaz M. 2011. Severe alterations in lipid composition of frontal cortex lipid rafts from Parkinson's disease and incidental Parkinson's disease. Mol. Med. 17: 1107–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han X., Holtzman D. M., McKeel D. W., Kelley J., Morris J. C. 2002. Substantial sulfatide deficiency and ceramide elevation in very early Alzheimer's disease: potential role in disease pathogenesis. J. Neurochem. 82: 809–818 [DOI] [PubMed] [Google Scholar]
- 51.Liu Q., Trotter J., Zhang J., Peters M. M., Cheng H., Bao J., Han X., Weeber E. J., Bu G. 2010. Neuronal LRP1 knockout in adult mice leads to impaired brain lipid metabolism and progressive, age-dependent synapse loss and neurodegeneration. J. Neurosci. 30: 17068–17078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strittmatter W. J., Roses A. D. 1996. Apolipoprotein E and Alzheimer's disease. Annu. Rev. Neurosci. 19: 53–77 [DOI] [PubMed] [Google Scholar]
- 53.Hill J. M., Steiner I., Matthews K. E., Trahan S. G., Foster T. P., Ball M. J. 2005. Statins lower the risk of developing Alzheimer's disease by limiting lipid raft endocytosis and decreasing the neuronal spread of Herpes simplex virus type 1. Med. Hypotheses. 64: 53–58 [DOI] [PubMed] [Google Scholar]
- 54.Carter C. J. 2008. Interactions between the products of the Herpes simplex genome and Alzheimer's disease susceptibility genes: relevance to pathological-signalling cascades. Neurochem. Int. 52: 920–934 [DOI] [PubMed] [Google Scholar]
- 55.Tadano-Aritomi K., Hikita T., Fujimoto H., Suzuki K., Motegi K., Ishizuka I. 2000. Kidney lipids in galactosylceramide synthase-deficient mice. Absence of galactosylsulfatide and compensatory increase in more polar sulfoglycolipids. J. Lipid Res. 41: 1237–1243 [PubMed] [Google Scholar]
- 56.Li G., Hu R., Kamijo Y., Nakajima T., Aoyama T., Ehara T., Shigematsu H., Kannagi R., Kyogashima M., Hara A. 2009. Kidney dysfunction induced by protein overload nephropathy reduces serum sulfatide levels in mice. Nephrology (Carlton). 14: 658–662 [DOI] [PubMed] [Google Scholar]
- 57.Zhang X., Nakajima T., Kamijo Y., Li G., Hu R., Kannagi R., Kyogashima M., Aoyama T., Hara A. 2009. Acute kidney injury induced by protein-overload nephropathy down-regulates gene expression of hepatic cerebroside sulfotransferase in mice, resulting in reduction of liver and serum sulfatides. Biochem. Biophys. Res. Commun. 390: 1382–1388 [DOI] [PubMed] [Google Scholar]
- 58.Wang L., Kamijo Y., Matsumoto A., Nakajima T., Higuchi M., Kannagi R., Kyogashima M., Aoyama T., Hara A. 2011. Kidney transplantation recovers the reduction level of serum sulfatide in ESRD patients via processes correlated to oxidative stress and platelet count. Glycoconj. J. 28: 125–135 [DOI] [PubMed] [Google Scholar]
- 59.Shikata K., Suzuki Y., Wada J., Hirata K., Matsuda M., Kawashima H., Suzuki T., Iizuka M., Makino H., Miyasaka M. 1999. L-selectin and its ligands mediate infiltration of mononuclear cells into kidney interstitium after ureteric obstruction. J. Pathol. 188: 93–99 [DOI] [PubMed] [Google Scholar]
- 60.Ogawa D., Shikata K., Honke K., Sato S., Matsuda M., Nagase R., Tone A., Okada S., Usui H., Wada J., et al. 2004. Cerebroside sulfotransferase deficiency ameliorates L-selectin-dependent monocyte infiltration in the kidney after ureteral obstruction. J. Biol. Chem. 279: 2085–2090 [DOI] [PubMed] [Google Scholar]
- 61.Lande M. B., Priver N. A., Zeidel M. L. 1994. Determinants of apical membrane permeabilities of barrier epithelia. Am. J. Physiol. 267: C367–C374 [DOI] [PubMed] [Google Scholar]
