Abstract
Pathomechanisms underlying vascular calcification biogenesis are still incompletely understood. Biomineral from human atherosclerotic intimal plaques; human, equine, and bovine medial vascular calcifications; and human and equine bone was released from collagenous organic matrix by sodium hydroxide/sodium hypochlorite digestion. Solid-state 13C NMR of intimal plaque mineral shows signals from cholesterol/cholesteryl esters and fatty acids. In contrast, in mineral from pure medial calcifications and bone mineral, fatty acid signals predominate. Refluxing (chloroform/methanol) intimal plaque calcifications removes the cholesterylic but not the fatty acyl signals. The lipid composition of this refluxed mineral now closely resembles that of the medial and bone mineral, which is unchanged by reflux. Thus, intimal and medial vascular calcifications and bone mineral have in common a pool of occluded mineral-entrained fatty acyl-rich lipids. This population of fatty acid may contain methyl-branched fatty acids, possibly representing lipoprotein particle remnants. Cell signaling and mechanistic parallels between physiological (orthotopic) and pathological (ectopic) calcification are also reflected thus in the NMR spectroscopic fingerprints of mineral-associated and mineral-entrained lipids. Additionally the atherosclerotic plaque mineral alone shows a significant independent pool of cholesterylic lipids. Colocalization of mineral and lipid may be coincidental, but it could also reflect an essential mechanistic component of biomineralization.
Keywords: cholesterol, fatty acid, matrix vesicle, hydroxyapatite, osteogenesis, biomineralization, atherosclerosis, diabetes mellitus, renal insufficiency, nuclear magnetic resonance
Calcifications, which often occur in atherosclerosis, are almost exclusively in the intimal layer of the arterial wall. Small mineral deposits in the fibrous cap are associated with plaque rupture, whereas large calcium phosphate crystals may stabilize it (1). On the other hand, renal insufficiency and diabetes mellitus (2), in particular, provoke calcification in the medial layer of the arterial wall, which is also associated with increased cardiovascular morbidity and mortality (3).
Lipids are widely believed to be important participants in some apatitic biomineralization processes (4), in both normal hard tissue and tissue of pathological origin (5), although the source of lipids is far from clear in either case. In atherosclerotic calcification, thinking focuses on the role of matrix vesicles (MV), small (20–200 nm) spherical bodies bounded by a lipid layer that often contains particles of calcium phosphate. MVs are released by vascular smooth muscle cells (VSMC) after prolonged exposure in vitro to calcium and phosphate levels, mimicking the mineral imbalance in chronic kidney disease (6). In vivo, ultrastructural studies have identified hydroxyapatite-containing MVs in human atherosclerotic aortas with calcified lesions, suggesting that these structures may serve as a first nidus for vascular calcification (7). Moreover, MVs are enriched in acidic phospholipids, such as phosphatidylserine, that could coordinate calcium ions in the initial mineral nucleation stages (8, 9), and they have been found to be loaded with mineralization-supportive proteins and other macromolecules (9).
Comparative mass spectrometric lipidomics of nonmineralized human atherosclerotic plaques shows abundant unsaturated cholesteryl esters and some phosphatidylcholines and sphingomyelins, with elevated concentrations of lipid species, particularly cholesteryl esters, in plaque relative to plasma (10). Cholesteryl esters are nearly always associated with atherosclerosis, but what their involvement is in the calcification process is still a matter of considerable debate. Crystallites of cholesterol are found in close proximity to apatitic mineral in atherosclerotic plaques (11), and in vitro, apatitic crystals are found to grow from cholesterol crystal surfaces (12), prompting suggestions of a possible templating role. Conversely, hydroxyapatite seed crystals added to phosphatidyl/cholesterol liposomes caused the liposomes to aggregate, suggesting that the lipid role may be much more complex than simple templating. The situation is further complicated by the fact that most cholesterol in the body is carried in low-density lipoprotein (LDL) particles (13), which also have their own phospholipid membrane. In turn, LDL particles binding to arterial walls is recognized as a key initiator of atherosclerosis (14), although their possible roles in calcification of atherosclerotic plaques is less clear.
