Abstract
Accumulation of excess lipids is associated with heart failure. The effects of transgenic expression of diacylglycerol acyl transferase 1 (DGAT1) in cardiomyocytes is controversial. We explored whether mice expressing DGAT1 via the myosin heavy chain (MHC) promoter develop heart dysfunction with aging or after crossing with mice over expressing peroxisome proliferator-activated receptor γ (PPARγ) in the heart. MHC-DGAT1 transgenic mice had increased heart triglyceride but no evidence of heart dysfunction, even up to age 12 months. The MHC-DGAT1 transgene improved heart dysfunction and survival of MHC-PPARγ-expressing transgenic mice. Both diacylglycerol and ceramide levels in the heart were reduced by this cross, as were the levels of several mRNAs of genes involved in lipid metabolism. There were fewer large lipid droplets in MHC-DGAT1×MHC-PPARγ mice compared with MHC-PPARγ, but total lipid content was not changed. Therefore, overexpression of DGAT1 is not toxic to the heart but reduces levels of toxic lipids and improves lipotoxic cardiomyopathy. Moreover, the beneficial effects of DGAT1 illustrate the interrelationship of several lipid metabolic pathways and the difficulty of assigning benefit to an isolated change in one potentially toxic lipid species.
Keywords: triglyceride, ceramide, cardiomyopathy
Fatty acids (FA) are the principal fuel of normal adult hearts and supply ∼70% of the ATP necessary for cardiac function (1). When there is an imbalance between lipid uptake and oxidation, lipids accumulate in organs. And when they exceed the storage capacity of each organ, they create a group of diseases classified as lipotoxicities. These diseases include nonalcoholic fatty liver disease, type 2 diabetes, and metabolic cardiomyopathy (2).
The major storage form of lipids within the body is triglyceride (TG), a lipid that is believed to be biologically inert (3). Diacylglycerol (DAG) acyl transferase 1 (DGAT1) is one of two enzymes that catalyze the final step in TG synthesis. Dgat1 belongs to a gene family that includes acyl-CoA:cholesterol acyltransferases 1 and 2 (4). DGAT1 has been overexpressed in mice to elucidate the function of this gene in the development of metabolic disease. In skeletal muscle, overexpression of DGAT1 increased TG stores but reduced DAG and ceramide, increased FA oxidation, and improved insulin sensitivity (5); this mimics the biology of chronic exercise and is sometimes referred to as the “athlete paradox” (6, 7). Overexpression of DGAT1 in adipose tissue using an AP2 promoter led to greater obesity but not insulin resistance in C57BL6 mice (8). FVB mice, however, still had insulin resistance (9), a finding that might reflect genotype differences or variation in macrophage DGAT1 expression with the AP2 promoter. Overexpression of DGAT1 in the liver (10) and heart (11) increased TG content of those tissues but did not cause insulin resistance or heart dysfunction. Similarly, overexpression of DGAT1 in macrophages ameliorated FA-induced inflammation and high-fat feeding-induced insulin resistance (12). These data support the hypothesis that conversion of intermediary toxic lipids to TG via DGAT1 can be a detoxifying process (3).
Lipotoxic cardiomyopathy models have been created in which lipid oxidation is insufficient to balance lipid uptake, leading to increased accumulation of TG, free FA (FFA), and other potentially toxic lipids, and in some cases, to premature death (13, 14). The peroxisome proliferator-activated receptors (PPAR) are a group of three nuclear receptor proteins that work as transcription factors regulating the expression of genes involved in lipid oxidation and uptake (15). Transgenic cardiomyocyte overexpression of PPARα or PPARγ leads to lipotoxic cardiomyopathy (13, 16). PPARδ does not cause increased lipid accumulation and does not cause cardiomyopathy (17), probably because it also induces angiopoietin-like protein 4 expression, which inhibits lipoprotein lipase and reduces heart lipid uptake (18). Although there is no obvious human cardiac muscle phenotype associated with activation of PPARα, PPARγ agonist treatment causes symptomatic heart failure either due to greater fluid retention or direct effects on the heart (19).
One unanswered question is which lipid(s) are toxic. The most obvious candidates are FAs, fatty acyl CoAs, DAGs, and ceramides. A second issue is whether a common lipid-altering process will alleviate lipotoxicity in more than one model. We had created mice with heart-specific DGAT1 expression using the myosin heavy chain (MHC) promoter and showed that the MHC-DGAT1 transgene increased the survival of mice overexpressing acyl CoA synthetase 1 (ACS1). This improvement was associated with better mitochondrial function, increased FA oxidation, and decreased apoptosis (11). However, Glenn et al. reported another MHC-DGAT1 transgenic model that displayed a late cardiomyopathy with fibrosis in the heart (20). This report suggested that the level of expression, the mouse genetic background, or technical issues related to the type of construct used could affect cardiomyocyte biology. Thus, the effects of DGAT1 expression in the heart are controversial. To determine whether our MHC-DGAT1 transgene would improve a second lipotoxic model and to determine whether this transgene led to heart dysfunction in older mice, we studied MHC-DGAT1 transgenic mice on the wild-type (WT) and MHC-PPARγ background. In addition, we obtained more comprehensive lipidomics in this model.
MATERALS AND METHODS
Mice and diets
Animal protocols were in compliance with accepted standards of animal care and were approved by the Columbia University Institutional Animal Care and Use Committee. Male mice were used in experiments unless otherwise indicated. WT C57BL/6J mice were purchased from the Jackson Laboratory. MHC-PPARγ and MHC-DGAT1 mice were created as described (11, 13). MHC-DGAT1 FVB mice were backcrossed to C57BL/6 background for six generations before use. Mice were housed in a barrier facility with 12 h light/12 h dark cycles and had ad libitum access to chow diet (5053 PicoLab Rodent Diet 20; Purina Mills).
Lipid measurements
To measure FFA, TG, DAG, and ceramide, lipids were first extracted from hearts using chloroform/methanol/HCl (v/v/v, 2:1:0.01) (5). Butylated hydroxytoluene (0.01%) was included in the extraction solution as an antioxidant, and [3H]triolein (0.25 μCi) was used as an internal control for TG recovery. TG and FA concentrations in lipid extracts were determined enzymatically with colorimetric kits (Sigma-Aldrich). DAG and ceramide levels were measured using a DAG kinase-based method. Lipids extracted from muscle specimens were dried under a stream of N2, redissolved in 7.5% octyl-β-D-glucoside containing 5 mM cardiolipin and 1 mM diethylenetriamine pentaacetate, in which DAG and ceramide are quantitatively phosphorylated to form 32P-labeled phosphatidic acid and ceramide-1-phosphate, respectively, which were then quantified. The reaction was carried out at room temperature for 30 min in 100 mM imidazole HCl, 100 mM NaCl, 25 mM MgCl2, 2 mM EGTA (pH 6.6), 2 mM DTT, 10 μg/100 μl DAG kinase (Sigma-Aldrich), 1 mM ATP, and 1 μCi/100 μl [γ-32P]ATP. The reaction was stopped by addition of chloroform/methanol (v/v, 2:1) and 1% HClO4, and lipids were extracted and washed twice with 1% HClO4. Lipids were resolved by thin layer chromatography (TLC, Partisil K6 adsorption TLC plates, Whatman catalog no. LK6D); mobile phase contained chloroform/methanol/acetic acid (v/v/v, 65:15:5). The bands corresponding to phosphatidic acid and ceramide-1-phosphate were identified with known standards, and silicon was scraped into a scintillation vial for radioactivity measurement. [3H]triolein bands from the same TLC plates were identified and quantified in the same way and were used as controls for lipid recovery. LC/MS analyses were performed as described previously (21).
