Abstract
Cholesteryl ester transfer protein (CETP) is important clinically and is the current target for new drug development. Its structure and mechanism of action has not been well understood. We have combined current new structural and functional methods to compare with relevant prior data. These analyses have led us to propose several steps in CETP's function at the molecular level, in the context of its interactions with lipoproteins, e.g., sensing, penetration, docking, selectivity, ternary complex formation, lipid transfer, and HDL dissociation. These new molecular insights improve our understanding of CETP's mechanisms of action.
Keywords: molecular biology of CETP, functional domains of CETP, lipid transfer biology, lipid transfer tunnel hypothesis
Recent innovative X-ray crystallographic, electron microscopic (EM), and bioinformatics observations significantly improve our understanding of the physical relationships of cholesteryl ester transfer protein's (CETP) interactions with lipoproteins and lipid transfer processes (1, 2). Two major observations derived using these novel methods are i) CETP connects with or forms bridges between two lipoproteins, e.g., HDL and LDL, with resultant neutral lipid transfer, and ii) CETP appears to contain a hydrophobic tunnel along its entire long axis capable of neutral lipid transfer. Using this new paradigm, prior data regarding CETP and lipoprotein interactions can be reevaluated from current perspectives. Here, we focus on molecular details of CETP that appear relevant to its interactions with lipoproteins, including sensing, penetration, docking, selectivity, ternary complex formation, lipid transfer, and HDL dissociation.
First, a summary of the biological and clinical importance of CETP is in order, as it has a central role in lipoprotein remodeling and as CETP inhibitors are in various stages of drug development.
CETP is capable of proatherogenic activities during remodeling through both triglyceride and cholesteryl ester (CE) pathways. CETP mediates transfer of triglycerides from VLDL-1 to HDL and/or LDL in exchange for CE, resulting in larger, relatively triglyceride-enriched HDL and LDL species (3, 4). Hepatic lipase remodels these larger lipoprotein species into i) smaller and denser HDL remnants destined for removal from the circulation by the hepatic holo receptor and/or by renal clearance (5) and ii) smaller, denser LDL destined for the subendothelial space of arteries to initiate plaque formation, as these LDL species react weakly with LDL receptors, thus prolonging their half-lives (6–8). These pathological endpoints are observed clinically as circulating i) high normal to moderately elevated triglyceride levels, ii) low total HDL cholesterol (HDL-C) levels, and iii) high levels of small, dense LDL. This triad is referred to as the atherogenic lipid profile, which is observed in ∼25% of the general population in Western countries due to insulin resistance, including type 2 diabetes (9, 10).
Further, but related, proatherogenic activity for CETP involves its redirection of CE to more atherogenic lipoprotein species, rather than to direct CE disposal by the liver via SRB-1 or the LDL receptor (4, 6). The latter two, in part, mediate reverse cholesterol transport (RCT), in which cholesterol obtained from peripheral tissue is esterified by LCAT. Most CEs derived from LCAT do not return to the liver via the HDL SRB-1 pathway but, rather, through more atherogenic pathways (4, 7). CETP mediates the transfer of most CE from HDL to VLDL or to other more atherogenic intermediate-density lipoproteins and remnants (3). Apolipoprotein F may inhibit direct transfer of some, but not all, of CE from HDL to the potentially more atherogenic LDL (11). Inhibiting the above two proatherogenic remodeling events could be advantageous. Indeed, in vitro studies using human plasma indicate an antiatherogenic behavior of antibody-mediated CETP inhibition (12).
Transfer of CE from HDL directly to LDL by CETP could also be antiatherogenic if the LDL is cleared by the liver LDL receptor. This role of RCT is especially important if the original source of cholesterol is from plaque (4). As this process is potentially antiatherogenic, inhibition could be disadvantageous.
An additional concern is that excessive CETP inhibition increases HDL-C to supra physiological levels (>70 mg/dl), which appear to result in paradoxically high rates of cardiovascular disease (CVD), as shown in several epidemiological studies (13, 14) and in one CETP inhibitor interventional trial (15) but not in another (dal-OUTCOMES). The dal-OUTCOMES trial was terminated for lack of efficacy, and the details of this study were not available at the time of this review. Regarding the former study, increased CVD was attributed to hypertension, and an unusual number of patients had fatal sepsis; HDL is known to be crucial for innate immunity, e.g., lipopolysaccharide sequestration (16). High levels of circulating HDL-C may also be associated with dysfunctional HDL species in some studies (17). Finally, as CETP activity decreases in the general population, increased rates of CVD are observed (18). From the entire pro- and antiatherogenic concepts of CETP inhibition and clinical intervention trial results, no clear conclusions are apparent.
Most recently, the focus has been on the potential for positive outcomes of CETP inhibition (17, 19, 20). Because CETP inhibitors appear to have conflicting biochemical activities with respect to CVD and because two recent large, international clinical trials have failed to show efficacy, an improved understanding of CETP's molecular interactions could be beneficial to more definitive descriptions of CETP function and drug design.
SENSING AND PENETRATION
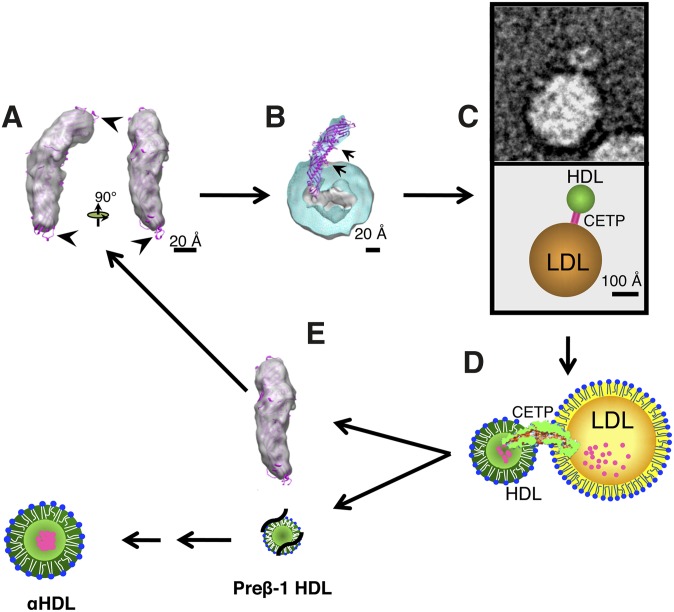
Before CETP can engage in lipid transfer, it must first recognize or sense the surfaces of potential donor or recipient lipoproteins, and then penetrate into their surface and/or core. We believe sensing is an important facet of CETP function and may be susceptible to new drug design. With respect to sensing and penetration, superimposing the X-ray crystal structure CETP on the EM-generated three-dimensional CETP image reveals exposed and flexible loops protruding beyond the ∼130 Å long, density-rich protein structure at the extreme ends of the N and C termini (Fig. 1A) (2). These loops are flexible according to molecular dynamics simulation studies (2). The N-terminal domain of CETP also has other interesting molecular features vis a vis sensing and penetration: i) it has all 47 amino acids that are 100% conserved among 26 CETP orthologs; ii) a 100% conserved hydrophobic tryptophan 105 is present in the mini helix at the end of one loop; and iii) the region adjacent to the mini helix is highly enriched with hydrophobic amino acids (1, 21). Linker insertion (two nucleotide insertions) scanning mutants’ data of CETP also indicates that four regions in the N-terminal extreme end (amino acid positions 48, 53, 98, and 165) are important for CETP lipid transfer function and presumably also for sensing and/or penetration (22). Unfortunately, mutations in the highly conserved mini helix have not been examined, as the mutagenicity data were gathered decades before the helix's discovery.