- 62.Trick D., Decker J., Groene H. J., Schulze M., Wiegandt H. 1999. Regional expression of sulfatides in rat kidney: immunohistochemical staining by use of monospecific polyclonal antibodies. Histochem. Cell Biol. 111: 143–151 [DOI] [PubMed] [Google Scholar]
- 63.Fredman P., Mânsson J. E., Rynmark B. M., Josefsen K., Ekblond A., Halldner L., Osterbye T., Horn T., Buschard K. 2000. The glycosphingolipid sulfatide in the islets of Langerhans in rat pancreas is processed through recycling: possible involvement in insulin trafficking. Glycobiology. 10: 39–50 [DOI] [PubMed] [Google Scholar]
- 64.Buschard K., Blomqvist M., Månsson J. E., Fredman P., Juhl L., Gromada J. 2006. C16:0 sulfatide inhibits insulin secretion in rat β-cells by reducing the sensitivity of KATP channels to ATP inhibition. Diabetes. 55: 2826–2834 [DOI] [PubMed] [Google Scholar]
- 65.Blomqvist M., Osterbye T., Månsson J. E., Horn T., Buschard K., Fredman P. 2003. Selective lack of the C16:0 fatty acid isoform of sulfatide in pancreas of type II diabetic animal models. APMIS. 111: 867–877 [DOI] [PubMed] [Google Scholar]
- 66.Buschard K., Høy M., Bokvist K, Olsen H. L., Madsbad S., Fredman P., Gromada J. 2002. Sulfatide controls insulin secretion by modulation of ATP-sensitive K(+)-channel activity and Ca(2+)-dependent exocytosis in rat pancreatic beta-cells. Diabetes. 51: 2514–2521 [DOI] [PubMed] [Google Scholar]
- 67.Osterbye T., Jorgensen K. H., Fredman P., Tranum-Jensen J., Kaas A., Brange J., Whittingham J. L., Buschard K. 2001. Sulfatide promotes the folding of proinsulin, preserves insulin crystals, and mediates its monomerization. Glycobiology. 11: 473–479 [DOI] [PubMed] [Google Scholar]
- 68.Andersson K., Buschard K., Fredman P., Kaas A., Lidström A. M., Madsbad S., Mortensen H., Jan-Eric M. 2002. Patients with insulin-dependent diabetes but not those with non-insulin-dependent diabetes have anti-sulfatide antibodies as determined with a new ELISA assay. Autoimmunity. 35: 463–468 [DOI] [PubMed] [Google Scholar]
- 69.Buschard K., Hanspers K., Fredman P., Reich E. P. 2001. Treatment with sulfatide or its precursor, galactosylceramide, prevents diabetes in NOD mice. Autoimmunity. 34: 9–17 [DOI] [PubMed] [Google Scholar]
- 70.Buschard K., Diamant M., Bovin L. E., Mansson J. E., Fredman P., Bendtzen K. 1996. Sulphatide and its precursor galactosylceramide influence the production of cytokines in human mononuclear cells. APMIS. 104: 938–944 [DOI] [PubMed] [Google Scholar]
- 71.Bovin L. F., Fredman P., Månsson J. E., Buschard K., Bendtzen K. 1999. In vitro production of cytokines is influenced by sulfatide and its precursor galactosylceramide. FEBS Lett. 455: 339–343 [DOI] [PubMed] [Google Scholar]
- 72.Roeske-Nielsen A., Fredman P., Mansson J. E., Bendtzen K., Buschard K. 2004. Beta-galactosylceramide increases and sulfatide decreases cytokine and chemokine production in whole blood cells. Immunol. Lett. 91: 205–211 [DOI] [PubMed] [Google Scholar]
- 73.Suzuki Y., Toda Y., Tamatani T., Watanabe T., Suzuki T., Nakao T., Murase K., Kiso M., Hasegawa A., Tadano-Aritomi K., et al. 1993. Sulfated glycolipids are ligands for a lymphocyte homing receptor, L-selectin (LECAM-1), binding epitope in sulfated sugar chain. Biochem. Biophys. Res. Commun. 190: 426–434 [DOI] [PubMed] [Google Scholar]
- 74.Yang X. D., Karin N., Tisch R., Steinman L., McDevitt H. O. 1993. Inhibition of insulitis and prevention of diabetes in nonobese diabetic mice by blocking L-selectin and very late antigen 4 adhesion receptors. Proc. Natl. Acad. Sci. USA. 90: 10494–10498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roeske-Nielsen A., Dalgaard L. T., Månsson J. E., Buschard K. 2010. The glycolipid sulfatide protects insulin-producing cells against cytokine-induced apoptosis, a possible role in diabetes. Diabetes Metab. Res. Rev. 26: 631–638 [DOI] [PubMed] [Google Scholar]