There is considerable evidence that vascular calcification is associated with phenotypic changes of VSMCs toward osteoblast/chondrocyte-like differentiation (15). Physiological growth-plate calcification of cartilage and bone may involve MVs, although the detail of that involvement is still a subject of considerable research (4). By analogy, vascular calcification might also be seen as a “growth phase,” albeit a pathological one. There are further similarities between physiological calcification of bone and cartilage and pathological vascular calcification (e.g., the respective compositions and crystallinities of the mineral components and the nature of the biomolecules, such as collagen and sugar species (16), closely associated with the mineral). Detailed atomic-level characterization and comparison of the lipid associated with bone and pathological vascular biomineral deposits could provide further insight into the role of lipids in both normal and pathological mineralization and, thus, suggest new approaches to regulating mineralization processes.
Solid-state NMR is a powerful technique for characterizing the chemical composition and molecular structure of biological materials. It is often effective with little sample manipulation and extraction. This can be particularly useful for composite mineralized tissue because freeing organic material from the mineral phase can require severe and possibly destructive treatments. NMR methods have been extensively applied to vascular deposits and their molecular precursors. Hamilton et al. identified 13C signals from cholesteryl esters and the choline head groups of phosphatidylcholines in intact LDL and in arterial tissue containing fibrotic lesions (17). Numerous lipid 1H (18) and 13C (19) NMR assignments have been made in cholesterol-fed rabbit atherosclerotic lesions and lipoproteins, and they have been used to investigate lipid compositions and ratios. In model phospholipid-cholesterol binary mixtures, nonesterified cholesterol decreases phospholipid mobility and lipid NMR signal linewidths (20). Solid-state 13C and 31P NMR, respectively, characterize the organic and the mineral components of human carotid artery plaques (21). NMR quantification of crystalline cholesterol and hydroxyapatite in intact human atherosclerotic plaques correlates with chemical analyses and shows that some cholesterol takes the form of crystalline cholesterol monohydrate (22).
In this study, we use chemical treatment of pathologically and naturally calcified tissue to remove extracellular matrix organic material, leaving only the mineral deposit itself, thus allowing detailed characterization of its organic contents. We find fatty acyl lipids that resist chemical digestion and subsequent organic solvent extraction in all the biomineral types. However, cholesterol and cholesteryl esters are abundant only in mineral from calcified intimal vascular plaque, and they can be mobilized by organic solvents, suggesting significant differences in the roles of these two lipid populations in the calcification process. Moreover, the form of acyl fatty acids we identify in both vascular tissue and bone raises further questions about the source and role of this lipid population.
MATERIALS AND METHODS
All human material was obtained and used with full institutional ethical approval. Intimal calcifications were obtained from carotid endarterectomies; NMR was performed on four samples, three of which were from different individuals, and one of which was prepared from intimal calcifications from three other individuals, pooled. Calcifications judged to be predominantly medial from six different individuals were obtained from femoral arteries of diabetic patients after amputation. Intimal calcified atherosclerotic plaque was pretreated with collagenase and elastase as described in detail in Duer et al. (16). Calcified medial vascular material was frozen at −20 C as soon as possible after surgery and subjected to sodium hydroxide and sodium hypochlorite digestion (see below) without further treatment. A single sample of human bone was obtained from the femoral head of a patient undergoing hip replacement surgery. Equine tibial cortical bone (from six different horses ranging in age from fetus to 35 years) and calcified pulmonary artery medial plaque (from five different horses and one cow) was obtained from animals which died naturally or were euthanized humanely for reasons unrelated to this study.
Pure lipids were purchased from Sigma (Poole, Dorset, UK) and used without further purification.
Calcifications were released from organic matrix and other connective tissue (collagen and other biomacromolecules) by prolonged digestion in concentrated sodium hydroxide and sodium hypochlorite at 4°C. At intervals of a few weeks, samples were filtered, thoroughly washed with distilled water, air dried, and interrogated by 13C SSNMR. If signals from collagenous proteins were observed, particularly the signature signal at ca. 70 ppm from the γ-carbon of hydroxyproline, the sample was resuspended in concentrated sodium hydroxide and sodium hypochlorite for another few weeks. This procedure was repeated until continued digestion resulted in no further spectroscopic changes. Yields of calcified material free of organic matrix and connective tissue material varied widely, between a few milligrams and a few hundreds of milligrams, depending on the mass of original available material.
Lipids were extracted from digested mineral by refluxing calcifications from intimal plaque and bone, in 1:1 chloroform:methanol for 30 min. The solvent was decanted, and the refluxed mineral was washed twice with fresh chloroform/methanol and air dried. The washings were added to the original decanted refluxate, which was dried under low vacuum on a rotary evaporator and redissolved in deuterochloroform for liquid-state NMR characterization.