LC/MS
Lipid analyses by LC/MS were performed as recently reported (21). Briefly, all solutions and solvents used for lipid extraction were precooled at 4°C. A 0.25 ml aliquot of 0.1 M KH2PO4 and 0.25 ml of 2-propanol were added to the weighed, frozen tissue aliquots (15–20 mg). A 20 μl aliquot of 50 μM heptadecanoyl-CoA, dissolved in 0.1 M KH2PO4/2-propanol/acetonitrile (1:1: 2), was added per sample as internal standard. The sample was homogenized using a bead beater (BioSpec) using 1.0 mm glass beads. A 30 μl aliquot of saturated aqueous ammonium sulfate and 0.5 ml of acetonitrile were added to the homogenate, and the mixture was vortexed well. After centrifugation at 2,500 g for 10 min, the supernatant was transferred to MS vials for analyses. All analyses were carried out on a Waters Xevo TQ MS ACQUITY UPLC system (Waters) controlled by Mass Lynx Software 4.1.
Electron microscopy
Left ventricles from 16 h fasted 3-month-old mice were isolated, fixed with 2.5% glutaraldehyde in 0.1M Sorenson's buffer (pH 7.2), and treated with 1% OsO4 also in Sorenson's buffer for 1 h. After dehydration and embedment in Lx-112 (Ladd Research Industries), thin sections (60 nm) were cut using a MT-7000 ultramicrotome, stained with uranyl acetate and lead citrate, and examined under a JEOL JEM-1200 EXII electron microscope. Pictures were captured by an ORCA-HR digital camera (Hamamatsu) and recorded with an AMT Image Capture Engine.
Real-time PCR
Total RNA was extracted using a Trizol kit from Invitrogen. 1 μg of RNA was initially treated with DNase I. The RNA samples were then reverse-transcribed using the SuperScript III First-Strand Synthesis System for RT-PCR. Real-time amplification was performed using iQ SYBR Green Supermix (Bio-Rad). Primers used for PCR amplification are listed in Table I. Analysis was performed using iCycler iQ Real-Time Detection System software (Bio-Rad).
TABLE 1.
Ceramide composition
| Ceramide (nmol/g) | WT | MHC-DGAT1 | MHC-PPARγ | MHC-DGAT1×MHC-PPARγ |
| C14:0 | 0.027 ± 0.005 | 0.020 ± 0.006 | 0.034 ± 0.013 | 0.019 ± 0.002ab |
| C16:0 | 2.0 ± 0.4 | 2.25 ± 0.39 | 3.26 ± 0.74 | 2.39 ± 0.58c |
| C18:0 | 1.89 ± 0.57 | 2.65 ± 0.77 | 3.32 ± 0.81 | 1.98 ± 0.32a,c |
| C18:1 | 0.069 ± 0.017 | 0.072 ± 0.016 | 0.099 ± 0.027 | 0.070 ± 0.018b |
| C20:0 | 0.081 ± 0.070 | 0.027 ± 0.007 | 0.040 ± 0.014 | 0.037 ± 0.012 |
| C20:1 | 0.013 ± 0.011 | 0.003 ± 0.001 | 0.004 ± 0.001 | 0.004 ± 0.001b |
| C22:0 | 0.139 ± 0.109 | 0.055 ± 0.018 | 0.075 ± 0.019 | 0.069 ± 0.022 |
| C22:1 | 0.021 ± 0.019 | 0.006 ± 0.002 | 0.011 ± 0.003 | 0.013 ± 0.001 |
| C24:0 | 2.12 ± 0.77 | 1.31 ± 0.29 | 1.88 ± 0.54 | 1.56 ± 0.18b |
| C24:1 | 1.00 ± 0.76 | 0.47 ± 0.13 | 0.94 ± 0.24 | 0.82 ± 0.15 |
| C26:0 | 0.035 ± 0.014 | 0.036 ± 0.001 | 0.031 ± 0.012 | 0.023 ± 0.010 |
| C26:1 | 0.034 ± 0.010 | 0.026 ± 0.004 | 0.034 ± 0.009 | 0.023 ± 0.004ab |
| Total | 7.44 ± 1.69 | 7.11 ± 1.03 | 9.42 ± 2.13 | 7.02 ± 1.07ab |
P< 0.05 versus MHC-PPAR of LSD t-test followed one-way ANOVA, n = 4–6.
P< 0.05 of one-way ANOVA, n = 4–6.
P< 0.01 of one-way ANOVA, n = 4–6.
Echocardiography
Two-dimensional echocardiography was performed using a high-resolution imaging system with a 30-MHz imaging transducer (Vevo 770; VisualSonics) in unconscious 3- to 4-month-old mice. The mice were anesthetized with 1.5–2% isoflurane and thereafter maintained on 1–1.5% isoflurane throughout the procedure. Two-dimensional echocardiographic images were obtained and recorded in a digital format. Images were analyzed offline by a researcher blinded to the murine genotype. Left ventricular end-diastolic dimension (LVDd) and left ventricular end-systolic dimension (LVDs) were measured. Percentage fractional shortening (FS), which quantifies contraction of the ventricular wall and is an indication of muscle function, was calculated as % FS = ([LVDd − LVDs] / LVDd) × 100.
Western blotting
Tissue (10–30 mg) was homogenized in lysis buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% Nonidet P40, 0.5% sodium deoxycholate, and 1 protease inhibitor cocktail tablet (Roche)/25–50 ml. Cell lysates (50 μg per sample) obtained after centrifugation at 15,000 g for 10 min at 4°C. To obtain membrane and cytosol fractions, muscle tissues were homogenized in the homogenization buffer containing 20 mM HEPES, pH 7.4, 1 mM CaCl2, 1 mM MgCl2, 5 mM Na3VO4, 10 mM NaF, 1 mM DTT, and a cocktail of protease inhibitors. One-fourth volume of 30% sucrose was added to the samples immediately following homogenization. The mixtures were then centrifuged at 1,500 g for 10 min at 4°C. The supernatants were collected and centrifuged at 150,000 g for 1 h at 4°C. The membrane pellets were homogenized and resuspended in a 400 μl buffer containing 20 mM HEPES, pH 7.4, 0.25 M sucrose, 5 mM Na3VO4, 10 mM NaF, 1 mM DTT, and protease inhibitors. The cytosol fraction was obtained by concentrating the supernatants by acetone precipitation. Proteins (50 μg) were resolved by SDS-PAGE and then transferred onto nitrocellulose membranes. Immunoblotting was carried out using the following primary antibodies: PKC-α, PKC-δ, Glut1, P-IRS1 307, total-IRS1, P-AKT 473, total-AKT, and PDK4 (Cellsignaling).
Statistics
All data are presented as mean ± SD, unless indicated otherwise. Comparisons between two groups were performed using unpaired two-tailed Student t-test; comparisons more than two groups were performed with one-way ANOVA with post hoc LSD t-test. A P value less than 0.05 was used to determine statistical significance.