- 76.Miyazaki Y., Pipek R., Mandarino L. J., DeFronzo R. A. 2003. Tumor necrosis factor alpha and insulin resistance in obese type 2 diabetic patients. Int. J. Obes. Relat. Metab. Disord. 27: 88–94 [DOI] [PubMed] [Google Scholar]
- 77.Buschard K., Fredman P., Bog-Hansen E., Blomqvist M., Hedner J., Rastam L., Lindblad U. 2005. Low serum concentration of sulfatide and presence of sulfated lactosylceramid are associated with Type 2 diabetes. The Skaraborg Project. Diabet. Med. 22: 1190–1198 [DOI] [PubMed] [Google Scholar]
- 78.Roeske-Nielsen A., Buschard K., Manson J. E., Rastam L., Lindblad U. 2009. A variation in the cerebroside sulfotransferase gene is linked to exercise-modified insulin resistance and to type 2 diabetes. Exp. Diabetes Res. 2009: 429593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shamshiev A., Gober H. J., Donda A., Mazorra Z., Mori L., De Libero G. 2002. Presentation of the same glycolipid by different CD1 molecules. J. Exp. Med. 195: 1013–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cernadas M., Cavallari M., Watts G., Mori L., De Libero G., Brenner M. B. 2010. Early recycling compartment trafficking of CD1a is essential for its intersection and presentation of lipid antigens. J. Immunol. 184: 1235–1241 [DOI] [PubMed] [Google Scholar]
- 81.Blomqvist M., Rhost S., Teneberg S., Löfbom L., Osterbye T., Brigl M., Månsson J. E., Cardell S. L. 2009. Multiple tissue-specific isoforms of sulfatide activate CD1d-restricted type II NKT cells. Eur. J. Immunol. 39: 1726–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Arrenberg P., Halder R., Dai Y., Maricic I., Kumar V. 2010. Oligoclonality and innate-like features in the TCR repertoire of type II NKT cells reactive to a beta-linked self-glycolipid. Proc. Natl. Acad. Sci. USA. 107: 10984–10989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Arrenberg P., Halder R., Kumar V. 2009. Cross-regulation between distinct natural killer T cell subsets influences immune response to self and foreign antigens. J. Cell. Physiol. 218: 246–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Halder R. C., Jahng A., Maricic I., Kumar V. 2007. Mini review: immune response to myelin-derived sulfatide and CNS-demyelination. Neurochem. Res. 32: 257–262 [DOI] [PubMed] [Google Scholar]
- 85.Arrenberg P., Maricic I., Kumar V. 2011. Sulfatide-mediated activation of type II natural killer T cells prevents hepatic ischemic reperfusion injury in mice. Gastroenterology. 140: 646–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang S. H., Lee J. P., Jang H. R., Cha R. H., Han S. S., Jeon U. S., Kim D. K., Song J., Lee D. S., Kim Y. S. 2011. Sulfatide-reactive natural killer T cells abrogate ischemia-reperfusion injury. J. Am. Soc. Nephrol. 22: 1305–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tam F. W. 2002. Role of selectins in glomerulonephritis. Clin. Exp. Immunol. 129: 1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Garcia J., Callewaert N., Borsig L. 2007. P-selectin mediates metastatic progression through binding to sulfatides on tumor cells. Glycobiology. 17: 185–196 [DOI] [PubMed] [Google Scholar]
- 89.Simonis D., Schlesinger M., Seelandt C., Borsig L., Bendas G. 2010. Analysis of SM4 sulfatide as a P-selectin ligand using model membranes. Biophys. Chem. 150: 98–104 [DOI] [PubMed] [Google Scholar]
- 90.Duchesneau P., Gallagher E., Walcheck B., Waddell T. K. 2007. Up-regulation of leukocyte CXCR4 expression by sulfatide: an L-selectin-dependent pathway on CD4+ T cells. Eur. J. Immunol. 37: 2949–2960 [DOI] [PubMed] [Google Scholar]