Solid-state NMR
All experiments were performed using standard SSNMR methodology on a Bruker 9.4 Tesla Avance-400 wide bore spectrometer, at frequencies of 400.1 MHz (1H) and 100.5 MHz (13C) and magic angle spinning (MAS) rate of 12.5 kHz. One-dimensional datasets were acquired using cross polarization (CP) MAS (1H π/2 pulse length 2.5 μs, 1H cross polarization field 70 kHz, 1H-13C cross-polarization contact time 2.5 ms, broadband TPPM decoupling during signal acquisition at a 1H field strength of 100 kHz). Repetition time was 2 s in all experiments. 13C chemical shifts were referenced to the signal of the methylene carbon of solid α-glycine at 43.1 ppm relative to tetramethylsilane at 0 ppm. Where there was insufficient material to fill a 4 mm outer diameter rotor unfilled volume was taken up by PTFE tape. Number of scans acquired depended on available sample and was generally between 10,000 and 100,000.
Liquid-state NMR
All 13C experiments were performed in deuterochlorofom (C2HCl3) using standard methodology on a Bruker 9.4 Tesla Avance-500 standard bore spectrometer at frequencies of 500.1 MHz (1H) and 125.6 MHz (13C). Standard pulse-acquire with continuous broadband decoupling was used (π/6 excitation pulses, repetition time 2.25 s). Chemical shifts were referenced to the central component of the deuterochloroform triplet at 77.23 ppm relative to tetramethylsilane at 0 ppm.
RESULTS
Vascular calcifications
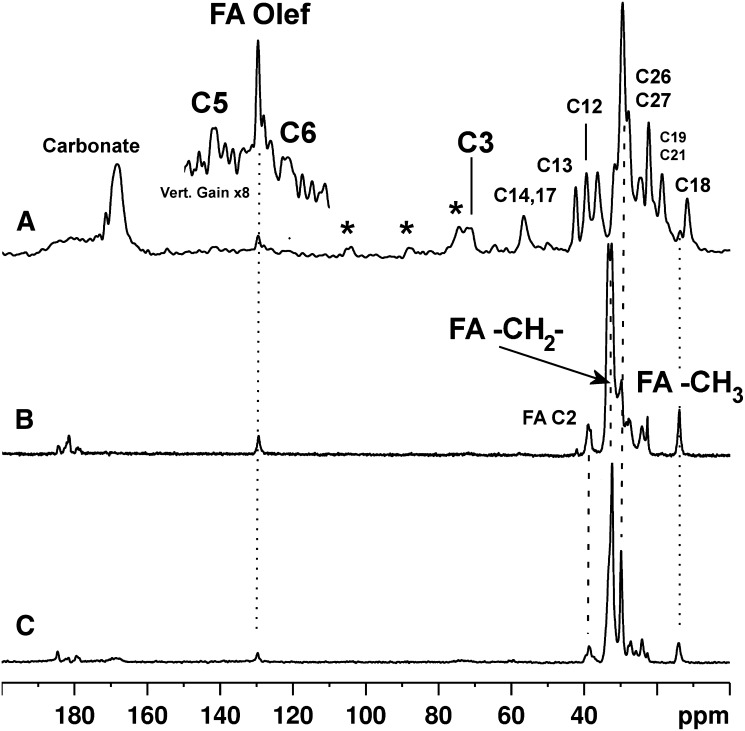
13C CP-MAS spectra of mineral from a calcified human intimal plaque (carotid) and from a human medial (femoral) calcification are shown in Fig. 1A, B, respectively. The spectrum of the mineral from the intimal calcification shows signals from cholesterol-related compounds, fatty acids, and carbonate substituted into the hydroxyapatite matrix. In contrast, the spectrum of mineral from the one medial calcification shown only displays signals from fatty acids and no detectable signal from cholesterol. It was pos sible to digest and interrogate a total of four intimal calcifications, one of which was obtained by pooling three separate earlier samples prior to sodium hydroxide and sodium hypochlorite digestion. The 13C spectra from each were very similar (see supplementary Fig. I). Results for medial calcifications were less consistent because of the presence of both medial and intimal calcifications in the vessel walls of most patients and because of our inability to separate these layers, with the exception of the sample shown in Fig. 1B. Probably because of this, there is more variation across the spectra from the medial calcifications with respect to the relative proportions of cholesterol to fatty acid (supplementary Fig. II), but in all cases, there is less cholesterol relative to fatty acid than in any of the pure intimal calcifications. To interrogate more samples of pure medial calcifications uncontaminated by intimal calcifi cation, we obtained samples from nonprimate species (equine and bovine), which are prone to medial rather than intimal vascular calcification (23–25). A typical 13C CP-MAS solid-state NMR spectrum of an equine medial calcification is shown in Fig. 1C. There is much less intersample variation in the 13C NMR spectral profile among the nonhuman medial calcifications (shown in supplementary Fig. III). With the exception of a signal of low and variable intensity from mineral carbonate ion, all the spectra are dominated by fatty acid signals with no detectable signals from cholesterol.