Fig. 1.
CETP's molecular and functional mechanisms (2). (A) Sensing. Three-dimensional EM molecular envelope of CETP shown in gray, and the X-ray crystal structure of CETP overlay is shown in magenta. Arrows indicate free loops extending out of the electron-dense region of CETP, with one lower loop showing the mini helix. (B) Penetration and docking. Cross-section of three-dimensional EM molecular envelope of CETP penetrating into the CE core (gray) of spherical rHDL (turquoise), with overlay of X-ray crystal structure of CETP (magenta) and arrows indicating the positions of each phospholipid pore; lower arrow also indicates the region of α-helix X. (C) Ternary complex formation. EM image (top) and diagram (bottom) of ternary complex showing CETP bridging the smaller spherical rHDL particle and the larger spherical LDL particle. (D) Lipid exchange. Illustration of CETP's interactions with lipoproteins in the ternary complex and resulting lipid transfer, in which small magenta circles represent CE moving from the smaller HDL particle to the larger LDL particle. We propose the following sequence of events: i) CETP's binding to lipoproteins may create forces resulting in the twisting of the two distal domains of CETP. ii) Twisting is the key to opening the tunnel for lipid exchange between lipoproteins. iii) Neutral lipid, e.g., CE, would migrate through the hydrophobic tunnel structure. iv) Flow and direction of lipid transfer would be established by the following dominant thermodynamic forces: differential CE concentrations, changes in hydrophobicity within CETP's central cavity favoring CE transfer from the N-terminal to C-terminal domain, and entropic energy created by the chemical potential of CE in HDL, by reason of its more ordered packing, forces CE to the less ordered (molten liquid) CE-rich (LDL) or triglyceride-rich (VLDL) lipoproteins. (E) HDL dissociation. Illustration showing the dissociation of HDL into free CETP (gray), and dissociation of relatively CE-free HDL resulting in the formation of preβ-1 HDL (black lines and small lipid structure), which is subsequently converted (arrows) into maturing α-HDL species.
With respect to sensing, the exposed loops could contain important sensing elements, similar to exchangeable lipoproteins. Locusta migratoria's apolipophorin III (apo Lp-III), an insect exchangeable lipoprotein, is composed of several α-helices when lipid free, some of which are connected by exposed loops that are structurally similar to those observed in CETP (23). Mutagenicity studies of apo Lp-III indicate that loop positions, leucine 32, 34, and 95, are essential for sensing lipoprotein (24). Experiments using radiolabeled apo Lp-III binding to phospholipase C-treated human LDL, which has some phosphate groups removed from the surface phospholipids, show that, whereas wild-type, unlabeled apo Lp-III competitively displaces most radiolabeled exchangeable lipoprotein, mutant proteins L32R, L34R, and L95R are associated with substantially less displacement of labeled apo Lp-III binding to LDL. The triple mutant has almost no activity in displacing labeled apo Lp-III. These data suggest that specific leucine loop positions are important for sensing surface defects on LDL, which lack surface-charged groups, thus exposing the more hydrophobic diacylglycerol; however, these studies did not distinguish potential differences between other adjacent hydrophobic amino acids. Further insect studies of a loop mini helix may be analogous to CETP's conserved loop mini helix (25). In moth (Manduca sexta) apo Lp-III, in which a 5 amino acid mini helix exists in a loop bridging α-helices, nonhydrophobic amino acid mutants of valine 97 of the helix show decreased displacement of labeled apo Lp-III compared with wild-type apo Lp-III. Even a charged amino acid at position 96, if modified to an uncharged amino acid, has no effect in displacing labeled apo Lp-III. Thus, a single amino acid position of a loop or a loop mini helix can be important to sense surface defects in modified LDL. Finally, bilayer membrane model systems indicate that just one diacylglycerol molecule present in a phospholipid membrane surface can be detected by apo Lp-III, indicating the sensitivity of the recognition function in this exchangeable lipoprotein (26). In summary, i) insect apo Lp-III structure (interhelical loops and a mini helix) is remarkably similar to CETP structure (inter strand loops and a mini helix); ii) insect apo Lp-III loops and the mini helix have exquisite sensitivity for LDL binding, i.e., just one amino acid appears critical for function; and iii) CETP has an unusual 100% conserved amino acid at a crucial and analogous position to that described for the mini helix of the insect apolipoprotein, implying that this location of the molecule is especially important. These structural similarities between exchangeable lipoproteins and CETP suggest analogous functions could exist in CETP's loops and its mini helix to sense surface defects in lipoprotein surfaces. These highly conserved areas of CETP could be targets for new drug design.
