Abstract
Cholesterol is an essential component of eukaryotic cell membranes, regulating fluidity and permeability of the bilayer. Outside the membrane, cholesterol is esterified to fatty acids forming cholesterol esters (CEs). Metabolism of CEs is characterized by recurrent hydrolysis and esterification as part of the CE cycle; however, since recombinant 15-lipoxygenase (15-LO) was shown to oxidize cholesteryl linoleate of LDL, there has been interest in CE oxidation, particularly in the context atherogenesis. Studies of oxidized CE (oxCE) metabolism have focused on hydrolysis and subsequent reverse cholesterol transport with little emphasis on the fate the newly released oxidized fatty acyl component. Here, using mass spectrometry to analyze lipid oxidation products, CE metabolism in murine peritoneal macrophages was investigated. Ex vivo macrophage incubations revealed that cellular 15-LO directly oxidized multiple CE substrates from intracellular stores and from extracellular sources. Freshly harvested murine macrophages also contained 15-LO-specific oxCEs, suggesting the enzyme may act as a CE-oxidase in vivo. The metabolic fate of oxCEs, particularly the hydrolysis and remodeling of oxidized fatty acyl chains, was also examined in the macrophage. Metabolism of deuterated CE resulted in the genesis of deuterated, oxidized phosphatidylcholine (oxPC). Further experiments revealed these oxPC species were formed chiefly from the hydrolysis of oxidized CE and subsequent reacylation of the oxidized acyl components into PC.
Keywords: 15-lipoxygenase, oxidized phospholipids, atherosclerosis, oxidized LDL
Cholesteryl esters are common lipid molecules that are central for cellular homeostasis. There are numerous molecular species of CE present in animals, with the most abundant in human blood being cholesteryl esters of linoleate [CE(18:2)], arachidonate [CE(20:4)], and docosahexaenoate [CE(22:6)], all polyunsaturated fatty acids (1). Intracellular CE is stored in unique organelles called lipid bodies, which consist of a neutral lipid core (CEs and triacylglycerides) surrounded by an amphipathic monolayer chiefly comprised of phosphatidylcholine (PC) (2). In the circulation, CEs are concentrated in LDL, which deliver cholesterol to extrahepatic cells through recognition by the LDL receptor at the plasma membrane of target cells followed by endocytosis. Thus, CEs constitute the storage and transport form of cholesterol as well as sequester fatty acids for energy storage. Generally, discussions of CE metabolism are limited to esterification and deesterification, as part of the intercellular CE cycle or during lipoprotein formation and metabolism (3, 4). However, there has been heightened interest in CE oxidation since in vitro studies revealed that CE(18:2) within LDL was converted to cholesteryl 13(S)-hydroxy-9,11-octadecadienoic acid [CE(13(S)-HODE)] by purified rabbit reticulocyte 15-LO after reduction of the initial hydroperoxide product, cholesteryl 13(S)-hydroperoxy-9,11-octadecadienoic acid [CE(13(S)-HpODE)] (5). The vast majority of CE oxidation studies have examined the potential role of 15-LO in the generation oxidized LDL, and there is now substantial evidence that 15-LO expression contributes to the oxidation of LDL particles during coincubations with cultured cells (6, 7). In several of these studies, concurrent CE peroxidation was observed as the dominant lipid modification suggesting direct CE oxidation by intracellular 15-LO (8–10). However, chiral CE oxidation products were not shown, and therefore oxidation of CEs by radical mechanisms could not be ruled out. In fact, nonenzymatic LDL oxidation results in a similar profile of lipid oxidation products, with oxCEs being the dominant species. Furthermore, it is not fully understood how the formation of extracellular oxidized LDL could proceed by direct oxidation of CE by intercellular 15-LO. Thus, it remains unclear whether 15-LO, in the intact cell, plays an important role in direct CE oxidation
Previous studies demonstrated that oxCEs were substrates for hydrolysis, even preferred substrates over their nonoxidized counterparts (11). These investigations went on to show that CE oxidation enhances free cholesterol efflux (12); however, the fate of the newly freed oxylipins was not examined. In separate studies, where particularly high levels of oxidized CEs were formed during coincubations of LDL with 15-LO-expressing murine fibroblasts, oxidized PL (oxPL) species were observed as minor products (8). This finding suggested that radical propagation had occurred or that 15-LO was directly oxidizing PLs (13). An alternate mechanism of oxPL formation, which was not examined, was further metabolism of the abundant oxCE species leading to the esterification of oxidized acyl chains in the PL pool. These separate observations of oxCE hydrolysis and the genesis oxPL in conditions of high oxCE suggest that some proportion of oxPL might originate from the hydrolysis and remodeling of abundant oxCEs. Oxidized PLs, particularly oxidized phosphatidylcholine (oxPC), are ligands for macrophage scavenger receptors (14) implicated in vascular plaque progression (15) where oxCEs are often abundant (16, 17).