- 91.Sandhoff R., Grieshaber H., Djafarzadeh R., Sijmonsma T. P., Proudfoot A. E., Handel T. M., Wiegandt H., Nelson P. J., Gröne H. J. 2005. Chemokines bind to sulfatides as revealed by surface plasmon resonance. Biochim. Biophys. Acta. 1687: 52–63 [DOI] [PubMed] [Google Scholar]
- 92.Popovic Z. V., Sandhoff R., Sijmonsma T. P., Kaden S., Jennemann R., Kiss E., Tone E., Autschbach F., Platt N., Malle E., et al. 2007. Sulfated glycosphingolipid as mediator of phagocytosis: SM4s enhances apoptotic cell clearance and modulates macrophage activity. J. Immunol. 179: 6770–6782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Altomare E., Fallarini S., Battaglini C. O., Mossotti M., Panza L., Lombardi G. 2011. Synthetic isoforms of endogenous sulfatides differently modulate indoleamine 2,3-dioxygenase in antigen presenting cells. Life Sci. 89: 176–181 [DOI] [PubMed] [Google Scholar]
- 94.Kyogashima M., Sakai T., Onaya J., Hara A. 2001. Roles of galactose and sulfate residues in sulfatides for their antagonistic functions in the blood coagulation system. Glycoconj. J. 18: 245–251 [DOI] [PubMed] [Google Scholar]
- 95.Ida M., Satoh A., Matsumoto I., Kojima-Aikawa K. 2004. Human annexin V binds to sulfatide: contribution to regulation of blood coagulation. J. Biochem. 135: 583–588 [DOI] [PubMed] [Google Scholar]
- 96.Merten M., Thiagarajan P. 2001. Role for sulfatides in platelet aggregation. Circulation. 104: 2955–2960 [DOI] [PubMed] [Google Scholar]
- 97.Merten M., Thiagarajan P. 2004. P-selectin in arterial thrombosis. Z. Kardiol. 93: 855–863 [DOI] [PubMed] [Google Scholar]
- 98.Hu R., Li G., Kamijo Y., Aoyama T., Nakajima T., Inoue T., Node K., Kannagi R., Kyogashima M., Hara A. 2007. Serum sulfatides as a novel biomarker for cardiovascular disease in patients with end-stage renal failure. Glycoconj. J. 24: 565–571 [DOI] [PubMed] [Google Scholar]
- 99.Drahos K. E., Welsh J. D., Finkielstein C. V., Capelluto D. G. 2009. Sulfatides partition disabled-2 in response to platelet activation. PLoS ONE. 4: e8007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Welsh J. D., Charonko J. J., Salmanzadeh A., Drahos K. E., Shafiee H., Stremler M. A., Davalos R. V., Capelluto D. G., Vlachos P. P., Finkielstein C. V. 2011. Disabled-2 modulates homotypic and heterotypic platelet interactions by binding to sulfatides. Br. J. Haematol. 154: 122–133 [DOI] [PubMed] [Google Scholar]
- 101.Osawa H., Sugano K., Igari T., Tai T., Iwamori M., Kawakami M. 1997. Immunohistochemical study of sulfatide expression in gastric carcinoma: alteration of sulfatide expression. J. Clin. Gastroenterol. 25 (Suppl.): 135–140 [DOI] [PubMed] [Google Scholar]
- 102.Kobayashi T., Honke K., Tsunematsu I., Kagaya H., Nishikawa S., Hokari K., Kato M., Takeda H., Sugiyama T., Higuchi A., et al. 1999. Detection of cerebroside sulfotransferase mRNA in human gastric mucosa and adenocarcinoma. Cancer Lett. 138: 45–51 [DOI] [PubMed] [Google Scholar]
- 103.Gasa S., Casl M. T., Jin T., Kamio K., Uehara Y., Miyazaki T., Makita A. 1991. Elevated serum level of glycolipid sulfotransferase in patients with hepatocellular carcinoma. Cancer Lett. 59: 19–24 [DOI] [PubMed] [Google Scholar]
- 104.Kamio K., Jin T., Gasa S., Ohhira M., Honke K., Kasai N., Makita A. 1992. Inconsistent expression of glycolipid sulfotransferase activity between hepatoma and serum. Tohoku J. Exp. Med. 168: 29–35 [DOI] [PubMed] [Google Scholar]
- 105.Honke K., Tsuda M., Hirahara Y., Miyao N., Tsukamoto T., Satoh M., Wada Y. 1998. Cancer-associated expression of glycolipid sulfotransferase gene in human renal cell carcinoma cells. Cancer Res. 58: 3800–3805 [PubMed] [Google Scholar]