With respect to penetration, EM studies of binary complexes show the N-terminal domain of CETP penetrating ∼50 Å into both spherical reconstituted HDL (rHDL) and native human HDL (Fig. 1B) (2). The C-terminal end appears to penetrate into LDL ∼25 Å and into VLDL ∼20 Å. As the lipoprotein phospholipid surface layer is 18–27 Å thick, it appears that CETP penetrates the HDL surface and core, whereas for LDL and VLDL, only the surface appears penetrated. Shallow CETP penetration into liposomes is also observed (G. Ren and M. A. Charles, unpublished observations), and it is known that CETP can transfer lipids using liposomes (27, 28). Thus apoA-1, apoB, or other proteins are apparently not essential, and the hydrophobic nature of the N- and C-terminal ends of CETP may permit penetration into the hydrophobic environment of the lipoprotein surface and/or core. EM studies show virtually no ternary complexes of CETP bridging two HDLs, LDLs, or VLDLs (2), implying CETP domain specificity for HDL (N-terminal) versus LDL and VLDL (C-terminal). Antibody studies appear to confirm these observations (2). Substantial differences are observed between the N- and C-terminal domains’ extreme ends; e.g., i) the N-terminal domain has a tapered configuration and is highly hydrophobic, ii) the C-terminal domain has a more globular configuration and is less hydrophobic (1, 2), iii) a conserved mini helix amino acid is present in the N-terminal domain (Fig. 1A), which could be involved in permitting deeper penetration into lipoproteins, in addition to or exclusive of its putative sensing function, and iv) the amphipathic α-helix X at the carboxyl-terminal end of the C-terminal domain that folds back into the region of the N-terminal domain is probably also involved in lipoprotein sensing and attachment, analogous to apolipoproteins.
DOCKING
The α-helix X of CETP is located about 50–60 Å in from the N-terminal domain end, which is similar to the length of CETP that penetrates HDL (Fig. 1B, arrow) (1, 2). This amphipathic helix is not conserved in various CETP orthologs (21), which is typical; what's important is the presence of similar hydrophilic and/or hydrophobic amino acids in the correct positions of the helix to ensure amphipathigenicity. Importantly the amino acid structure of this section of CETP was correctly deduced to be an amphipathic helix and a significant functional component of CETP long before the X-ray crystallographic findings showed its existence (29, 30). Thus, the α-helix X has not escaped mutant analysis. A deletion mutant, Δ470–475, eliminates most of the distal end of the helix. Kinetic analy ses of CE transfer from HDL to LDL indicate that the mutant's Km for CE transfer is similar to wild-type CETP; however, the wild-type Vmax is more than three times greater than the mutant's (29). Similar studies of longer deletion mutants at the C-terminal end of CETP show similar binding and transfer results (30). These data are consistent with the concept that mutant CETP can penetrate HDL to some extent, permitting half-maximal lipid transfer occurring normally; however, to achieve maximal lipid transfer, the mutant is defective, which leads us to suggest that α-helix X may perform a docking function permitting more sustained CE transfer. This putative docking function of CETP seems restricted to HDL, as LDL, VLDL, or liposome CETP penetration is only 20–25 Å, not nearly close enough for α-helix X's interaction with the lipoprotein surface.
Rationale supporting the docking function of α-helix X are i) it is highly flexible (2), ii) it is located out of the electron-dense envelope of CETP (Fig. 1B) (2), iii) its amphipathic nature permits it to attach or dock to the lipoprotein surface, similar to exchangeable lipoproteins (23), iv) the deletion mutant is shown to bind and/or penetrate into HDL, an early phase, but not to achieve maximal transfer, a later phase (29), and v) more polar point mutations of α-helix X's hydrophobic amino acids also block long-term lipid transfer (31). Finally, this docking function may have a major role in lipoprotein selectivity.
LIPOPROTEIN SELECTIVITY
If CETP senses, penetrates, and docks with lipoproteins primarily based on hydrophobic and amphipathic properties, then one would expect similar interactions or a lack of specificity between CETP's selectivity for various lipoproteins. As noted above, penetration lengths of CETP into HDL versus LDL or VLDL are quite different, suggesting additional selective determinants at the CETP or lipoprotein level.
First, let's explore the lipoprotein selectivity question from a CETP-centric viewpoint, realizing it is difficult to distinguish the latter from lipoprotein determinants. A series of insightful CETP lipoprotein-binding studies, some performed 25–30 years ago, assist in interpreting CETP's lipoprotein selectivity in the context of new crystal structure and EM observations.
Studies of purified CETP and individual lipoproteins incubated for 4-6 h at room temperature and separated using Sephadex G 200 columns indicate that CETP exchange activity is retained in the column, whereas LDL or VLDL elute in the void volume (32). Incubations with HDL combined with CETP show the two coeluting in the void volume, indicating that CETP binds to HDL. When CETP was incubated with either LDL or VLDL, CETP exchange activity did not appear in the void volume but, rather, was retained as if no lipoprotein incubation occurred, indicating that CETP does not bind to these two lipoproteins under these conditions. Thus during these long incubation times, fundamental differences in CETP's interaction with LDL and VLDL occur when compared with HDL. During longer incubation times, CETP's binding to lipoproteins is potentially ambiguous. Thus similar studies using non-steady-state, short-term (minutes) assays with Sepharose covalently and separately bound to each of the lipoproteins were performed (33). These studies indicate that HDL-Sepharose columns bind 100% of CETP's transfer activity in excess of 90 min, whereas LDL-Sepharose columns release 50% of CETP by 45 min, and with VLDL-Sepharose columns, 50% of CETP elutes by 15 min. These binding studies are congruous with the penetration depth (∼50 Å) and potential docking of CETP with HDL and reduced penetration (20–25 Å) into LDL and VLDL.
CETP's N and C termini have genetic conservation and structural polarity, as described above. CETP also appears to have functional polarity. Thus the N-terminal domain clearly penetrates HDL as shown by EM three-dimensional reconstructions, but it is not clear whether that domain also penetrates LDL or VLDL, as no three-dimensional reconstructions have been performed (2). Also, the absence of ternary complexes observed after CETP incubations with individual lipoprotein subsets, i.e., either HDL, LDL, or VLDL, indirectly argues for CETP functional polarity. For example, after incubations with CETP and HDL, the C-terminal domain does not appear to interact with HDL, forming a HDL-CETP-HDL ternary complex. C-terminal domain-specific antibody EM data rarely show antibody binding to CETP during LDL-CETP interactions, whereas after HDL-CETP incubations, antibody is readily observed binding the free end of the C-terminal domain (2). These data suggest that the N-terminal domain may not preferentially penetrate LDL. Further, and in line with the prior direct visual observations (2), the percentage of LDL-CETP binary complexes observed in the absence of antibody is 38%, whereas with antibody, only 15% of these binary complexes are observed, again indicating that the C-terminal antibody for CETP interferes with LDL binding (2). These antibody data indicate that the C-terminal domain preferentially binds to LDL, whereas the N-terminal domain preferentially interacts with HDL. Although none of the above clearly excludes the possibility of the N-terminal domain interacting with LDL, the evidence quite strongly favors functional polarity. A caveat with the LDL antibody data is that antibody experiments are performed at 4°C, a temperature at which LDL has a much different external shape and internal structure than at room temperature or 37°C (34, 35). At low temperatures, LDL's core CEs develop a definitively ordered, stacking structure as close as 25–30 Å from the surface (35). This stacking might play a role in the low-temperature antibody experiments described above related to CETP's functional polarity. Thus, some ambiguity remains regarding CETP's potential selectivity for LDL, because at 4°C the N-terminal domain may not penetrate as effectively as the C-terminal domain. Similar VLDL studies have not been performed. Although ternary complexes between similar lipoproteins (e.g., LDL) are not regularly observed using EM, it is well established that lipid exchange can occur between two LDL species or between two HDL species (36). Thus, isotopic lipid exchange assays may be more sensitive than visual observation using electron micrographs, and/or there may be other mechanisms of CETP-mediated lipid transfer. In summary, it appears that the physicochemical structure of the two CETP domains, in part, determines HDL versus LDL or VLDL selectivity. Further, the EM data may more strongly reflect preferential, rather than absolute, selectivity. New mutagenicity and/or domain peptide chimeric/hybrid studies should delineate the CETP determinants responsible for polarity and selectivity.