Using isotopically labeled substrate coincubated with murine peritoneal macrophages, we determined whether cellular 15-LO was capable of directly oxidizing CE within endogenous lipid bodies as well as deuterated CE originating from the culture medium. By LC-MS/MS we examined the resulting oxCE profile for evidence of 15-LO-specific oxidation products of isotopically labeled and unlabeled CEs. To determine if 15-LO acts as a CE oxidase in vivo, CE oxidation products were examined in freshly harvested murine macrophages. To investigate biosynthetic coupling of CE oxidation to oxPL synthesis, we examined PLs isolated from murine macrophages after incubation with isotopically labeled CE substrate. To determine if this pathway was specific to oxCE remodeling, we compared oxPL products derived from CE oxidation and remodeling to oxPL generated by macrophage incubation with free d4-18:2.
EXPERIMENTAL PROCEDURES
Materials
Isotopically labeled 17,17,18,18-d4-linoleate (d4-18:2) was synthesized by Dr. Howard Sprecher (18). Free cholesterol was obtained from Sigma-Aldrich (St. Louis, MO). Cholesteryl 17,17,18,18-d4-linoleate [CE(d4-18:2)] was synthesized as previously reported (19). 1-Palmitoyl-2-oleoyl-phosphocholine was obtained from Avanti Polar Lipids (Alabaster, AL). All solvents used were HPLC or Optima grade and were purchased from Fischer Scientific (Fair Lawn, NJ). Female 6- to 10-week-old wild-type (C57BL/6J) and 15-LO-deficient (B6.129S2-Alox15tm1Fun/J) mice were purchased from Jackson Laboratory (Bar Harbor, ME).
Preparation of lipid vesicles
Synthetic lipid vesicles (LVs) were formed in aqueous buffer essentially by the method of Chapman and Trelease (20). Briefly, a mixture of CE(d4-18:2), palmitoyl-oleoyl-phosphocholine, and cholesterol (0.60, 0.15, and 0.10 μmol, respectively) was dried under a stream of N2 and diluted in ether (0.5 ml). This lipid composition corresponded to a neutral lipid to amphipathic lipid ratio similar to that of VLDL. Calcium- and magnesium-free (Ca/Mg-free) HBSS (1.6 ml) was added, and the biphasic system was sonicated under a stream N2. When the ether evaporated completely, the aqueous phase became uniformly turbid, indicating a stable emulsion of lipid vesicles.
Human lipoprotein
Whole blood was obtained from healthy volunteers with approval from the Colorado Multi-institutional Review Board. Platelet-poor plasma was prepared from whole blood as previously described (21) and centrifuged at 45,000 rpm for 10 min at 18°C. The top layer of chylomicrons was discarded, and the remaining chylomicron-free, platelet-poor plasma was used immediately. This material is hereafter referred to as human lipoprotein (LP).
Resident peritoneal macrophage incubations
All research involving animals was done in accordance with Public Health Service policy and was approved by the Colorado Multi-institutional Review Board. Murine peritoneal macrophages were isolated essentially as previously described (22). Briefly, 5 ml of Ca/Mg-free HBSS was injected into the peritoneal cavity of euthanized mice; the cavity was massaged and gently agitated before the lavage fluid was recovered. Macrophages in peritoneal lavage fluid were counted and diluted with a convenient amount (no less than 0.5 vols) of calcium- and magnesium-containing HBSS. Cells were then aliquoted at 5 × 105 cells/well into 24-well plates, which were incubated at 37°C for 1 h. Nonadherent cells were removed by washing once with HBSS. To the adherent cells, 0.5 ml of HEPES buffered HBSS (10 mM; pH 7.2) was added along with 25 μl of synthetic lipid vesicles (4.7 μmol/l CE(d4-18:2) final conc.), 1 μl of human LP (approximately 3 μmol/l CE final conc [1]), or d4-18:2 (4.7 μM final conc.). Incubations with d4-18:2 included fatty acid-free BSA (0.5% w/w) in the medium (MP Biomedicals, Solon, OH). After incubation at 37°C for various times, the medium was removed, and the cells were washed with 500 μl of medium. Adherent cells were then scraped twice with 500 μl of 90% MeOH in water containing SnCl2 (2 mM). Samples remained at room temperature for 1.5 h to allow complete reduction of hydroperoxide species before lipids were extracted. This step halted enzymatic activity by denaturing proteins and prevented further propagation of peroxy-radical oxygenation. Time courses were performed twice, each time using pooled macrophages from three mice and duplicate time points. When human LP was used, the two time courses were performed with material derived from the blood of separate donors.