- 106.Sakakibara N., Gasa S., Kamio K., Makita A., Nonomura K., Togashi M., Koyanagi T., Hatae Y., Takeda K. 1991. Distinctive glycolipid patterns in Wilms’ tumor and renal cell carcinoma. Cancer Lett. 57: 187–192 [DOI] [PubMed] [Google Scholar]
- 107.Balbaa M., Honke K., Makita A. 1996. Regulation of glycolipid sulfotransferase by tyrosine kinases in human renal cancer cells. Biochim. Biophys. Acta. 1299: 141–145 [DOI] [PubMed] [Google Scholar]
- 108.Yabunaka N., Honke K., Ishii A., Ogiso Y., Kuzumaki N., Agishi Y., Makita A. 1997. Involvement of Ras in the expression of glycolipid sulfotransferase in human renal cancer cells. Int. J. Cancer. 71: 620–623 [DOI] [PubMed] [Google Scholar]
- 109.Kobayashi T., Honke K., Gasa S., Kato N., Miyazaki T., Makita A. 1993. Epidermal growth factor elevates the activity levels of glycolipid sulfotransferases in renal-cell-carcinoma cells. Int. J. Cancer. 55: 448–452 [DOI] [PubMed] [Google Scholar]
- 110.Kobayashi T., Honke K., Gasa S., Miyazaki T., Tajima H., Matsumoto K., Nakamura T., Makita A. 1994. Hepatocyte growth factor elevates the activity levels of glycolipid sulfotransferases in renal cell carcinoma cells. Eur. J. Biochem. 219: 407–413 [DOI] [PubMed] [Google Scholar]
- 111.Abul-Milh M., Paradis S. E., Dubreuil J. D., Jacques M. 1999. Binding of Actinobacillus pleuropneumoniae lipopolysaccharides to glycosphingolipids evaluated by thin-layer chromatography. Infect. Immun. 67: 4983–4987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brennan M. J., Hannah J. H., Leininger E. 1991. Adhesion of Bordetella pertussis to sulfatides and to the GalNAc beta 4Gal sequence found in glycosphingolipids. J. Biol. Chem. 266: 18827–18831 [PubMed] [Google Scholar]
- 113.Piao H., Minohara M., Kawamura N., Li W., Mizunoe Y., Umehara F., Goto Y., Kusunoki S., Matsushita T., Ikenaka K., et al. 2010. Induction of paranodal myelin detachment and sodium channel loss in vivo by Campylobacter jejuni DNA-binding protein from starved cells (C-Dps) in myelinated nerve fibers. J. Neurol. Sci. 288: 54–62 [DOI] [PubMed] [Google Scholar]
- 114.Piao H., Minohara M., Kawamura N., Li W., Matsushita T., Yamasaki R., Mizunoe Y., Kira J. 2011. Tissue binding patterns and in vitro effects of Campylobacter jejuni DNA-binding protein from starved cells. Neurochem. Res. 36: 58–66 [DOI] [PubMed] [Google Scholar]
- 115.Zhu G., Chen H., Choi B. K., Del Piero F., Schifferli D. M. 2005. Histone H1 proteins act as receptors for the 987P fimbriae of enterotoxigenic Escherichia coli. J. Biol. Chem. 280: 23057–23065 [DOI] [PubMed] [Google Scholar]
- 116.Jansson L., Tobias J., Jarefjäll C., Lebens M., Svennerholm A. M., Teneberg S. 2009. Sulfatide recognition by colonization factor antigen CS6 from enterotoxigenic Escherichia coli. PLoS ONE. 4: e4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pantzar M., Teneberg S., Lagergård T. 2006. Binding of Haemophilus ducreyi to carbohydrate receptors is mediated by the 58.5-kDa GroEL heat shock protein. Microbes Infect. 8: 2452–2458 [DOI] [PubMed] [Google Scholar]
- 118.Hartmann E., Lingwood C. A., Reidl J. 2001. Heat-inducible surface stress protein (Hsp70) mediates sulfatide recognition of the respiratory pathogen Haemophilus influenzae. Infect. Immun. 69: 3438–3441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kamisago S., Iwamori M., Tai T., Mitamura K., Yazaki Y., Sugano K. 1996. Role of sulfatides in adhesion of Helicobacter pylori to gastric cancer cells. Infect. Immun. 64: 624–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mukai T., Asasaka T., Sato E., Mori K., Matsumoto M., Ohori H. 2002. Inhibition of binding of Helicobacter pylori to the glycolipid receptors by probiotic Lactobacillus reuteri. FEMS Immunol. Med. Microbiol. 32: 105–110 [DOI] [PubMed] [Google Scholar]