RESULTS
MHC-DGAT1 improves survival of MHC-PPARγ mice
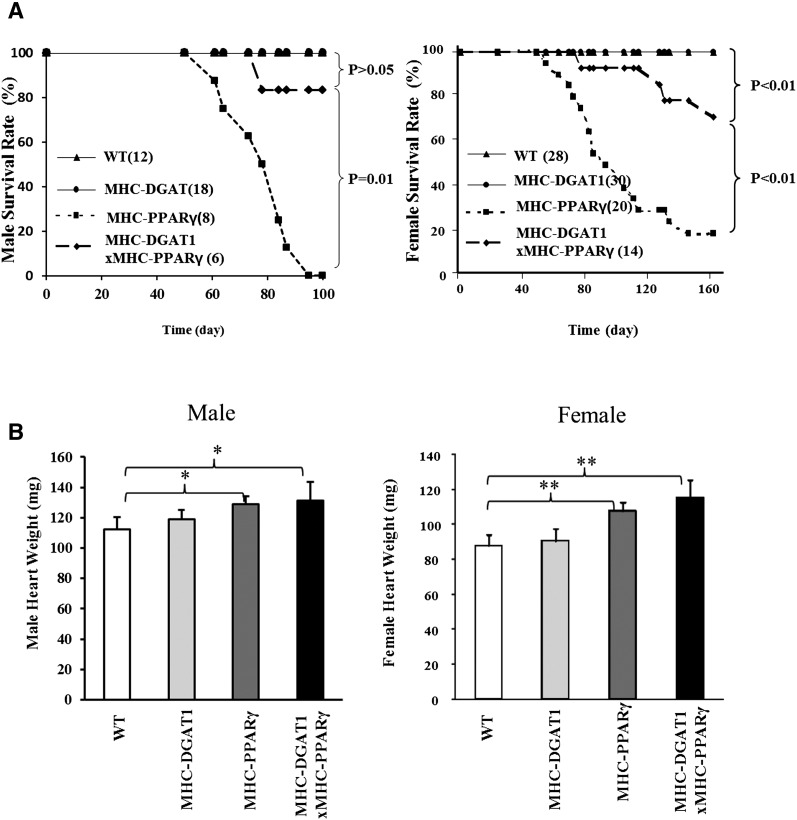
To test whether DGAT1 was generally beneficial for lipotoxic cardiomyopathy, we bred the MHC-DGAT1 transgene onto the C57BL/6 background and then crossed these mice with MHC-PPARγ transgenic mice. MHC-DGAT1×MHC-PPARγ mouse survival was significantly improved in both males and females (log-rank test, P ≤ 0.01 in both males and females) (Fig. 1A) compared with MHC-PPARγ mice.
Fig. 1.
Survival rate and heart weight in WT, MHC-DGAT1, MHC-PPARγ, and MHC-DGAT1×MHC-PPARγ mice. (A) Male and female MHC-PPARγ mouse survival was increased by the MHC-DGAT1 transgene (Log-rank test, P ≤ 0.01). (B) Male and female heart weights were significantly increased in MHC-PPARγ and MHC-DGAT1×MHC-PPARγ mice (one-way ANOVA, *P < 0.05, **P < 0.01 of LSD t-test).
Heart weights did not correlate with heart dysfunction. MHC-PPARγ mice had significantly increased heart weights (by ∼15–20% in both males and females, one-way ANOVA, P < 0.05), whereas MHC-DGAT1 mouse heart weights did not differ from WT littermates (Fig. 1B). MHC-DGAT1 did not reduce heart weight of the MHC-PPARγ mice. The reason for this appeared to be a lack of reduction in stored lipids. In male mice, heart TG was ∼10% increased in MHC-DGAT1, ∼23% increased in MHC-PPARγ mice, and ∼15% increased in MHC-DGAT1×MHC-PPARγ mice compared with their WT littermates (Fig. 2A); the difference between the MHC-PPARγ and MHC-DGAT1×MHC-PPARγ mice was not statistically different. This result indicated that the improvement of survival of MHC-DGAT1×MHC-PPARγ mice was probably not due to reduced TG content. Because more females were maintained for the survival curve, male mice were used to determine the mechanism of the rescue.
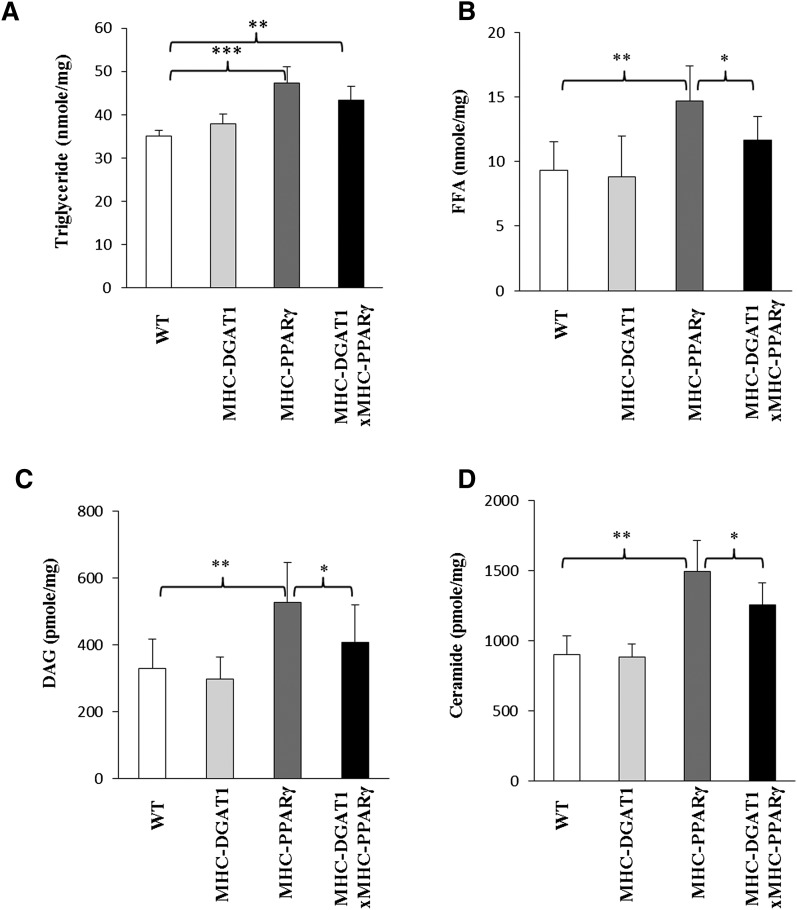
Fig. 2.
Lipid content in WT, MHC-DGAT1, MHC-PPARγ, and MHC-DGAT1×MHC-PPARγ mice. (A) TG was increased in MHC-PPARγ and MHC-DGAT1×MHC-PPARγ mice compared with their WT litter mates (n = 5–6, one-way ANOVA, **P < 0.01, ***P < 0.001 of LSD t-test). (B–D) Increased FFA, DAG, and ceramide levels in MHC-PPARγ mice were reduced by MHC-DGAT1 transgene (n = 5–6, one-way ANOVA, *P < 0.05, **P < 0.01 of LSD t-test).
Some potential toxic FA derivatives were then measured in the cardiac muscle of these mice. In male mice, FFA, DAG, and ceramide were increased ∼57%, ∼78%, and ∼66%, respectively, in MHC-PPARγ mice compared with WT littermates (P < 0.01). These levels were decreased in MHC-DGAT1×MHC-PPARγ mice; FFA decreased ∼20%, DAG ∼27%, and ceramide ∼16% compared with MHC-PPARγ mice (one-way ANOVA, P ≤ 0.01) (Fig. 2BndashD). The reduction in ceramides was due to normalization of C14:0, C18:0, and C26:1 levels to the amounts found in the WT littermates (Table 1). Also C24 ceramides were reduced in the double transgenic mice. In contrast, most acyl CoAs, which were decreased by the MHC-PPARγ gene, remained low (Table II).
TABLE 2.
Male gene expression
| Gene | WT | MHC-DGAT1 | MHC-PPARγ | MHC-DGAT1×MHC-PPARγ |
| Genes involved in lipid metabolism | ||||
| Ppara | 100 ± 24 | 150 ± 33 | 160 ± 87 | 117 ± 20 |
| Ppard | 100 ± 54 | 181 ± 48 | 74 ± 36 | 70 ± 22 |
| Pparg | 100 ± 13 | 184 ± 61 | 1360 ± 526 | 1150 ± 298c |
| Atgl | 100 ± 36 | 198 ± 62 | 258 ± 134 | 72 ± 19a,b |
| Cd36 | 100 ± 36 | 176 ± 35 | 246 ± 55 | 82 ± 51a,c |
| Aco | 100 ± 43 | 161 ± 48 | 202 ± 107 | 59 ± 34a,b |
| Pdk4 | 100 ± 34 | 265 ± 26 | 75 ± 30 | 45 ± 7b |
| Cpt1 | 100 ± 29 | 146 ± 41 | 194 ± 72 | 161 ± 64b |
| Lpl | 100 ± 19 | 118 ± 26 | 95 ± 20 | 105 ± 14 |
| Genes involved in lipid droplet formation | ||||
| Adrp | 100 ± 17 | 126 ± 21 | 155 ± 39 | 137 ± 30b |
| Tip47 | 100 ± 28 | 471 ± 173 | 79 ± 23 | 211 ± 68b |
| Oxpat | 100 ± 51 | 303 ± 141 | 155 ± 42 | 580 ± 217a,c |
| Genes involved in heart failure | ||||
| Bnp | 100 ± 46 | 116 ± 12 | 187 ± 60 | 71 ± 41a,b |
P< 0.05 versus MHC-PPAR of LSD t-test followed one-way ANOVA, n = 4–5.