Lipid extractions
Neutral lipids were extracted as previously described using 75:25 (v/v) isooctane:ethyl acetate (17). Phospholipids were extracted with a modified Bligh and Dyer procedure replacing chloroform with dichloromethane (19). Free fatty acids and oxylipins were isolated by solid phase extraction (C18) as previously described (23).
Chromatography
Separation of CEs and oxCEs by normal phase (NP) LC-MS/MS as well as chiral separations of hydroxylated-CEs were performed as previously reported (17). Free fatty acids and oxylipins (e.g., HETEs and HODEs) were separated using reversed-phase LC-MS/MS (RP LC-MS/MS) as previously reported (23).
Phospholipids (PLs) were separated by two methods. To isolate PLs according to headgroup, NP LC was performed as previously described (24). To separate PLs species by acyl composition, a RP LC-MS/MS was used. Lipid extracts were loaded onto a 150 × 3 mm, 2.6 μm, Kinetex C18 column (Phenomenex, Torrance, CA), and PLs were eluted with an isocratic flow of 5:3:1:1 (v/v/v/v) MeOH:acetonitrile:dichloromethane:H2O containing ammonium acetate (1 mM) at 200 μl/min.
Mass spectrometry
Online MS/MS of CEs was performed essentially as previously described using an AB Sciex API 3200 triple quadrupole mass spectrometer (AB Sciex, Toronto, Canada) (17). Briefly, ammonium acetate (10 mM in 95:5 ACN:H2O) was added to the eluent postcolumn to form ammoniated molecular ions, [M+NH4]+, in the electrospray source. These adducts were detected in positive ion mode using precursor ion scanning (precursors of m/z 369.3) or multiple reaction monitoring (MRM).
Online MS/MS of phospholipids was performed on an AB Sciex 5500 triple quadrupole linear ion trap hybrid mass spectrometer operating in negative ion mode essentially as previously described (24). All species were detected using MRM transitions for deprotonated molecular ions decomposing to specific product carboxylate anions with the exception of phosphatidylcholine species, where the parent ions were molecular acetate adducts [M+OAc]−. A comprehensive table of parent and product ions monitored is presented in Supplementary Table I. In exploratory experiments (chiefly NP LC), all transitions were included in the MRM method; in subsequent experiments (chiefly RP LC), the number of transitions included was reduced (only monitoring palmitate- and oleate-containing PC species) to allow for greater sensitivity. To accommodate over 400 MRM transitions while maintaining a functional sampling rate (i.e., duty cycle <6 s), a “scheduled MRM” approach was used during certain experiments. This involved monitoring selected transitions during a discrete retention time window corresponding to the known elution behavior of specific PL analytes, thus limiting the total MRM transitions monitored during a given portion of the chromatogram.
Collisionally induced dissociation spectra were acquired online during HPLC by including an enhanced product ion scan in the duty of the mass spectrometer. Ion source and collisionally induced dissociation parameters were the same as those used for MRM analyses. Product ion spectra were acquired from m/z 250 to 870 scanning at 1,000 Da/s. MS3 spectra were acquired using an activation energy of 0.1 V, scanning products ions from m/z 150 to 300 at 1,000 Da/s.
RESULTS
Oxidation of CE(d4-18:2) LVs by macrophage 15-lipoxygenase
15-Lipoxygenase was previously shown to stereospecifically oxygenate CE(18:2) forming CE(13(S)-HODE) after reduction of the initial peroxide product, CE(13(S)-HpODE) (5). In contrast, auto-oxidation of CE(18:2) produced an array of nonspecific oxidation products lacking regio- and stereospecificity. Therefore, detailed characterization of CE oxidation products (oxCE) provides insight into mechanisms of CE oxygenation. To determine if macrophage 15-LO oxygenated CE delivered in extracellular LVs, CE(d4-18:2)-laden LVs were incubated with murine peritoneal macrophages for 45, 90, 180, or 1200 min. After incubation, the medium was removed, and the cells were washed before intracellular lipids were extracted and analyzed by NP LC-MS/MS.