Exploring selectivity from a lipoprotein-centric viewpoint reveals significant differences in lipoprotein structure when comparing HDL to LDL or VLDL at 37°C, e.g., large areas of surface phospholipids unencumbered by proteins and thus available for CETP penetration. Despite the size differences between HDL and LDL, the phospholipid surface dimensions free of apolipoproteins appear similar (33). Further increased lipoprotein surface curvature (e.g., for HDL) could affect selectivity, as phospholipid-charged head groups might be further apart, permitting sensing elements of CETP to penetrate into the lipoprotein's hydrophobic surface. Although some data are consistent with this notion (37), other results are not (38, 39). Thus, lipoprotein structure could, in part, be involved in CETP's selectivity for lipoproteins.
Lipid exchange, and thus selectivity, can be altered by a variety of lipoprotein chemical and physical factors, such as lipoprotein surface charge (33, 36, 40, 41). Increasing the negative charge of lipoproteins can be achieved by acetylation to reduce the positive charge of lysine residues or by the addition of negatively charged free fatty acids to the surface of lipoproteins. When acetylated LDL is the donor for an LDL acceptor, there is a small (∼20%) increase in CETP activity over a broad range of increasingly negatively charged lipoprotein (36). By contrast, addition of oleate to the donor LDL species results in an initial increase (∼60%) of CETP exchange activity followed by a marked reduction in activity at higher levels of negatively charged lipoprotein (36). Similar experiments using lauric or palmitic acid show similar results (41). Thus, it appears that different methods used to increase the negative charge of lipoproteins can result in different effects on CETP function. Complementary binding studies have also been performed after increasing the negative charge of lipoproteins. Progressive acetylation or oleate concentration both result in increased binding of CETP using LDL (36). These studies clearly indicate that perturbations of the lipoprotein surface negative charge can dissociate CETP binding from lipid transfer. One implication of these studies emphasizes the importance of combining methods of CETP binding with appropriate lipid exchange function to insure relevance, as was done in the newer EM studies (2).
Perhaps the most important physiological determinant of CETP's selectivity for lipoproteins is circulating molar ratios of CETP and lipoproteins. Plasma levels of VLDL (∼0.1 µM), LDL (∼2 µM), HDL (∼13 µM), and CETP (∼60 nM) suggest that levels of each lipoprotein and CETP would appear to provide a kinetic argument for CETP's predominant association with HDL and its lower association with LDL and VLDL (32, 33, 40–42).
Modulatory elements may also affect selectivity, but these roles are unclear. Despite apoF's lipid transfer inhibition, substantial lipid transfer occurs with VLDL to LDL; the latter are present in native whole plasma presumably containing apoF and CETP (43). ApoC-1 has inhibitory in vitro and in vivo roles with CETP's interactions with HDL (44, 45). Modulatory elements require a substantial amount of additional in vivo studies to determine their physiological roles.
Thus CETP's molecular selectivity for lipoproteins appear multifactorial: i) CETP structure appears most critical, ii) circulating molar concentrations of CETP and lipoproteins favor CETP's physiological preference for HDL, iii) lipoprotein structure may be involved, and iv) modulatory proteins have an unclear status.
TERNARY COMPLEX FORMATION AND LIPID EXCHANGE
Substantial lipid exchange biochemical information is in the literature (9, 11, 28, 29, 36, 43), but here we primarily focus on recent X-ray crystal and EM structural studies. It is important to indicate at this point that lipid transfer via CETP is usually a heteroexchange mechanism (e.g., CE exchanging with triglyceride), rather than a net flow of either lipid between lipoproteins (11).
X-ray crystallographic studies reveal several important physical attributes of CETP, including its central cavity accommodating two CE molecules (1). There are two pores on the surface of CETP that communicate with the central cavity; these pores are ∼25 Å apart and aligned longitudinally, and each is occupied by a phospholipid molecule. The N- and C-terminal domains’ phospholipid pores are located ∼60 and ∼45 Å from each end, respectively. Because the central cavity communicates with the outside aqueous environment via these pores, it was speculated that these pores gate the central cavity for neutral lipid exchange (1). This formulation was consistent with the CETP shuttle hypothesis described over three decades ago (46). Thus, free CETP interacts with a lipoprotein along the former's length, permitting both phospholipid pores to be adjacent to the lipoprotein surface. Two neutral lipid molecules could enter CETP, and then CETP would dissociate from the donor lipoprotein. Once again free in the circulation, CETP would find an acceptor lipoprotein, attach to it near these two phospholipid pores, and exchange these neutral lipids (1). As no direct evidence showing CETP attaching along its length to lipoproteins or liposomes has been observed (2) and as circulating free CETP levels are difficult to measure because of its low concentration (33, 41), the CETP shuttle hypothesis appears to require more direct proof.
More recent EM and X-ray crystallographic studies combined with molecular dynamics simulation provide substantial direct (ternary complex formation) and indirect evidence for the tunnel hypothesis (Fig. 1C, D and Table 1), which was also predicted nearly three decades ago (47). We speculate that new, additional pores permitting lipid transfer exchange may exist at the extreme ends of the N- and C-terminal domains of CETP. Another putative function of the highly conserved mini helix amino acid at the extreme end of the N-terminal domain could be pore formation; a similar but less conserved mini helix also exists at the C-terminal end.