P< 0.05 of one-way ANOVA, n = 4–5.
P< 0.01 of one-way ANOVA, n = 4–5.
MHC-PPARγ mice die from ventricular arrhythmias (22). Although a number of lipids could alter ion channels, in vitro and animal experiments suggest that n-3 PUFAs directly influence atrial and ventricular myocyte electrophysiology, potentially mediated by effects on membrane ion channels or cell-cell connexins (23, 24). C20:5 acyl CoAs were reduced in MHC-PPARγ transgenic mice but recovered to normal levels in MHC-DGAT1×PPARγ mice (Table II).
Heart function was improved in MHC-DGAT1timesMHC-PPARγ mice
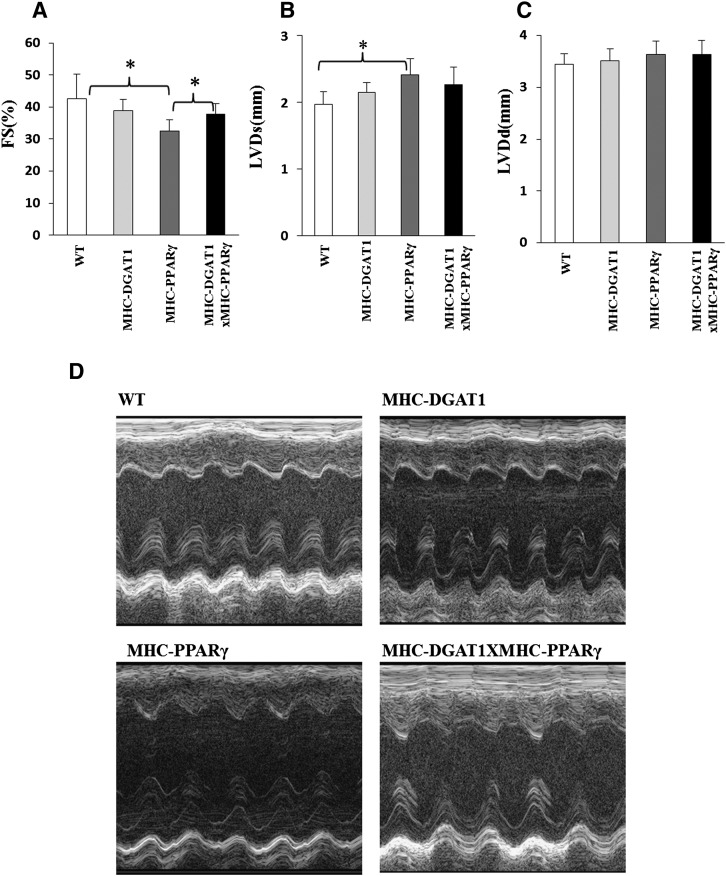
C57BL/6 MHC-DGAT transgenic mice did not show any heart dysfunction at 4 months of age. MHC-PPARγ transgenic mice had a ∼24% reduction in FS and ∼22% increase in LVDs compared with controls (Fig. 3A, B). FS was enhanced ∼15% in MHC-DGAT1×MHC-PPARγ animals compared with MHC-PPARγ mice, although the variability between mice led to no statistical change in LVDs or LVDd in these mice (one-way ANOVA, P < 0.05) (Fig. 3B, C). Fig. 3D shows representative echocardiography pictures.
Fig. 3.
Cardiac function in WT, MHC-DGAT1, MHC-PPARγ, MHC-DGAT1×MHC-PPARγ. (A) Fraction shorting (FS), (B) left ventricular systolic dimension (LVDs), and (C) left ventricular diastolic dimension (LVDd) (n = 5–6, one-way ANOVA, *P < 0.05 of LSD t-test) are shown. (D) Representative photographs of echocardiograms.
Lipid droplet changes in MHC-DGAT1×MHC-PPARγ
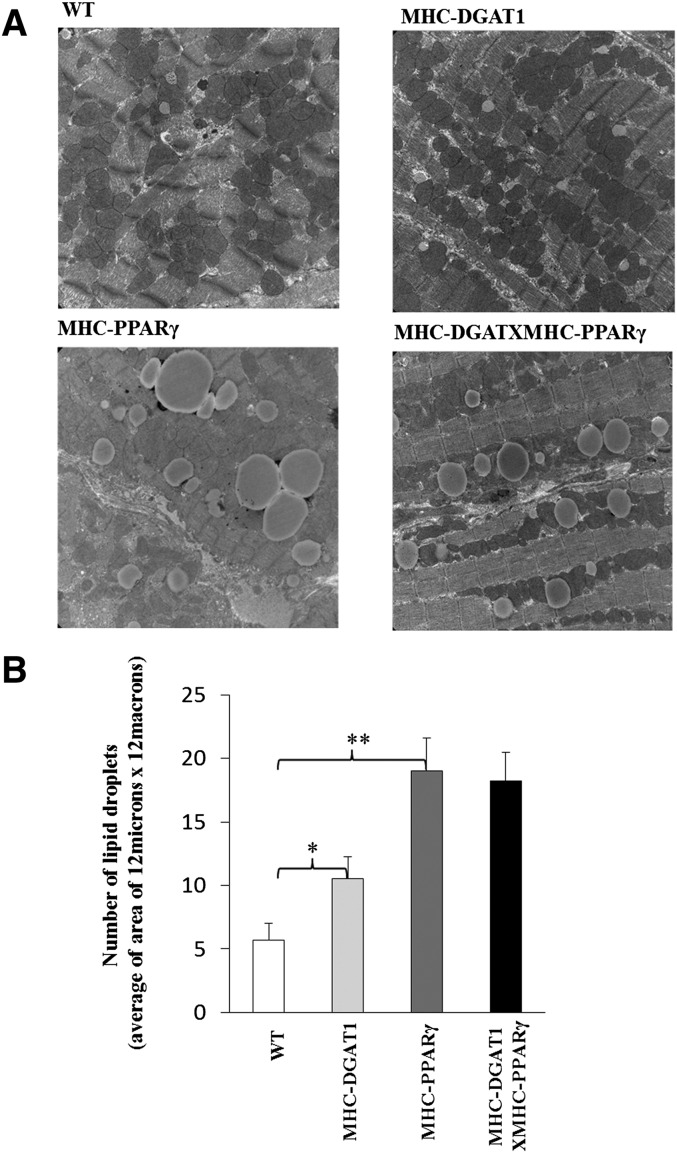
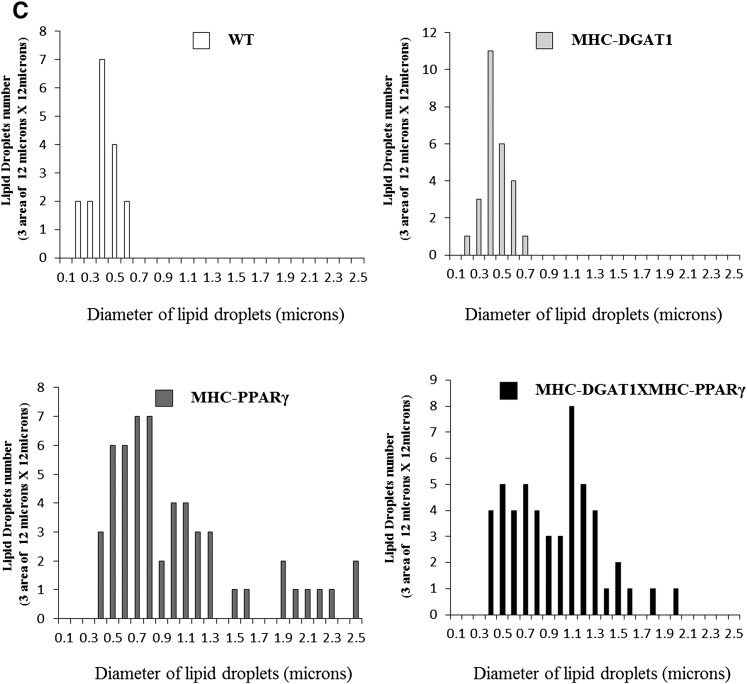
Several processes could have improved the heart dysfunction in these mice, most obviously the reduction in DAG and ceramide or a change in storage of cardiomyocyte lipids. As shown in Fig. 2A, MHC-PPARγ hearts had more TG than WT littermates, but this accumulation was not significantly decreased by the MHC-DGAT1 transgene. We then assessed lipid droplet size and number as an indication of lipid storage. Electron microscopy showed that the number of lipid droplets was increased ∼3-fold in MHC-PPARγ mice (Fig. 4A, B). MHC-DGAT1 slightly increased lipid droplet number compared with WT (Fig. 4B). Lipid droplet size distribution was altered in the double transgenic mice, even though the total number of droplets did not change (Fig. 4B). The number of large lipid droplets (diameter > 2 μm) was greater in MHC-PPARγ than MHC-DGAT1×MHC-PPARγ, whereas the number of medium-sized droplets (1–2 μm) was greater in the double transgenics (Fig. 4C). This is why the total TG remained the same between MHC-PPARγ mice and MHC-DGAT1×MHC-PPARγ mice.