Fig. 1.
13C solid-state NMR spectra of mineral from (A) human intimal, (B) human medial, and (C) equine medial calcifications. Some key signal assignments appear on the spectra. Where present, the signals from small quantities of filter paper cellulose are indicated by asterisks. C, cholesterol/cholesterol ester; FA, fatty acid; Olef, olefinic.
Bone mineral
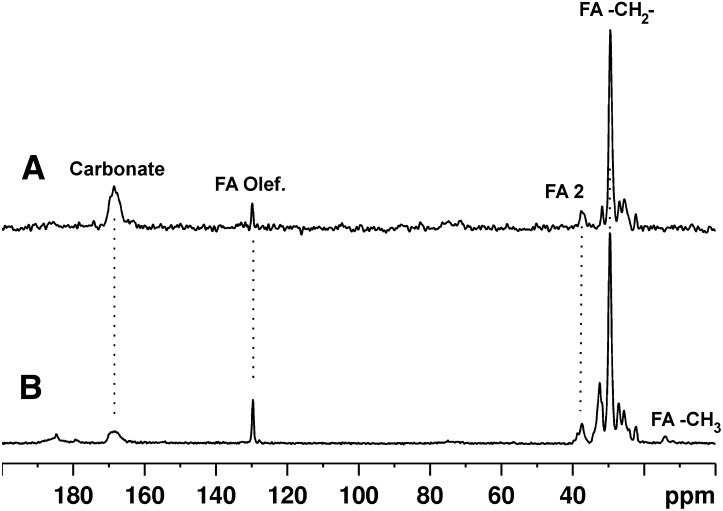
To interrogate the properties of nonpathological mineralized material, several samples of equine bone and a sample of adult human bone were also digested with sodium hydroxide and sodium hypochlorite to free the mineral from the collagenous organic matrix. 13C CP-MAS spectra from a human sample and an equine bone mineral sample are shown in Fig. 2A, B, respectively. They are very similar to each other and to five other equine bone samples (supplementary Fig. IV), with the exception of large variations in the proportion of mineral carbonate ion.
Fig. 2.
13C solid-state NMR spectra of mineral from (A) human bone and (B) equine bone.
Organic solvent extraction
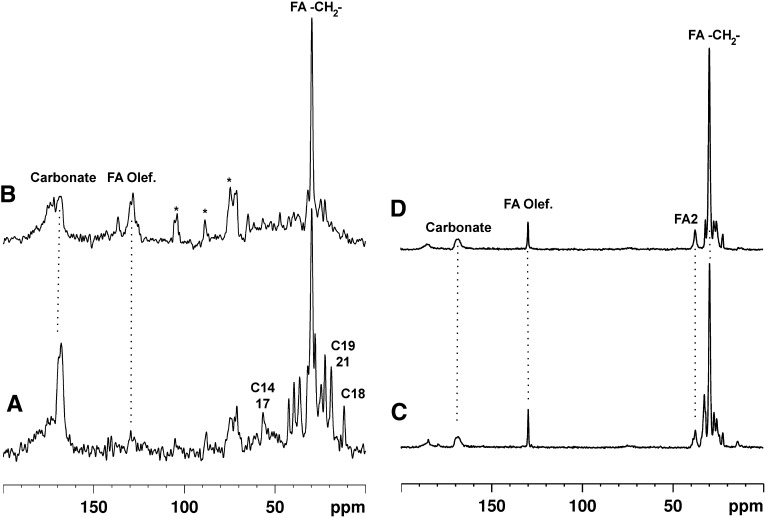
Changes in the spectral characteristics of intimal plaque mineral and bone on chloroform/methanol reflux are exemplified in Fig. 3. Reflux considerably reduces the signals due to cholesterolic compounds in intimal calcifications relative to those from fatty acids. In contrast, the content and proportions of lipid in bone hardly change on reflux. Liquid-state 13C spectra of the compounds released from the calcifications are shown in Fig. 4.