- 121.Ozcelik P., Bezirci F. B., Suzuki Y., Uzawa H., Nishida Y., Kobayashi K., Suzuki T., Miyamoto D., Nagatake T., Ahmed K. 2006. Sulfatide and its synthetic analogues recognition by Moraxella catarrhalis. Microbiol. Immunol. 50: 967–970 [DOI] [PubMed] [Google Scholar]
- 122.Olson L. D., Gilbert A. A. 1993. Characteristics of Mycoplasma hominis adhesion. J. Bacteriol. 175: 3224–3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang Q., Young T. F., Ross R. F. 1994. Glycolipid receptors for attachment of Mycoplasma hyopneumoniae to porcine respiratory ciliated cells. Infect. Immun. 62: 4367–4373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Krivan H. C., Olson L. D., Barile M. F., Ginsburg V., Roberts D. D. 1989. Adhesion of Mycoplasma pneumoniae to sulfated glycolipids and inhibition by dextran sulfate. J. Biol. Chem. 264: 9283–9288 [PubMed] [Google Scholar]
- 125.Yagci A., Yagci T., Sener B., Suziki Y., Ahmed K. 2007. Sulfatide mediates attachment of Pseudomonas aeruginosa to human pharyngeal epithelial cells. New Microbiol. 30: 167–171 [PubMed] [Google Scholar]
- 126.Kaneda K., Masuzawa T., Yasugami K., Suzuki T., Suzuki Y., Yanagihara Y. 1997. Glycosphingolipid-binding protein of Borrelia burgdorferi sensu lato. Infect. Immun. 65: 3180–3185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mamelak D., Mylvaganam M., Whetstone H., Hartmann E., Lennarz W., Wyrick P. B., Raulston J., Han H., Hoffman P., Lingwood C. A. 2001. Hsp70s contain a specific sulfogalactolipid binding site. Differential aglycone influence on sulfogalactosyl ceramide binding by recombinant prokaryotic and eukaryotic hsp70 family members. Biochemistry. 40: 3572–3582 [DOI] [PubMed] [Google Scholar]
- 128.Xia B., Sachdev G. P., Cummings R. D. 2007. Pseudomonas aeruginosa mucoid strain 8830 binds glycans containing the sialyl-Lewis x epitope. Glycoconj. J. 24: 87–95 [DOI] [PubMed] [Google Scholar]
- 129.Blomqvist M., Osterbye T., Månsson J. E., Buschard K., Fredman P. 2006. Uptake of the glycosphingolipid sulfatide in the gastrointestinal tract and pancreas in vivo and in isolated islets of Langerhans. Lipids Health Dis. 5: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Huesca M., Borgia S., Hoffman P., Lingwood C. A. 1996. Acidic pH changes receptor binding specificity of Helicobacter pylori: a binary adhesion model in which surface heat shock (stress) proteins mediate sulfatide recognition in gastric colonization. Infect. Immun. 64: 2643–2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Takamatsu K., Kamei K., Kubushiro K., Kiguchi K., Nozawa S., Iwamori M. 1993. Luteal phase-characteristic induction of I3SO3-GalCer in human cervical epithelia and uterine endometria, and follicular phase-characteristic formation of a ganglioside-derived negative charge gradient in different regions of fallopian tubes. Biochim. Biophys. Acta. 1170: 232–236 [DOI] [PubMed] [Google Scholar]
- 132.Pancake S. J., Holt G. D., Mellouk S., Hoffman S. L. 1992. Malaria sporozoites and circumsporozoite proteins bind specifically to sulfated glycoconjugates. J. Cell Biol. 117: 1351–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Toda G., Ikeda Y., Kashiwagi M., Iwamori M., Oka H. 1990. Hepatocyte plasma membrane glycosphingolipid reactive with sera from patients with autoimmune chronic active hepatitis: its identification as sulfatide. Hepatology. 12: 664–670 [DOI] [PubMed] [Google Scholar]