Fig. 4.
Lipid droplet changes in WT, MHC-DGAT1, MHC-PPARγ, and MHC-DGAT1×MHC-PPARγ mice. (A) Electron microscopy pictures of lipid droplets, (B) lipid droplet numbers per area (n = 3, one-way ANOVA, *P < 0.05, **P < 0.01 of LSD t-test), and (C) lipid droplet size distribution in WT, MHC-DGAT1, MHC-PPARγ, and MHC-DGAT1×MHC-PPARγ mice.
Expression of genes regulating lipid uptake and storage
We first assessed whether MHC-DGAT1 improved cardiac function and mortality because it altered expression of PPARγ or other PPARs. It did not (Table 2). In adipose, smaller lipid droplets are created during lipolysis (25), so we assessed lipolysis enzymes in the four groups of mice. ATGL is the primary enzyme responsible for hydrolysis of lipid droplet in hearts (26). Expression of this enzyme was decreased with the expression of the MHC-DGAT1 transgene in MHC-PPARγ-expressing cardiac muscle. So increased ATGL was not the reason for the smaller lipid droplets, but less enzyme activity could have prevented release of toxic FAs from stored TG. However, ATGL is responsible for regulation of PPARα in the heart (27). In agreement with this, mRNA levels of CD36, a gene involved in lipid uptake and TG hydrolysis, and acyl-CoA oxidase (AOX) were ∼2-fold higher in MHC-PPARγ mice and were reduced in MHC-DGAT1×MHC-PPARγ mice. In addition, PDK4, a regulator of FA versus glucose oxidation, was decreased in MHC-DGAT1×MHC-PPARγ mice (Table 2). This finding implies that the double transgenic mice had more glucose oxidation. Other genes in these pathways, CPT1 and LpL, were not altered. Male and female mice showed similar changes (supplementary Table III). Therefore, MHC-DGAT1×MHC-PPARγ hearts appeared to have reduced uptake and oxidation of FAs and greater glucose oxidation.
mRNA levels of genes involved in lipid droplet formation in heart were measured. ADRP (Plin2) was increased (∼26%) in MHC-DGAT1, ∼55 in MHC-PPARγ, and ∼37% in MHC-DGAT1XMHC-PPARγ transgenic mice. Compared with WT littermates, TIP47 (Plin3) was increased ∼4 and OXPAT (Plin5) increased ∼3-fold in MHC-DGAT1 cardiac muscle. In double transgenic mice, OXPAT was the most significantly increased of these genes(Table 2).
MHC-DGAT1×MHC-PPARγ had gene expression changes consistent with the improved function and mortality. BNP mRNA levels were increased in MHC-PPARγ mice. Consistent with the functional results, BNP was reduced to normal levels by DGAT1(Table 2).
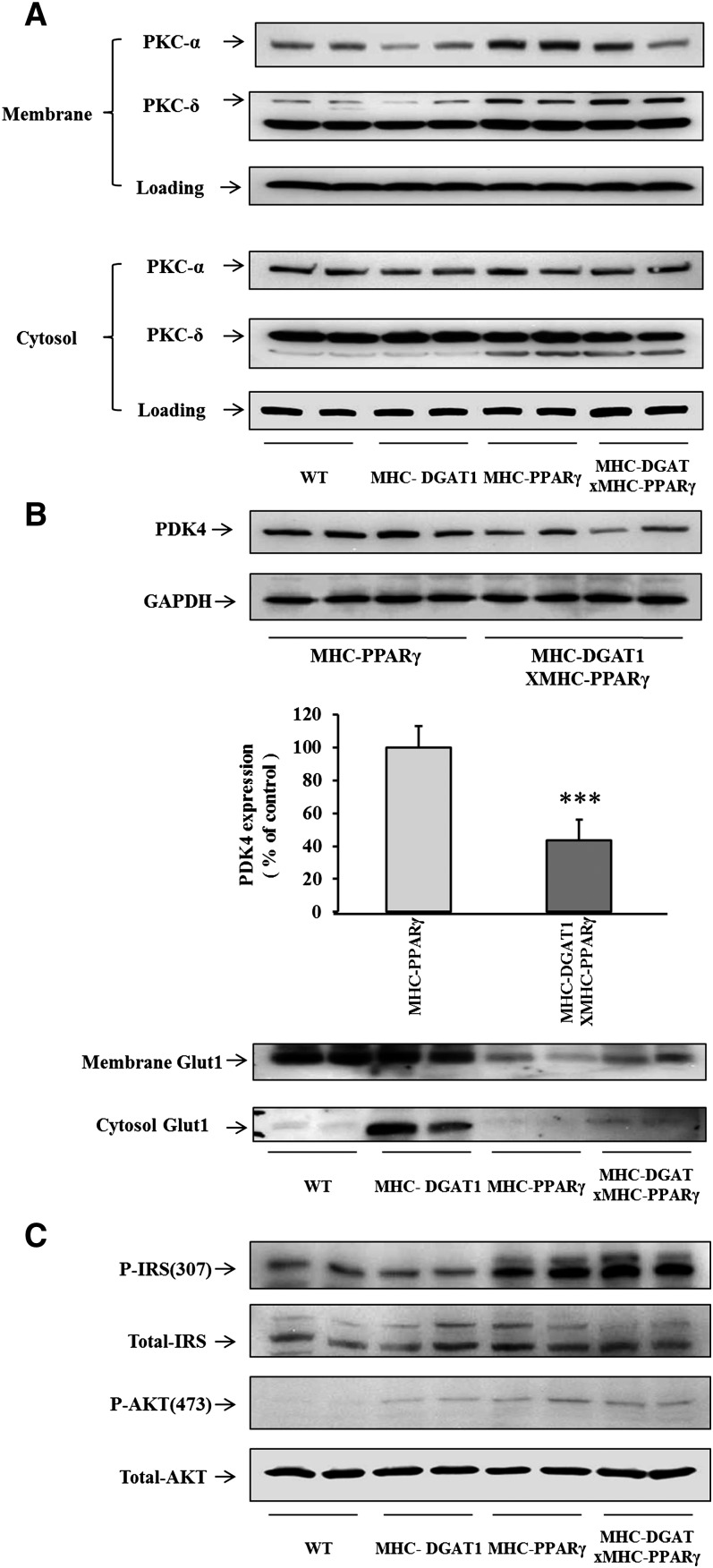
Regulation of PKC and insulin signaling pathways
We next assessed whether changes in DAG and ceramide altered membrane (active) levels of protein kinase C (PKC) in cardiac muscle. Membrane PKCα protein was reduced in MHC-DGAT1 and increased in heart tissue from MHC-PPARγ mice. The increased PKCα protein in cardiac membranes was partially decreased by the MHC-DGAT1 transgene. PKCδ, another DAG activated PKC isoform, was not reduced in MHC-DGAT1×PPARγ cardiac muscles (Fig. 5A).