Fig. 3.
13C solid-state NMR spectra of mineral from a human intimal calcification (A) before and (B) after reflux in 1:1 chloroform:methanol, compared with equine bone mineral before (C) and (D) after the same treatment. FA, fatty acid; FA2, fatty acid 2-carbon.
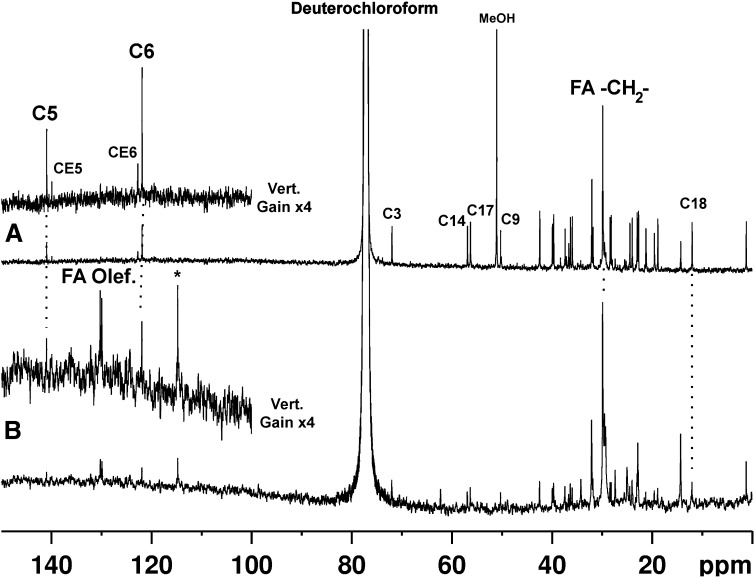
Fig. 4.
13C liquid-state NMR spectra of material released by reflux in 1:1 chloroform:methanol from (A) human intimal and (B) equine bone mineral.
DISCUSSION
Mineral from vascular calcifications closely resembles bone hydroxyapatite by X-ray powder diffraction and 31P NMR (22), consistent with the biological mechanistic parallels between normal osteogenesis and pathological vascular mineralization (26, 27). However, our work shows that, after chemical removal of the collagenous organic matrix, intimal atherosclerotic calcifications, on the one hand, and medial calcifications and bone, on the other, are strikingly different. All three mineral types produce 13C NMR signals, indicating the presence of partly unsaturated (signal at ca. 130 ppm) long-chain fatty acids on or in the mineral. However, stripping the collagenous matrix from intimal calcifications leaves significant amounts of cholesterol and cholesteryl esters, which can be removed by reflux in chloroform/methanol. Our spectral assignments are based on literature, e.g., Kroon et al. (19), and comparison with pure model compounds (see supplementary Figs. V and VI). Head group and glyceryl signals from phospholipids, e.g., in MVs or lipoprotein particles, such as phosphatidylcholine and phosphatidylserine, expected between ca. 50 and 70 ppm, are not observed. These phospholipids may not be present in detectable quantities, or signals may be broadened by chemical and structural heterogeneity and immobilization. Although reflux removes cholesterylic signals from intimal calcifications, it leaves signals from long-chain fatty acids. In the pure medial calcifications and bone mineral, reflux mobilizes a small proportion of the total lipid, leaving strong fatty acid signals. Thus, NMR shows all three mineral types associated with pools of mineral-entrained fatty acids resistant to alkali, oxidizing agents, and lipid-solubilizing solvents.
After reflux, the intimal plaque mineral lipid spectra now resemble those of pure medial calcifications and bone mineral. This argues for two distinct pools of hydrophobic lipid in or associated with the intimal mineral: one cholesterylic and accessible to organic solvents and possibly a remnant of lipoprotein precursors, and the other less accessible to organic extraction and consisting predominantly of fatty acyl structures.