TABLE 1.
Data supporting the tunnel hypothesis for CETP-mediated lipid exchange (2)
| 1. EM studies show HDL visually connected or bridged to LDL or VLDL by CETP (Fig. 1C). |
| 2. CETP's ends penetrate the lipoproteins, forming a ternary complex (Fig. 1C). |
| 3. X-ray crystallographic analyses show unconnected hydrophobic cavities located in the central axis of both the N- and C-terminal domains and lateral to the 60 Å long central cavity. |
| 4. Molecular dynamics simulation data suggest that only minor twisting (15°) along the long axis of CETP creates 10° of tilt within the β-barrel strands. |
| 5. Torsional analysis suggests that 10° of tilting causes these cavities to become connected to each other and to the central cavity, forming a long, continuous, hydrophobic tunnel that extends the full length of CETP. |
| 6. The tunnel has appropriate space and hydrophobicity capable of accommodating neutral lipids, e.g., CE and triglyceride. |
| 7. Functional EM studies indicate that after CETP ternary complex formation between HDL and LDL, HDL particles decrease in size in the ternary complex, presumably due to CE transfer to LDL (Fig. 1D). |
| 8. EM HDL size analyses of the ternary complexes permit an approximate CE transfer rate constant of 0.58 ± 0.19/ h (r > 0.94) to be calculated. |
| 9. CETP C-terminal domain-specific antibody, which inhibits CETP's association with LDL, inhibits functional CE transfer. |
| 10. CETP is deeply embedded into HDL and apparently has no off-rate after binding to HDL after several hours. |
CETP three-dimensional EM imaging (Fig. 1B) indicates that the N-terminal domain penetrates (∼50 Å) through HDL's surface (∼17–28 Å) into its CE-rich core (2). The N-terminal phospholipid pore (∼60 Å from the end) is thus adjacent to the HDL surface, permitting the possibility of surface lipid transfer as well. We propose that this pore could be important for surface phospholipid transfer when the size of HDL is decreasing (CE efflux) or increasing (triglyceride influx), thus potentially stabilizing the increasing or decreasing curvature of the HDL surface. The C-terminal domain phospholipid pore appears too far removed (∼45 Å) from the LDL surface to participate in lipid transfer because the CETP C-terminal domain only penetrates LDL and VLDL by 20–25 Å. Thus, we propose a new model for lipid transfer (Fig. 1D).
As described earlier, VLDL's relationship with HDL and LDL is important, and combining recent EM studies with previous observations is revealing. Experiments coincubating LDL and VLDL isolated from normal subjects and CETP (the latter present in lipoprotein-deficient plasma) for 18 h indicate that LDL CE content decreases ∼50%, whereas LDL triglyceride increases 5-fold (3). VLDL analyses after the same incubation show reciprocal increases in CE content and decreasing triglyceride mass. Similar results have been observed with other triglyceride-rich lipoproteins serving as triglyceride donors to LDL (3). Interestingly, these experiments were evaluated by gel electrophoresis, which indicated the presence of apoA-1 in the incubation medium. From separate studies, it is known that spherical HDL can be generated during 18 h incubations containing lipoprotein-deficient plasma, i.e., LCAT, apoA-1, and LDL (48). One would presume that VLDL exchange of triglyceride to LDL should occur directly by the CETP ternary complex (Fig. 2A); however, these two lipoproteins were not examined by EM for CETP ternary complex formation (2). Usually experiments showing triglyceride exchange from VLDL to LDL are either conducted in the presence of added HDL (9, 11, 43), or “native” (12) or “natural” (48, 49) plasma containing HDL, and/or they are of long incubation times permitting the formation of HDL from apoA-1 present on VLDL (3, 11, 12, 43, 48, 49).
Fig. 2.
Alternate pathways for triglyceride exchange from VLDL to LDL. (A) The most straightforward model illustrating how triglyceride would be exchanged from VLDL to LDL using the VLDL-CETP-LDL ternary complex depicted by the asterisk. (B) We propose that existing data are more compatible with the following schema: i) VLDL interacts with the more stable HDL-CETP binary complex forming a VLDL-CETP-HDL ternary complex, (directly observed and depicted by an asterisk on the first line of Fig. 2B). ii) The ternary complex permits triglyceride enrichment of HDL and relative CE enrichment of VLDL. iii) The triglyceride-enriched HDL-CETP binary complex (depicted by a double asterisk) dissociates from VLDL. iv) Acting as a shuttle, the binary complex locates and interacts with LDL. v) Another ternary complex lipid exchange, HDL-CETP-LDL (directly observed and depicted by an asterisk on the lower line of Fig. 2B), results in triglyceride-enriched LDL species. vi) The HDL-CETP binary complex dissociates from LDL. vii) The triglyceride-enriched LDL would have a fate described in the text.
In the absence of direct evidence for VLDL-mediated triglyceride enrichment of LDL in experiments excluding the presence of HDL, it appears that the more straightforward CETP ternary complex with LDL and VLDL mediating heteroexchange requires continued investigation. Although short-term isotopic studies of CETP-mediated lipid exchange show VLDL donor to LDL acceptor lipid exchange, in the absence of HDL (50), the qualitative lipid changes are different from the mass transfer, nonisotopic lipid studies described above (3). Thus, CE exchange to LDL from VLDL exceeds triglyceride exchange (50). Further, when VLDL is examined for mass transfer after incubations with LDL, VLDL CE content increases ∼80% and triglyceride content decreases ∼20% (3), whereas using the isotopic method, lipid exchange from LDL to VLDL shows triglyceride exchange exceeding CE (50). These differences could indicate that isotopic methods are more sensitive and might reflect other potential mechanisms of lipid exchange, e.g., lipoprotein surface exchange, compared with mass transfer studies that may be more related to lipoprotein core exchange. Also note that isotopic transfer methods clearly show that lipid exchange exists between LDL donors and LDL acceptors, as well as between HDL donor and acceptor molecules, and that even liposome acceptor/donor exchange is mediated by CETP (28, 36). On the other hand, EM evidence suggests that ternary complexes consisting only of HDL and CETP or only of LDL and CETP are very rarely observed (2), whereas liposome/CETP ternary complexes are frequently observed (G. Ren and M. A. Charles, unpublished observations). Thus, there may be more than one mechanism of lipid exchange mediated by CETP. Because of the sensitivity of isotope methods, the circulating concentrations of lipoproteins described in the prior section, and the preferential binding of CETP to HDL compared with LDL and VLDL, we speculate that HDL may participate in these VLDL/LDL triglyceride and CE exchanges (Fig. 2B). This concept invokes both the shuttle and the tunnel hypotheses, although the shuttle would be the HDL-CETP binary complex in which ternary complexes permit two heteroexchanges to occur. The prediction of a HDL-CETP binary complex was also derived nearly three decades ago from insightful kinetic studies describing the tunnel hypothesis (47).