Fig. 5.
Western blot of PKCs, Glut1, and insulin signaling pathways in WT, MHC-DGAT1, MHC-PPARγ, and MHC-DGAT1×MHC-PPARγ mice. A: Membrane associated PKCα and PKCδ were increased in MHC-PPARγ cardiac muscle but only PKCα was down-regulated in MHC-DGAT1XPPARγ mice. B: The MHC-DGAT1 transgene also decreased PDK4 protein (***P<0.001) and elevated membrane and cytosol Glut1, which was significantly decreased in MHC-PPARγ cardiac muscle. C: P-IRS1 and P-AKT levels in hearts.
Several indicators of glucose metabolism were altered by this cross. Because PDK4 mRNA levels as well as PDK4 protein (Fig. 5B) were lower in MHC-DGAT1×PPARγ than MHC-PPARγ cardiac muscle, we speculated that these double transgenic mice have reduced uptake and oxidation of FAs and greater glucose oxidation. Membrane associated Glut1 protein, which was reduced in MHC-PPARγ cardiac muscle, may have slightly recovered in MHC-DGAT1×PPARγ hearts (Fig. 5B). The insulin signaling pathway, which has been reported to be defective in lipotoxic cardiomyopathy (28, 29), was assessed. P-IRS1 levels were increased with PPARγ expression and were even greater in the double transgenic mice (Fig. 5C). P-AKT levels were not altered when we compared MHC-PPARγ with MHC-DGAT1×MHC-PPARγ mice(Fig. 5C).
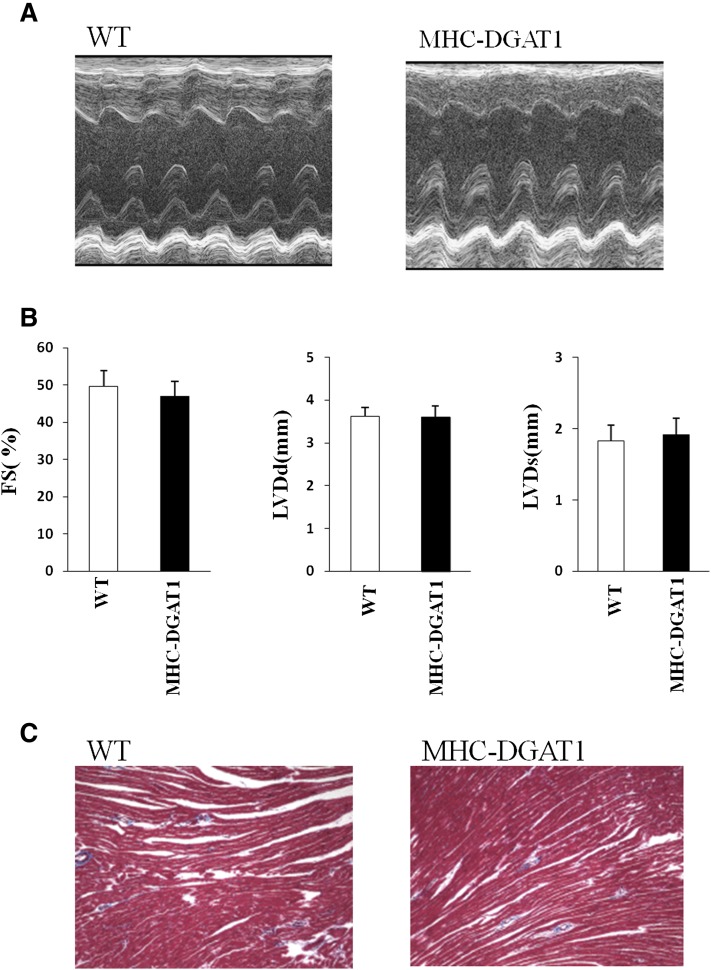
Heart function is normal in old MHC-DGAT1 transgenic mice
Glenn et al. reported that their MHC-DGAT1 transgenic mice developed significant heart dysfunction over time (20), unlike what we had reported in younger mice on the FVB background. To clarify this issue, we maintained our MHC-DGAT1 transgenic mice to 52 weeks, an age that led to dysfunction in their animals. However, we still did not detect heart dysfunction in our MHC-DGAT1 transgenic mice (Fig. 6A, B). We also did not find increased fibrosis by collagen staining (Fig. 6C).
Fig. 6.
Cardiac function in 12-month-old WT and MHC-DGAT1 mice. (A) Representative photographs of echocardiograms of 12-month-old WT and MHC-DGAT1 mice, and (B) FS, LVDd, and LVDd of these mice (n = 6 and 8). (C) Two representative collagen staining pictures of mice.
DISCUSSION
DGAT1, one of two identified enzymes for TG synthesis, uses DAG and FA to create TG. In animal models, DGAT1 overexpression is beneficial in some situations (5, 11). Our previous study demonstrated that overexpression of DGAT1 in skeletal muscle improved high-fat-treated insulin sensitivity; this occurred as more FA substrates were stored as TG and oxidized and as concentrations of lipotoxic FA-derivatives were reduced (5, 30). In our MHC-DGAT1 transgenic model, DGAT1 also reduced DAG and ceramide in the heart. Although no heart function change was found in MHC-DGAT1 transgenic mice, the MHC-DGAT1 transgene significantly improved heart function and increased survival of a lipotoxic cardiomyopathy model created by overexpression of ACS1 (11). There is, however, some controversy on the role of DGAT1 in the heart.
In the current study, MHC-DGAT1×MHC-PPARγ mice had increased survival and reduced heart content of DAG and ceramide. DAG and ceramide are believed to be toxic (31, 32); the improved function and survival are, we speculate, due to their reduction. In a recent publication, we had shown that MHC-PPARγ mice die of ventricular fibrillation associated with reduced activity of a potassium channel (22). Such channels may be modulated by adrenergic signaling, which is defective in these hearts, probably due to increased lipid-driven PKC activation (2). In support of that, membrane-associated PKCα was reduced in the rescued mice.
CD36 modulates FFA uptake into muscles and is especially important in delivery of FFA and lipoprotein FAs to the heart (33, 34). CD36 is a PPARγ-regulated gene, and its expression was decreased in MHC-DGAT1×MHC-PPARγ despite no reduction in expression of the MHC-PPARγ transgene. Perhaps the reduction in ATGL expression in the double transgenic mice led to reduced production of a PPAR ligand (26). Reduced CD36 expression in MHC-DGAT1×MHC-PPARγ mice may have reduced FA uptake, which assisted in the reduced production of DAG and ceramide. Others have shown that deletion of CD36 will improve lipid toxicity in mice overexpressing PPARα in cardiomyocytes (35). We note that CD36 expression significantly increased in MHC-DGAT1 transgenic mice, but these mice oxidized more FA (11) and, thus, were in lipid balance.
In contrast to increased FA uptake and oxidation in MHC-PPARγ heart, we speculated that the hearts of MHC-DGAT1xPPARγ mice switched from FA to more glucose oxidation because PDK4 gene expression was decreased. The decreased membrane-associated Glut1 level in MHC-PPARγ cardiac muscle was partially recovered in MHC-DGAT1×PPARγ double transgenic mice. Overexpression of Glut1 in cardiac muscle prevents the development of heart failure attributable to pressure overload in mice (36), and the increase in Glut1 might also improve heart function in the MHC-PPARγ mice. In addition, we found an increase in P-IRS1 and a decrease in PDK4 in these hearts. Although P-AKT, another usual downstream target of insulin signaling was not altered, P-AKT is also a marker for cardiac hypertrophy (37). However, overall, the improved survival is likely to be associated with greater glucose use. Reductions in either or both ceramide (32) and DAG (38) should improve insulin signaling. Our study, however, shows the difficulty of trying to alter just one of these potentially toxic lipids.