The 13C NMR fatty acid signals for straight-chain fatty acids occur at 180 ppm for the COOH acid carbon, with the chain carbons occurring between 22 and 34 ppm, plus the end methyl group at 14–15 ppm, with very little variation in this distribution of signals (28) except for the unsaturated C = C carbons, which routinely occur close to 130 ppm. However, the spectra of both intimal and medial mineral and bone show strong signals at 38–39 ppm and ca. 183–185 ppm. These arise from 2-methyl-branched fatty acids (28), of which pristanic acid is a typical example found in mammalian tissues. Formed from metabolism of other branched fatty acids, such as phytanic acid in cell perioxisomes, the role of such branched-chain fatty acids in mammalian physiology is not clear, although they are clearly an energy source; pristanic acid can be taken up by mitochondria and metabolized to carbon dioxide and water. Crucially though, branched fatty acids are not common components of phospholipid membranes, so it seems unlikely that this is the source of this lipid component in these calcified tissues.
Interestingly, a detailed atomic-force microscopy study (29) found an abundance of small (∼145 nm) lipid particles decorating collagen microfibrils in bone. The nature of these could not be identified for certain, but their size led the authors of that study to propose that they were lipoprotein particles. Methyl-branched fatty acids are transported in lipoprotein particles, so this would be consistent with our finding here. We can infer that this fatty acid pool must be entrained within mineral structures. These fatty acids may be relics of lipoprotein particles, which give rise to a host of questions about their possible role in matrix calcification.
The liquid-state NMR spectra reveal the composition of the lipids released by reflux. In the refluxate from intimal plaque mineral (Fig. 4A), each of the olefinic carbons of unesterified and esterified cholesterol gives rise to a pair of well-resolved distinct signals (C5 and C6, and CE5 and CE6, respectively, in Fig. 4). Unesterified cholesterol predominates over esterified cholesterol in the organic solvent-exposed lipid pool, at least after digestion.
The much weaker spectrum of the refluxate from bone mineral shows a relatively much higher proportion of fatty acid signals, such as from long-chain terminal methyl groups (ca. 14 ppm) and from olefinic carbons (at ca. 130 ppm). There are no detectable signals around 62 and 69 ppm (which would correspond to the glyceryl carbons of intact triglycerides and phosphatidyl glycerides); it is, of course, possible that the sample preparation has leached these components out of the mineral deposits. Liquid-state spectra of model cholesterylic compounds and glycerides are shown for comparison in supplementary Figs. VII and VIII, respectively. We note there are signals consistent with both 2-methyl (37–40 ppm) and n-methyl-branched fatty acids [n ≠ 2 (36–38, 42 ppm)]. These signals arise from carbons with or next to methyl substitutions, and the net intensity of these signals compared with that between 29 and 30 ppm, which represents the remaining fatty acid chain carbons, suggests that such carbons are relatively abundant; in other words, that there is a preponderance of methyl-substituted fatty acids associated with mineral. A spectrum of pristanic acid, a model methyl branched fatty acid, is shown in supplementary Fig. IX. Neutral (zwitterionic) phospholipids predominate over acidic phospholipids in normally calcifying and calcified tissue; the former, but not the latter, are extractable without demineralization (30, 31), and our results are consistent with this. What is equally interesting is that calcified vascular tissue appears to contain a similar distribution of mineral-associated fatty acids, suggesting that these fatty acids play a role in calcification. A possible synergy between cholesterylic compounds and phospholipids in supporting calcification is supported by model liposome-hydroxyapatite systems (32); it would be interesting to examine how cholesterylic lipids and lipids containing branched-chain fatty acids compare in this regard.
In conclusion, intimal atherosclerotic vascular calcifications contain a pool of cholesterylic lipids and a pool of fatty acyl lipids that resist strong alkali and chemical oxidation. The cholesterylic, but not the fatty acyl, population is readily mobilized by organic solvents. In medial vascular calcifications, however, alkali and oxidation leave a predominantly fatty acyl lipid pool closely resembling that in bone mineral and the fatty acyl lipids left behind after organic solvent reflux in intimal calcifications. It is likely that this predominantly fatty acyl pool is common to all three calcification types (intimal, medial vascular, and bone). It is characterized by a relative abundance of methyl-branched fatty acids, and we tentatively suggest that these may represent the remnants of lipoprotein particles. The cholesterylic lipids observed only in intimal calcifications may play an independent role in mineralization, or their presence may be merely a coincidental function of the abundance of such lipids in atherosclerotic lesions. Thus, solid-state NMR shows that participation of fatty acyl lipids in calcification is another factor common to all three families of mineralized tissue, in spite of their different orthotopic or ectopic origins, locations, and pathological significance.