In summary, lipid exchange is shown to occur after a CETP ternary complex is formed, and the lipids traverse a hydrophobic tunnel in CETP driven by thermodynamic forces (Fig. 1C, D). Finally, a HDL-CETP shuttle is proposed as one option to account for lipid exchange between VLDL and LDL (Fig. 2B). Studies using isotope methods suggest that other mechanisms of lipid exchange may occur as well. Future studies combining direct visualization methods with physiologically relevant CETP-mediated lipid exchange systems should resolve these current ambiguities.
HDL DISSOCIATION
CETP does not appear to have an off-rate from HDL after several hours (32, 33), CETP penetrates deeply into HDL's core (2), and CETP may dock with HDL, all of which are in contrast to CETP's relationships with LDL and VLDL. Thus, the fate of the CETP-HDL binary complex is of interest. EM functional studies show spherical rHDL particles become smaller after 4 h incubations with recombinant CETP and LDL, all present in ternary complexes, and that spherical HDL particles are virtually absent after 24 h (2). These EM observations confirm prior data using size exclusion chromatography, in which incubations with HDL, LDL, or VLDL and lipoprotein-deficient plasma (CETP source) show that α-HDL particles virtually disappear after 24 h (51). During these incubations, the presence of first larger and then smaller HDL species becomes apparent. When these smaller HDL species were examined by agarose gel electrophoresis, there was an absence of α-migrating HDL species and presence of pre-β-migrating HDL species (51). Both EM and size exclusion chromatography experiments suggest that the CETP-HDL binary complex is destined for dissociation (disappearance) with the release of apoA-1 from the HDL particle and the spontaneous formation of pre-β HDL species, which in vivo could regenerate α-HDL particles (Fig. 1E). The above scenario is related to HDL's interaction to transfer CE to LDL. In another scenario described earlier in this article, HDL becomes enlarged with triglycerides from VLDL, and the HDL-CETP binary complex is potentially delipidated (denatured) by hepatic lipase, resulting in apoA-1 release and dissociation and/or reduced size to HDL remnants that are cleared by the hepatic holo receptor (5, 9).
Recent studies using thermal and chemical methods suggest that human spherical HDL particle stability appears more kinetic than thermodynamic (52). This hypothesis proposes that CE formation by LCAT is destabilizing and places HDL in a kinetic trap, where it remains until a mechanism for its escape occurs (53), e.g., CETP (54), hepatic lipase (via CETP), streptococcal opacity factor (53), or physical perturbations (52). CETP appears to induce similar size changes to HDL (51) as described above for thermal and chemical perturbations (52–54). The physical characteristics of apoA-1 may have a key role in these phenomena (54).
CONCLUSIONS
Key steps in CETP function include sensing, penetration, docking, lipoprotein selectivity, ternary complex formation, lipid transfer, and HDL dissociation (Fig. 1). This review is written in the context of illuminating areas of CETP structure and function requiring more clarity. Obviously, future binding, mutagenicity, and cross-linking studies coupled with evolutionary considerations will further refine CETP's multiple biological functions, and it is hoped, lead to positive clinical implications.
Acknowledgments
The authors thank Gary Ren for helpful discussions.
Footnotes
Abbreviations:
- CE
- cholesteryl ester
- CETP
- cholesteryl ester transfer protein
- CVD
- cardiovascular disease
- EM
- electron microscopy
- HDL-C
- HDL cholesterol
- RCT
- reverse cholesterol transport
REFERENCES
- 1.Qiu X., Mistry A., Ammirati M. J., Chrunyk B. A., Clark R. W., Cong Y., Culp J. S., Danley D. E., Freeman T. B., Geoghegan K. F., et al. 2007. Crystal structure of cholesteryl ester transfer protein reveals a long tunnel and four bound lipid molecules. Nat. Struct. Mol. Biol. 14: 106–113 [DOI] [PubMed] [Google Scholar]
- 2.Zhang L., Yan F., Zhang S., Lei D., Charles M. A., Cavigiollo G., Oda M., Krauss R. M., Weisgraber K. H., Rye K. A., et al. 2012. Structural basis of transfer between lipoproteins by cholesteryl ester transfer protein. Nat. Chem. Biol. 8: 342–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deckelbaum R. J., Eisenberg S., Oschry Y., Butbul E., Sharon I., Olivecrona T. 1982. Reversible modification of human plasma low density lipoproteins toward triglyceride-rich precursors. J. Biol. Chem. 257: 6509–6517 [PubMed] [Google Scholar]
- 4.Chapman M. J., Le Goff W., Guerin M., Kontush A. 2010. Cholesteryl ester transfer protein: at the heart of action of lipid-modulating therapy with statins, fibrates, niacin, and cholesteryl ester transfer protein inhibitors. Eur. Heart J. 31: 149–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao C., Watanabe T., Zhang Y., Trigatti B., Szeto L., Connelly P. W., Marcovina S., Vaisar T., Heinecke J. W., Lewis G. F. 2008. Enhanced cellular uptake of remnant high-density lipoprotein particles. Circ. Res. 103: 159–166 [DOI] [PubMed] [Google Scholar]
- 6.Packard C. J., Shepard J. 1997. Lipoprotein heterogeneity and apolipoprotein B metabolism. Arterioscler. Thromb. Vasc. Biol. 17: 3542–3556 [DOI] [PubMed] [Google Scholar]
- 7.Berneis K. K., Krauss R. M. 2002. Metabolic origins and clinical significance of LDL heterogeneity. J. Lipid Res. 43: 1363–1379 [DOI] [PubMed] [Google Scholar]