Recently, Glenn et al. reported that another MHC-DGAT1 transgenic model had increased cardiac myocyte lipid accumulation and heart dysfunction (20). Over time, expression of their DGAT1-FLAG-tagged transgene in the heart resulted in the development of a significant cardiomyopathy at 52 weeks associated with cardiac fibrosis. The toxicity was found only on a mixed background; transgene expression was lost in C57BL6 mice. The reasons for the cardiac toxicity were postulated to be due to excess TG accumulation, although TG is often viewed as a relatively inert storage form of lipid. The disparate results from our study might be due either to the time course of the observed responses or to the genetic background; our first studies used mice on the FVB rather than “mixed” background. To determine whether our mice would develop cardiomyopathy in old age on a uniform background, we crossed the MHC-DGAT1 transgene onto the C57BL6 background and maintained them to 52 weeks of age. We still found no cardiac dysfunction and no fibrosis. Aside from background, the location of insertion of these two MHC-DGAT1 transgenes might have led to altered phenotypes. Another possibility is that the FLAG epitope-tagged protein used affected heart function; some tagged proteins have been reported to cause dilated cardiomyopathy in vitro and in vivo (39) (40). FLAG epitope tags may also interfere with a particular target protein's function (41).
In the heart, FAs are likely acquired by both protein- and nonprotein-mediated uptake (42). FAs derived from each of these processes can be utilized for membrane biosynthesis, energy production through β-oxidation, and generation of lipid-signaling molecules. When cells accumulate more FAs than are required for anabolic or catabolic processes, excess lipid is esterified and stored as TG in lipid droplets (43); however, nonadipose tissues have a limited capacity for storage of lipids. Accumulation of excess lipids in nonadipose tissues leads to cell dysfunction or cell death. This phenomenon, known as lipotoxicity, may play an important role in the pathogenesis of heart failure in patients with diabetes and obesity (43). However, TG itself is a nonreactive stored lipid that is usually sequestered in lipid droplets (3). Our current and previous study and those of others have found that overexpression DGAT1 may have beneficial effects in specific tissues: DGAT1 may route more toxic FA derivates to TG synthesis and β-oxidation, reduce DAG and ceramide accumulation, and decrease PKC activation (2). Thus, activation of DGAT1 in the heart may be a pathway for lipotoxic cardiomyopathy treatment.
Supplementary Material
Acknowledgments
The authors thank Ni Son, M.D., Ph.D., for providing the MHC-PPARγ mice.
Footnotes
Abbreviations:
- ACS1
- acyl CoA synthetase 1
- DAG
- diacylglycerol
- DGAT1
- diacylglycerol acyl transferase 1
- FS
- fraction shorting
- LVDd
- left ventricular diastolic dimension
- LVDs
- left ventricular systolic dimension
- MHC
- myosin heavy chain
- PKC
- protein kinase C
- PPAR
- peroxisome proliferator-activated receptor
- TG
- triglyceride
- WT
- wild-type
This work was supported by National Institutes of Health Grants HL-45095 and HL-73029 (I. J. Goldberg) and Beijing Natural Science Foundation Major Project-5100003 (Y .Yin). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of three tables.
REFERENCES
- 1.Park T. S., Yamashita H., Blaner W. S., Goldberg I. J. 2007. Lipids in the heart: a source of fuel and a source of toxins. Curr. Opin. Lipidol. 18: 277–282 [DOI] [PubMed] [Google Scholar]
- 2.Drosatos K., Bharadwaj K. G., Lymperopoulos A., Ikeda S., Khan R., Hu Y., Agarwal R., Yu S., Jiang H., Steinberg S. F., et al. 2011. Cardiomyocyte lipids impair beta-adrenergic receptor function via PKC activation. Am. J. Physiol. Endocrinol. Metab. 300: E489–E499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Listenberger L. L., Han X., Lewis S. E., Cases S., Farese R. V., Jr, Ory D. S., Schaffer J. E. 2003. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc. Natl. Acad. Sci. USA. 100: 3077–3082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cases S., Smith S. J., Zheng Y. W., Myers H. M., Lear S. R., Sande E., Novak S., Collins C., Welch C. B., Lusis A. J., et al. 1998. Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc. Natl. Acad. Sci. USA. 95: 13018–13023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu L., Zhang Y., Chen N., Shi X., Tsang B., Yu Y. H. 2007. Upregulation of myocellular DGAT1 augments triglyceride synthesis in skeletal muscle and protects against fat-induced insulin resistance. J. Clin. Invest. 117: 1679–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodpaster B. H., He J., Watkins S., Kelley D. E. 2001. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J. Clin. Endocrinol. Metab. 86: 5755–5761 [DOI] [PubMed] [Google Scholar]
- 7.Bruce C. R., Thrush A. B., Mertz V. A., Bezaire V., Chabowski A., Heigenhauser G. J., Dyck D. J. 2006. Endurance training in obese humans improves glucose tolerance and mitochondrial fatty acid oxidation and alters muscle lipid content. Am. J. Physiol. Endocrinol. Metab. 291: E99–E107 [DOI] [PubMed] [Google Scholar]
- 8.Chen H. C., Stone S. J., Zhou P., Buhman K. K., Farese R. V., Jr 2002. Dissociation of obesity and impaired glucose disposal in mice overexpressing acyl coenzyme a:diacylglycerol acyltransferase 1 in white adipose tissue. Diabetes. 51: 3189–3195 [DOI] [PubMed] [Google Scholar]
- 9.Chen N., Liu L., Zhang Y., Ginsberg H. N., Yu Y. H. 2005. Whole-body insulin resistance in the absence of obesity in FVB mice with overexpression of Dgat1 in adipose tissue. Diabetes. 54: 3379–3386 [DOI] [PubMed] [Google Scholar]
- 10.Monetti M., Levin M. C., Watt M. J., Sajan M. P., Marmor S., Hubbard B. K., Stevens R. D., Bain J. R., Newgard C. B., Farese R. V., Sr, et al. 2007. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab. 6: 69–78 [DOI] [PubMed] [Google Scholar]
- 11.Liu L., Shi X., Bharadwaj K. G., Ikeda S., Yamashita H., Yagyu H., Schaffer J. E., Yu Y. H., Goldberg I. J. 2009. DGAT1 expression increases heart triglyceride content but ameliorates lipotoxicity. J. Biol. Chem. 284: 36312–36323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koliwad S. K., Streeper R. S., Monetti M., Cornelissen I., Chan L., Terayama K., Naylor S., Rao M., Hubbard B., Farese R. V., Jr 2010. DGAT1-dependent triacylglycerol storage by macrophages protects mice from diet-induced insulin resistance and inflammation. J. Clin. Invest. 120: 756–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Son N. H., Park T. S., Yamashita H., Yokoyama M., Huggins L. A., Okajima K., Homma S., Szabolcs M. J., Huang L. S., Goldberg I. J. 2007. Cardiomyocyte expression of PPARgamma leads to cardiac dysfunction in mice. J. Clin. Invest. 117: 2791–2801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiu H. C., Kovacs A., Ford D. A., Hsu F. F., Garcia R., Herrero P., Saffitz J. E., Schaffer J. E. 2001. A novel mouse model of lipotoxic cardiomyopathy. J. Clin. Invest. 107: 813–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michalik L., Auwerx J., Berger J. P., Chatterjee V. K., Glass C. K., Gonzalez F. J., Grimaldi P. A., Kadowaki T., Lazar M. A., O'Rahilly S., et al. 2006. International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol. Rev. 58: 726–741 [DOI] [PubMed] [Google Scholar]