Supplementary Material
Footnotes
Abbreviations:
- MAS
- magic angle spinning
- MV
- matrix vesicle
- VSMC
- vascular smooth muscle cell
This work was supported by the British Heart Foundation, the National Institute for Health Research, and the Biotechnology and Biological Sciences Research Council.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of nine figures.
REFERENCES
- 1.Motoyama S., Kondo T., Sarai M., Sugiura A., Harigaya H., Sato T., Inoue K., Okumura M., Ishii J., Anno H., et al. 2007. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J. Am. Coll. Cardiol. 50: 319–326 [DOI] [PubMed] [Google Scholar]
- 2.Demer L., Tintut Y. 2010. The bone-vascular axis in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 19: 349–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchell G. F., Hwang S. J., Vasan R. S., Larson M. G., Pencina M. J., Hamburg N. M., Vita J. A., Levy D., Benjamin E. J. 2010. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 121: 505–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Golub E. E. 2009. Role of matrix vesicles in biomineralization. Biochim. Biophys. Acta. 1790: 1592–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kapustin A., Shanahan C. M. 2009. Targeting vascular calcification: softening-up a hard target. Curr. Opin. Pharmacol. 9: 84–89 [DOI] [PubMed] [Google Scholar]
- 6.Reynolds J. L., Joannides A. J., Skepper J. N., McNair R., Schurgers L. J., Proudfoot D., Jahnen-Dechent W., Weissberg P. L., Shanahan C. M. 2004. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. J. Am. Soc. Nephrol. 15: 2857–2867 [DOI] [PubMed] [Google Scholar]
- 7.Hsu H. H. T., Camacho N. P. 1999. Isolation of calcifiable vesicles from human atherosclerotic aortas. Atherosclerosis. 143: 353–362 [DOI] [PubMed] [Google Scholar]
- 8.Genge B. R., Wu L. N. Y., Wuthier R. E. 2008. Mineralization of annexin-5-containing lipid-calcium-phosphate complexes - Modulation by varying lipid composition and incubation with cartilage collagens. J. Biol. Chem. 283: 9737–9748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu L. N. Y., Genge B. R., Wuthier R. E. 2008. Analysis and molecular modeling of the formation, structure, and activity of the phosphatidylserine-calcium-phosphate complex associated with biomineralization. J. Biol. Chem. 283: 3827–3838 [DOI] [PubMed] [Google Scholar]
- 10.Stegemann C., Drozdov I., Shalhoub J., Humphries J., Ladroue C., Didangelos A., Baumert M., Allen M., Davies A. H., Monaco C., et al. 2011. Comparative lipidomics profiling of human atherosclerotic plaques. Circ. Cardiovasc. Genet. 4: 232–242 [DOI] [PubMed] [Google Scholar]
- 11.Hirsch D., Azoury R., Sarig S., Kruth H. S. 1993. Colocalization of cholesterol and hydroxyapatite in human atherosclerotic lesions. Calcif. Tissue Int. 52: 94–98 [DOI] [PubMed] [Google Scholar]
- 12.Laird D. F., Mucalo M. R., Yokogawa Y. 2006. Growth of calcium hydroxyapatite (Ca-HAp) on cholesterol and cholestanol crystals from a simulated body fluid: a possible insight into the pathological calcifications associated with atherosclerosis. J. Colloid Interface Sci. 295: 348–363 [DOI] [PubMed] [Google Scholar]
- 13.Prassl R., Laggner P. 2009. Molecular structure of low density lipoprotein: current status and future challenges. Eur. Biophys. J. 38: 145–158 [DOI] [PubMed] [Google Scholar]
- 14.Skålén K., Gustafsson M., Rydberg E. K., Hulten L. M., Wiklund O., Innerarity T. L., Boren J. 2002. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature. 417: 750–754 [DOI] [PubMed] [Google Scholar]
- 15.Iyemere V. P., Proudfoot D., Weissberg P. L., Shanahan C. M. 2006. Vascular smooth muscle cell phenotypic plasticity and the regulation of vascular calcification. J. Intern. Med. 260: 192–210 [DOI] [PubMed] [Google Scholar]