- 8.Chen G. C., Liu W., Duchateau P., Allaart J., Hamilton R. L., Mendel D. M., Lau K., Hardman D. A., Frost P. H., Malloy M. J., et al. 1994. Conformational differences in human apolipoprotein B-100 among subspecies of low-density lipoproteins. J. Biol. Chem. 269: 29121–29128 [PubMed] [Google Scholar]
- 9.Griffin B. A., Freeman D. J., Tait G. W., Thomson J., Caslake M. J., Packard C. J., Shepherd J. 1994. Role of plasma triglyceride in the regulation of plasma low-density lipoprotein (LDL) subfractions: relative contribution of small, dense LDL to coronary heart disease risk. Atherosclerosis. 106: 241–253 [DOI] [PubMed] [Google Scholar]
- 10.Van J., Pan J., Charles M. A., Krauss R., Wong N., Wu X. 2007. Atherogenic lipid phenotype in a general group of subjects. Arch. Pathol. Lab. Med. 131: 1679–1685 [DOI] [PubMed] [Google Scholar]
- 11.Serdyuk A. P., Morton R. E. 1999. Lipid transfer inhibitor protein defines the participation of lipoproteins in lipid transfer reactions. Arterioscler. Thromb. Vasc. Biol. 19: 718–726 [DOI] [PubMed] [Google Scholar]
- 12.Morton R. E., Greene D. J. 2007. Partial suppression of CETP activity beneficially modifies the lipid transfer profile of plasma. Atherosclerosis. 192: 100–107 [DOI] [PubMed] [Google Scholar]
- 13.Stensvold I., Urdal P., Thurmer H., Tverdal A., Lund-Larson P. J., Foss O. P. 1992. High-density lipoprotein cholesterol and coronary, cardiovascular and all cause mortality among middle-aged Norwegian men and women. Eur. Heart J. 13: 1155–1163 [DOI] [PubMed] [Google Scholar]
- 14.van der Steeg W. A., Holme I., Boekholdt S. M., Larsen M. L., Lindahl C., Stroes E. S. G., Tikkanen M. J., Wareham N. J., Faergeman O., Olsson A. G., et al. 2008. High-density lipoprotein cholesterol, high-density lipoprotein particle size, and apolipoprotein A-1: significance for cardiovascular risk, the IDEAL and EPIC-Norfolk studies. J. Am. Coll. Cardiol. 51: 634–642 [DOI] [PubMed] [Google Scholar]
- 15.Barter P. J., Caulfield M., Eriksson M., Grundy S. M., Kastelein J. J. P., Komajda M., Lopez-Sendon J., Mosca L., Tardif J. C., Waters D. D., et al. 2007. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 357: 2109–2122 [DOI] [PubMed] [Google Scholar]
- 16.Thompson P. A., Berbee J. F. P., Rensen P. C. N., Kitchens R. L. 2008. Apolipoprotein A-II augments monocyte responses to LPS by suppressing the inhibitory activity of LPS-binding protein. Innate Immun. 14: 365–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niesor E. J. 2011. Different effects of compounds decreasing cholesteryl ester transfer protein activity on lipoprotein metabolism. Curr. Opin. Lipidol. 22: 288–295 [DOI] [PubMed] [Google Scholar]
- 18.Vasan R. S., Pencina M. J., Robins S. J., Zachariah J. P., Kaur G., D'Agostino R. B., Ordovas J. M. 2009. Association of circulating cholesteryl ester transfer protein activity with incidence of cardiovascular disease in the community. Circulation. 120: 2414–2420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barter P. J., Rye K. A. Cholesteryl ester transfer protein (CETP) inhibition as a strategy to reduce cardiovascular risk. J. Lipid Res. Epub ahead of print. May 1, 2012; doi: 10.1194/jlr.R024075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rye K. A., Barter P. J. 2012. The inhibition of cholesteryl ester transfer protein: a long and winding road. J. Lipid Res. 53: 1039–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall J., Qiu X. 2011. Structural and biophysical insight into cholesteryl ester-transfer protein. Biochem. Soc. Trans. 39: 1000–1005 [DOI] [PubMed] [Google Scholar]
- 22.Wang S., Deng L., Brown M. L., Agellon L. B., Tall A. R. 1991. Structure-function studies of human cholesteryl ester transfer protein by linker insertion scanning mutagenesis. Biochemistry. 30: 3484–3490 [DOI] [PubMed] [Google Scholar]
- 23.Weers P. M. M., Ryan R. O. 2006. Apolipophorin III: role model apolipoprotein. Insect Biochem. Mol. Biol. 36: 231–240 [DOI] [PubMed] [Google Scholar]
- 24.Weers P. M. M., Narayanaswami V., Kay C. M., Ryan R. O. 1999. Interaction of an exchangeable apolipoprotein with phospholipid vesicles and lipoprotein particles. J. Biol. Chem. 274: 21804–21810 [DOI] [PubMed] [Google Scholar]
- 25.Narayanaswami V., Wang J., Schieve D., Kay C. M., Ryan R. O. 1999. A molecular trigger of lipid binding-induced opening of a helix bundle exchangeable apolipoprotein. Proc. Natl. Acad. Sci. USA. 96: 4366–4371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soulages J. L., Salamon Z., Wells M. A., Tollin G. 1995. Low concentrations of diacylglycerol promote the binding of apolipophorin III to a phospholipid bilayer: a surface plasmon resonance spectroscopic study. Proc. Natl. Acad. Sci. USA. 92: 5650–5654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swenson T. L., Brocia R. W., Tall A. R. 1988. Plasma cholesteryl ester transfer protein has binding sites for neutral lipids and phophpolipids. J. Biol. Chem. 263: 5150–5157 [PubMed] [Google Scholar]
- 28.Morton R. E., Greene D. J. 2003. The surface cholesteryl ester content of donor and acceptor particles regulates CETP: a liposome-based approach to assess the substrate properties of lipoproteins. J. Lipid Res. 44: 1364–1372 [DOI] [PubMed] [Google Scholar]
- 29.Wang S., Kussie P., Deng L., Tall A. 1995. Defective binding of neutral lipids in a carboxyl-terminal deletion mutant of cholesteryl ester transfer protein. J. Biol. Chem. 270: 612–618 [DOI] [PubMed] [Google Scholar]
- 30.Au-Young J., Fielding C. J. 1992. Synthesis and secretion of wild-type and mutant human plasma cholesteryl ester transfer protein in baculovirus-transfected insect cells: the carboxyl-terminal region is required for both lipoprotein binding and catalysis of transfer. Proc. Natl. Acad. Sci. USA. 89: 4094–4098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang S., Wang X., Deng S., Rassart E., Milne R. W., Tall A. R. 1993. Point mutagenesis of carboxyl–terminal amino acids of cholesteryl ester transfer protein. J. Biol. Chem. 268: 1955–1959 [PubMed] [Google Scholar]