- 16.Finck B. N., Lehman J. J., Leone T. C., Welch M. J., Bennett M. J., Kovacs A., Han X., Gross R. W., Kozak R., Lopaschuk G. D., et al. 2002. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J. Clin. Invest. 109: 121–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burkart E. M., Sambandam N., Han X., Gross R. W., Courtois M., Gierasch C. M., Shoghi K., Welch M. J., Kelly D. P. 2007. Nuclear receptors PPARbeta/delta and PPARalpha direct distinct metabolic regulatory programs in the mouse heart. J. Clin. Invest. 117: 3930–3939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Georgiadi A., Lichtenstein L., Degenhardt T., Boekschoten M. V., van Bilsen M., Desvergne B., Muller M., Kersten S. 2010. Induction of cardiac Angptl4 by dietary fatty acids is mediated by peroxisome proliferator-activated receptor beta/delta and protects against fatty acid-induced oxidative stress. Circ. Res. 106: 1712–1721 [DOI] [PubMed] [Google Scholar]
- 19.Nesto R. W., Bell D., Bonow R. O., Fonseca V., Grundy S. M., Horton E. S., Le Winter M., Porte D., Semenkovich C. F., Smith S., et al. 2003. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. October 7, 2003. Circulation. 108: 2941–2948 [DOI] [PubMed] [Google Scholar]
- 20.Glenn D. J., Wang F., Nishimoto M., Cruz M. C., Uchida Y., Holleran W. M., Zhang Y., Yeghiazarians Y., Gardner D. G. 2011. A murine model of isolated cardiac steatosis leads to cardiomyopathy. Hypertension. 57: 216–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clugston R. D., Jiang H., Lee M. X., Piantedosi R., Yuen J. J., Ramakrishnan R., Lewis M. J., Gottesman M. E., Huang L. S., Goldberg I. J., et al. 2011. Altered hepatic lipid metabolism in C57BL/6 mice fed alcohol: a targeted lipidomic and gene expression study. J. Lipid Res. 52: 2021–2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrow J. P., Katchman A., Son N. H., Trent C. M., Khan R., Shiomi T., Huang H., Amin V., Lader J. M., Vasquez C., et al. 2011. Mice with cardiac overexpression of peroxisome proliferator-activated receptor gamma have impaired repolarization and spontaneous fatal ventricular arrhythmias. Circulation. 124: 2812–2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leaf A., Kang J. X., Xiao Y. F., Billman G. E. 2003. Clinical prevention of sudden cardiac death by n-3 polyunsaturated fatty acids and mechanism of prevention of arrhythmias by n-3 fish oils. Circulation. 107: 2646–2652 [DOI] [PubMed] [Google Scholar]
- 24.Dlugosova K., Okruhlicova L., Mitasikova M., Sotnikova R., Bernatova I., Weismann P., Slezak J., Tribulova N. 2009. Modulation of connexin-43 by omega-3 fatty acids in the aorta of old spontaneously hypertensive rats. J. Physiol. Pharmacol. 60: 63–69 [PubMed] [Google Scholar]
- 25.Brasaemle D. L. 2007. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J. Lipid Res. 48: 2547–2559 [DOI] [PubMed] [Google Scholar]
- 26.Haemmerle G., Lass A., Zimmermann R., Gorkiewicz G., Meyer C., Rozman J., Heldmaier G., Maier R., Theussl C., Eder S., et al. 2006. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 312: 734–737 [DOI] [PubMed] [Google Scholar]
- 27.Haemmerle G., Moustafa T., Woelkart G., Buttner S., Schmidt A., van de Weijer T., Hesselink M., Jaeger D., Kienesberger P. C., Zierler K., et al. 2011. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-alpha and PGC-1. Nat. Med. 17: 1076–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Witteles R. M., Fowler M. B. 2008. Insulin-resistant cardiomyopathy clinical evidence, mechanisms, and treatment options. J. Am. Coll. Cardiol. 51: 93–102 [DOI] [PubMed] [Google Scholar]
- 29.Banerjee S., Peterson L. R. 2007. Myocardial metabolism and cardiac performance in obesity and insulin resistance. Curr. Cardiol. Rep. 9: 143–149 [DOI] [PubMed] [Google Scholar]
- 30.Liu L., Shi X., Choi C. S., Shulman G. I., Klaus K., Nair K. S., Schwartz G. J., Zhang Y., Goldberg I. J., Yu Y. H. 2009. Paradoxical coupling of triglyceride synthesis and fatty acid oxidation in skeletal muscle overexpressing DGAT1. Diabetes. 58: 2516–2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu C., Chen Y., Cline G. W., Zhang D., Zong H., Wang Y., Bergeron R., Kim J. K., Cushman S. W., Cooney G. J., et al. 2002. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J. Biol. Chem. 277: 50230–50236 [DOI] [PubMed] [Google Scholar]
- 32.Holland W. L., Brozinick J. T., Wang L. P., Hawkins E. D., Sargent K. M., Liu Y., Narra K., Hoehn K. L., Knotts T. A., Siesky A., et al. 2007. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 5: 167–179 [DOI] [PubMed] [Google Scholar]
- 33.Coburn C. T., Knapp F. F., Jr, Febbraio M., Beets A. L., Silverstein R. L., Abumrad N. A. 2000. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J. Biol. Chem. 275: 32523–32529 [DOI] [PubMed] [Google Scholar]
- 34.Bharadwaj K. G., Hiyama Y., Hu Y., Huggins L. A., Ramakrishnan R., Abumrad N. A., Shulman G. I., Blaner W. S., Goldberg I. J. 2010. Chylomicron- and VLDL-derived lipids enter the heart through different pathways: in vivo evidence for receptor- and non-receptor-mediated fatty acid uptake. J. Biol. Chem. 285: 37976–37986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang J., Sambandam N., Han X., Gross R. W., Courtois M., Kovacs A., Febbraio M., Finck B. N., Kelly D. P. 2007. CD36 deficiency rescues lipotoxic cardiomyopathy. Circ. Res. 100: 1208–1217 [DOI] [PubMed] [Google Scholar]
- 36.Liao R., Jain M., Cui L., D'Agostino J., Aiello F., Luptak I., Ngoy S., Mortensen R. M., Tian R. 2002. Cardiac-specific overexpression of GLUT1 prevents the development of heart failure attributable to pressure overload in mice. Circulation. 106: 2125–2131 [DOI] [PubMed] [Google Scholar]
- 37.Matsui T., Nagoshi T., Rosenzweig A. 2003. Akt and PI 3-kinase signaling in cardiomyocyte hypertrophy and survival. Cell Cycle. 2: 220–223 [PubMed] [Google Scholar]
- 38.Morino K., Petersen K. F., Shulman G. I. 2006. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes. 55(Suppl. 2): S9–S15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang W. Y., Aramburu J., Douglas P. S., Izumo S. 2000. Transgenic expression of green fluorescence protein can cause dilated cardiomyopathy. Nat. Med. 6: 482–483 [DOI] [PubMed] [Google Scholar]
- 40.Agbulut O., Coirault C., Niederlander N., Huet A., Vicart P., Hagege A., Puceat M., Menasche P. 2006. GFP expression in muscle cells impairs actin-myosin interactions: implications for cell therapy. Nat. Methods. 3: 331. [DOI] [PubMed] [Google Scholar]
- 41.Funakoshi M., Hochstrasser M. 2009. Small epitope-linker modules for PCR-based C-terminal tagging in Saccharomyces cerevisiae. Yeast. 26: 185–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schaffer J. E. 2002. Fatty acid transport: the roads taken. Am. J. Physiol. Endocrinol. Metab. 282: E239–E246 [DOI] [PubMed] [Google Scholar]
- 43.Schaffer J. E. 2003. Lipotoxicity: when tissues overeat. Curr. Opin. Lipidol. 14: 281–287 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.