- 134.Rousset E., Harel J., Dubreuil J. D. 1998. Sulfatide from the pig jejunum brush border epithelial cell surface is involved in binding of Escherichia coli enterotoxin b. Infect. Immun. 66: 5650–5658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Beausoleil H. E., Dubreuil J. D. 2001. In vitro binding characteristics and affinity for sulfatide of Escherichia coli STb enterotoxin. Receptors Channels. 7: 401–411 [PubMed] [Google Scholar]
- 136.Bianco I. D., Fidelio G. D., Maggio B. 1990. Effect of sulfatide and gangliosides on phospholipase C and phospholipase A2 activity. A monolayer study. Biochim. Biophys. Acta. 1026: 179–185 [DOI] [PubMed] [Google Scholar]
- 137.Rivera-Marrero C. A., Ritzenthaler J. D., Newburn S. A., Roman J., Cummings R. D. 2002. Molecular cloning and expression of a novel glycolipid sulfotransferase in Mycobacterium tuberculosis. Microbiology. 148: 783–792 [DOI] [PubMed] [Google Scholar]
- 138.Miyazaki Y., Oka S., Yamaguchi S., Mizuno S., Yano I. 1995. Stimulation of phagocytosis and phagosome-lysosome fusion by glycosphingolipids from Sphingomonas paucimobilis. J. Biochem. 118: 271–277 [DOI] [PubMed] [Google Scholar]
- 139.Delézay O., Hammache D., Fantini J., Yahi N. 1996. SPC3, a V3 loop-derived synthetic peptide inhibitor of HIV-1 infection, binds to cell surface glycosphingolipids. Biochemistry. 35: 15663–15671 [DOI] [PubMed] [Google Scholar]
- 140.Cook D. G., Fantini J., Spitalnik S. L., Gonzalez-Scarano F. 1994. Binding of human immunodeficiency virus type I (HIV-1) gp120 to galactosylceramide (GalCer): relationship to the V3 loop. Virology. 201: 206–214 [DOI] [PubMed] [Google Scholar]
- 141.Laudanna C., Constantin G., Baron P., Scarpini E., Scarlato G., Cabrini G., Dechecchi C., Rossi F., Cassatella M. A., Berton G. 1994. Sulfatides trigger increase of cytosolic free calcium and enhanced expression of tumor necrosis factor-alpha and interleukin-8 mRNA in human neutrophils. Evidence for a role of L-selectin as a signaling molecule. J. Biol. Chem. 269: 4021–4026 [PubMed] [Google Scholar]
- 142.Schneider-Schaulies J., Schneider-Schaulies S., Brinkmann R., Tas P., Halbrugge M., Walter U., Holmes H. C., Ter Meulen V. 1992. HIV-1 gp120 receptor on CD4-negative brain cells activates a tyrosine kinase. Virology. 191: 765–772 [DOI] [PubMed] [Google Scholar]
- 143.Seddiki N., Ramdani A., Saffar L., Portoukalian J., Gluckman J. C., Gattegno L. 1994. A monoclonal antibody directed to sulfatide inhibits the binding of human immunodeficiency virus type 1 (HIV-1) envelope glycoprotein to macrophages but not their infection by the virus. Biochim. Biophys. Acta. 1225: 289–296 [DOI] [PubMed] [Google Scholar]
- 144.Delézay O., Koch N., Yahi N., Hammache D., Tourres C., Tamalet C., Fantini J. 1997. Co-expression of CXCR4/fusin and galactosylceramide in the human intestinal epithelial cell line HT-29. AIDS. 11: 1311–1318 [DOI] [PubMed] [Google Scholar]
- 145.Harouse J. M., Bhat S., Spitalnik S. L., Laughlin M., Stefano K., Silberberg D. H., Gonzalez-Scarano F. 1991. Inhibition of entry of HIV-1 in neural cell lines by antibodies against galactosyl ceramide. Science. 253: 320–323 [DOI] [PubMed] [Google Scholar]
- 146.Kensinger R. D., Catalone B. J., Krebs F. C., Wigdahl B., Schengrund C. L. 2004. Novel polysulfated galactose-derivatized dendrimers as binding antagonists of human immunodeficiency virus type 1 infection. Antimicrob. Agents Chemother. 48: 1614–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fantini J., Hammache D., Delézay O., Piéroni G., Tamalet C., Yahi N. 1998. Sulfatide inhibits HIV-1 entry into CD4-/CXCR4+ cells. Virology. 246: 211–220 [DOI] [PubMed] [Google Scholar]