- 32.Pattnaik N. M., Zilversmit D. B. 1979. Interaction of cholesteryl ester exchange protein with human plasma lipoproteins and phospholipid vesicles. J. Biol. Chem. 254: 2782–2786 [PubMed] [Google Scholar]
- 33.Morton R. E. 1985. Binding of plasma-derived lipid transfer protein to lipoprotein substrates. J. Biol. Chem. 260: 12593–12599 [PubMed] [Google Scholar]
- 34.Chen G. C., Krieger M., Kane J. P., Wu C. C., Brown M. S., Goldstein J. L. 1980. Beta-carotene as a probe of lipid domains of reconstituted human plasma Low-density lipoprotein: induced circular dichroism. Biochemistry. 19: 4706–4712 [DOI] [PubMed] [Google Scholar]
- 35.Ren G., Rudenko G., Ludtke S. J., Deisenhofer J., Chiu W., Pownall H. J. 2010. Model of human low-density lipoprotein and bound receptor based on Cryo EM. Proc. Natl. Acad. Sci. USA. 107: 1059–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morton R. E., Greene D. J. 2003. CETP and lipid transfer inhibitor protein are uniquely affected by the negative charge density of the lipid and protein domains of LDL. J. Lipid Res. 44: 2287–2296 [DOI] [PubMed] [Google Scholar]
- 37.Marcel Y. L., McPherson R., Hogue M., Czetnecka H., Zawadzki Z., Weech P. K., Whitlock M. E., Tall A. R., Milne R. W. 1990. Distribution and concentration of cholesteryl ester transfer protein in plasma of normolipemic subjects. J. Clin. Invest. 85: 10–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Francone O. L., Gurakar A., Fielding C. 1989. Distribution and functions of lecithin:cholesterol acyl-transferase and cholesteryl ester transfer protein in plasma lipoproteins. J. Biol. Chem. 264: 7066–7072 [PubMed] [Google Scholar]
- 39.Bruce C., Davidson W. S., Kussie P., Lund-Katz S., Phillips M. C., Ghosh R., Tall A. R. 1995. Molecular determinants of plasma cholesteryl ester transfer protein binding to high-density lipoproteins. J. Biol. Chem. 270: 11532–11542 [DOI] [PubMed] [Google Scholar]
- 40.Morton R. E., Zilversmit D. B. 1982. Purification and characterization of lipid transfer proteins from human lipoprotein-deficient plasma. J. Lipid Res. 23: 1058–1067 [PubMed] [Google Scholar]
- 41.Nishida H. I., Arai H., Nishida T. 1993. Cholesterol ester transfer mediated by lipid transfer protein as influenced by changes in the charge characteristics of plasma lipoproteins. J. Biol. Chem. 268: 16352–16360 [PubMed] [Google Scholar]
- 42.Jeyarajah E. J., Cromwell W. C., Otvos J. D. 2006. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin. Lab. Med. 26: 847–870 [DOI] [PubMed] [Google Scholar]
- 43.Morton R. E., Greene D. J. 1994. Regulation of lipid transfer between lipoproteins by an endogenous plasma protein: selective inhibition among lipoprotein classes. J. Lipid Res. 35: 836–847 [PubMed] [Google Scholar]
- 44.Gautier T., Masson D., de Barros J. P., Athias A., Gambert P., Metz-Boutique M. H., Lagrost L. 2000. Human apolipoprotein accounts for the ability of plasma high- density lipoprotein's to inhibit the cholesteryl ester transfer protein activity. J. Biol. Chem. 275: 37504–37509 [DOI] [PubMed] [Google Scholar]
- 45.Gautier T., Masson D., Jong M. C., Duverneuil L., Guern N. L., Deckert V., de Barros J. P., Dumont L., Bataille A., Zak Z., et al. 2002. Apolipoprotein CI deficiency markedly augments plasma lipoprotein changes mediated by human cholesteryl ester transfer protein (CETP) in CETP transgenic/apoCI- knocked out mice. J. Biol. Chem. 277: 31354–31363 [DOI] [PubMed] [Google Scholar]
- 46.Barter P. J., Jones M. E. 1980. Kinetic studies of the transfer of esterified cholesterol between human plasma low and high density lipoproteins. J. Lipid Res. 21: 238–249 [PubMed] [Google Scholar]
- 47.Ihm J., Quinn D. M., Busch S. J., Chataing B., Harmony J. A. K. 1982. Kinetics of plasma protein-catalyzed exchange of phosphatidylcholine and cholesteryl ester between plasma lipoproteins. J. Lipid Res. 23: 1328–1341 [PubMed] [Google Scholar]
- 48.Rye K. A., Barter P. J. 1994. The influence of apolipoproteins on the structure and function of spheroidal, reconstituted, high density lipoproteins. J. Biol. Chem. 269: 10298–10303 [PubMed] [Google Scholar]
- 49.Barter P. J., Lally J. I., Wattchow D. 1979. Metabolism of triglyceride in rabbit plasma low and high density lipoproteins: studies in vivo and in vitro. Metabolism. 28: 614–618 [DOI] [PubMed] [Google Scholar]
- 50.Morton R. E., Zilversmit D. B. 1981. A plasma inhibitor of triglyceride and cholesteryl ester transfer activities. J. Biol. Chem. 256: 11992–11995 [PubMed] [Google Scholar]
- 51.Liang H. Q., Rye K. A., Barter P. J. 1994. Dissociation of lipid-free apolipoprotein A-1 from high density lipoproteins. J. Lipid Res. 35: 1187–1199 [PubMed] [Google Scholar]
- 52.Mehta R., Gantz D. L., Gursky O. 2003. Human plasma high-density lipoproteins are stabilized by kinetic factors. J. Mol. Biol. 328: 183–192 [DOI] [PubMed] [Google Scholar]
- 53.Han M., Gillard B. K., Courtney H. S., Ward K., Rosales C., Khant H., Ludtke S. J., Pownall H. J. 2009. Disruption of human plasma high-density lipoproteins by streptococcal serum opacity factor requires labile apolipoprotein A-I. Biochemistry. 48: 1481–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pownall H. J., Ehnholm C. 2006. The unique role of apolipoprotein A-I in HDL remodeling and metabolism. Curr. Opin. Lipidol. 17: 209–213 [DOI] [PubMed] [Google Scholar]