- 16.Duer M. J., Friscic T., Proudfoot D., Reid D. G., Schoppet M., Shanahan C. M., Skepper J. N., Wise E. R. 2008. Mineral surface in calcified plaque is like that of bone: further evidence for regulated mineralization. Arterioscler. Thromb. Vasc. Biol. 28: 2030–2034 [DOI] [PubMed] [Google Scholar]
- 17.Hamilton J. A., Cordes E. H., Glueck C. J. 1979. Lipid dynamics in human low-density lipoproteins and human aortic tissue with fibrous plaques - study by high-field C-13 NMR-spectroscopy. J. Biol. Chem. 254: 5435–5441 [PubMed] [Google Scholar]
- 18.Kroon P. A., Seidenberg J. 1982. Organization of the core lipids of lipoproteins from normal and cholesterol-fed rabbits. A proton nuclear magnetic resonance study. Biochemistry. 21: 6483–6488 [DOI] [PubMed] [Google Scholar]
- 19.Kroon P. A., Quinn D. M., Cordes E. H. 1982. A carbon-13 nuclear magnetic resonance study of aortic lesions and cholesteryl ester rich lipoproteins from atherosclerotic rabbits. Biochemistry. 21: 2745–2753 [DOI] [PubMed] [Google Scholar]
- 20.Guo W., Hamilton J. A. 1995. A multinuclear solid-state NMR study of phospholipid-cholesterol interactions. Dipalmi toylphosphatidylcholine-cholesterol binary system. Biochemistry. 34: 14174–14184 [DOI] [PubMed] [Google Scholar]
- 21.Guo W., Morrisett J. D., Lawrie G. M., DeBakey M. E., Hamilton J. A. 1998. Identification of different lipid phases and calcium phosphate deposits in human carotid artery plaques by MAS NMR spectroscopy. Magn. Reson. Med. 39: 184–189 [DOI] [PubMed] [Google Scholar]
- 22.Guo W., Morrisett J. D., DeBakey M. E., Lawrie G. M., Hamilton J. A. 2000. Quantification in situ of crystalline cholesterol and calcium phosphate hydroxyapatite in human atherosclerotic plaques by solid-state magic angle spinning NMR. Arterioscler. Thromb. Vasc. Biol. 20: 1630–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cranley J. J. 1983. Focal medial calcification of the pulmonary-artery -- a survey of 1066 horses. Equine Vet. J. 15: 278–280 [DOI] [PubMed] [Google Scholar]
- 24.Arroyo L. G., Hayes M. A., DeLay J., Rao C., Duncan B., Viel L. 2008. Arterial calcification in race horses. Vet. Pathol. 45: 617–625 [DOI] [PubMed] [Google Scholar]
- 25.Van Vleet J. F., Ferrans V. J.2007. Cardiovascular system. In Pathologic Basis of Veterinary Pathology. M. D. McGavin and J. F. Zachary, editors. Mosby, St. Louis. 599–611.
- 26.Anderson H. C. 1983. Calcific diseases. A concept. Arch. Pathol. Lab. Med. 107: 341–348 [PubMed] [Google Scholar]
- 27.Sage A. P., Tintut Y., Demer L. L. 2010. Regulatory mechanisms in vascular calcification. Nat. Rev. Cardiol. 7: 528–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gunstone F. D. 1993. High-resolution C-13 NMR study of synthetic branched-chain acids and of wool wax acids and isostearic acid. Chem. Phys. Lipids. 65: 155–163 [Google Scholar]
- 29.Xu S., Yu J. J. 2006. Beneath the minerals, a layer of round lipid particles was identified to mediate collagen calcification in compact bone formation. Biophys. J. 91: 4221–4229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Irving J. T., Wuthier R. E. 1968. Histochemistry and biochemistry of calcification with special reference to the role of lipids. Clin. Orthop. Relat. Res. 56: 237–260 [PubMed] [Google Scholar]
- 31.Wuthier R. E. 1968. Lipids of mineralizing epiphyseal tissues in the bovine fetus. J. Lipid Res. 9: 68–78 [PubMed] [Google Scholar]
- 32.Wuthier R. E. 1975. Effect of phospholipids on the transformation of amorphous calcium phosphate to hydroxapatite in vitro. Calcif. Tissue Res. 19: 197–210 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.