- 148.Harouse J. M., Collman R. G., González-Scarano F. 1995. Human immunodeficiency virus type 1 infection of SK-N-MC cells: domains of gp120 involved in entry into a CD4-negative, galactosyl ceramide/3′-sulfo-galactosyl ceramide-positive cell line. J. Virol. 69: 7383–7390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Albright A. V., Strizki J., Harouse J. M., Lavi E., O'Connor M., González-Scarano F. 1996. HIV-1 infection of cultured human adult oligodendrocytes. Virology. 217: 211–219 [DOI] [PubMed] [Google Scholar]
- 150.Gisslén M., Fredman P., Norkrans G., Hagberg L. 1996. Elevated cerebrospinal fluid sulfatide concentrations as a sign of increased metabolic turnover of myelin in HIV type I infection. AIDS Res. Hum. Retroviruses. 12: 149–155 [DOI] [PubMed] [Google Scholar]
- 151.Gisslén M., Lekman A., Fredman P. 1999. High levels in serum, but no signs of intrathecal synthesis of anti-sulfatide antibodies in HIV-1 infected individuals with or without central nervous system complications. J. Neuroimmunol. 94: 153–156 [DOI] [PubMed] [Google Scholar]
- 152.Souayah N., Mian N. F., Gu Y., Ilyas A. A. 2007. Elevated anti-sulfatide antibodies in Guillain-Barré syndrome in T cell depleted at end-stage AIDS. J. Neuroimmunol. 188: 143–145 [DOI] [PubMed] [Google Scholar]
- 153.Sundell I. B., Halder R., Zhang M., Maricic I., Koka P. S., Kumar V. 2010. Sulfatide administration leads to inhibition of HIV-1 replication and enhanced hematopoeisis. J. Stem Cells. 5: 33–42 [PubMed] [Google Scholar]
- 154.Alpa M., Ferrero B., Cavallo R., Naretto C., Menegatti E., Di Simone D., Napoli F., La Grotta R., Rossi D., Baldovino S., et al. 2008. Anti-neuronal antibodies in patients with HCV-related mixed cryoglobulinemia. Autoimmun. Rev. 8: 56–58 [DOI] [PubMed] [Google Scholar]
- 155.Sakamoto H., Okamoto K., Aoki M., Kato H., Katsume A., Ohta A., Tsukuda T., Shimma N., Aoki Y., Arisawa M., et al. 2005. Host sphingolipid biosynthesis as a target for hepatitis C virus therapy. Nat. Chem. Biol. 1: 333–337 [DOI] [PubMed] [Google Scholar]
- 156.Suzuki T., Sometani A., Yamazaki Y., Horiike G., Mizutani Y., Masuda H., Yamada M., Tahara H., Xu G., Miyamoto D., et al. 1996. Sulphatide binds to human and animal influenza A viruses, and inhibits the viral infection. Biochem. J. 318: 389–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Suzuki T., Takahashi T., Nishinaka D., Murakami M., Fujii S., Hidari K. I., Miyamoto D., Li Y. T., Suzuki Y. 2003. Inhibition of influenza A virus sialidase activity by sulfatide. FEBS Lett. 553: 355–359 [DOI] [PubMed] [Google Scholar]
- 158.Takahashi T., Murakami K., Nagakura M., Kishita H., Watanabe S., Honke K., Ogura K., Tai T., Kawasaki K., Miyamoto D., et al. 2008. Sulfatide is required for efficient replication of influenza A virus. J. Virol. 82: 5940–5950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ogura K., Kohno K., Tai T. 1998. Molecular cloning of a rat brain cDNA, with homology to a tyrosine kinase substrate, that induces galactosylceramide expression in COS-7 cells. J. Neurochem. 71: 1827–1836 [DOI] [PubMed] [Google Scholar]
- 160.Takahashi T., Satoh H., Takaguchi M., Takafuji S., Yokoyama H., Fujii S., Suzuki T. 2010. Binding of sulfatide to recombinant hemagglutinin of influenza A virus produced by a baculovirus protein expression system. J. Biochem. 147: 459–462 [DOI] [PubMed] [Google Scholar]
- 161.Perino J., Foo C. H., Spehner D., Cohen G. H., Eisenberg R. J., Crance J. M., Favier A. L. 2011. Role of sulfatide in vaccinia virus infection. Biol. Cell. 103: 319–331 [DOI] [PubMed] [Google Scholar]