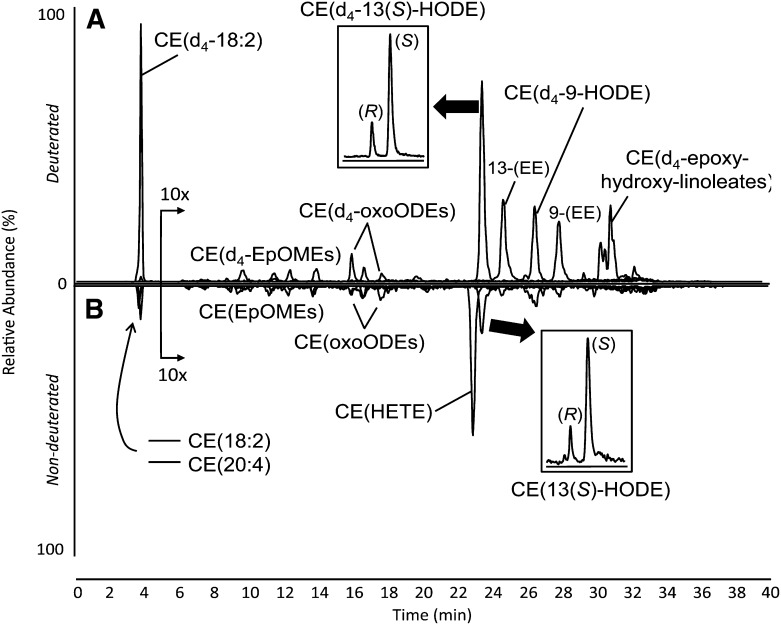
After shorter incubation times, the array of d4-oxCEs was indicative of 15-LO-driven oxygenation and auto-oxidation. The presence of numerous, deuterated oxidation products (all previously characterized [17]) indicated some nonspecific auto-oxidation occurred; however, the specific regioisomer, CE(d4-13-ZE-HODE), was the most abundant product, suggesting involvement of 15-LO in CE(d4-18:2) oxidation (Fig. 1A). When this compound was isolated and rechromatographed on a chiral column, a clear excess of the S enantiomer was observed, confirming oxygenation of the CE(d4-18:2) substrate by 15-LO to CE(d4-13(S)-ZE-HODE) (Fig. 1A, inset). When the incubation medium supernatant was analyzed separately (data not shown), only auto-oxidation products were detected, suggesting that 15-LO activity was confined to the macrophage cytoplasm. As incubation times increased, the preponderance of CE(d4-13(S)-ZE-HODE) was diminished relative to other oxidation products. After 20 h of substrate incubation, total oxidation products had clearly increased, but there was no longer an excess of the chiral 15-LO-specific product (Supplementary Fig. IA), suggesting nonspecific oxidation had overwhelmed the specific product profile. The inclusion of certain antioxidants was found to attenuate this auto-oxidation, but 15-LO-specific products were still readily formed (Supplementary Fig. III).
Fig. 1.
NP LC-MS/MS analysis of isotopically labeled (A) and endogenous (B) CE oxidation products after murine peritoneal macrophage incubation with synthetic lipid vesicles laden with CE(d4-18:2). The elution of deuterated species is shown on the positive axis (A), and the elution of endogenous, nondeuterated CEs is shown on the negative axis (B). The portions of the chromatograms from 5 to 40 min were amplified 10-fold. Insets show chiral separations after the indicated peaks were collected and rechromatographed. Absolute stereochemistry of CE(13(S)-HODE) was determined using chiral CE(13-HODE) standards.
Endogenous, nondeuterated CEs, mainly CE(18:2) and CE(20:4), were also present in peritoneal macrophages, albeit at much lower concentration than the CE(d4-18:2) taken up from the medium (Fig. 1B). Endogenous CE(18:2) was oxidized by 15-LO, forming the specific chiral product CE(13(S)-HODE) (Fig. 1B, inset). Additionally, endogenous CE(20:4) was oxidized to a single CE(HETE) regioisomer, which eluted at 23 min, just before CE(13(S)-HODE) (Fig. 1B). In separate experiments, described in detail in the supplementary materials, this product was isolated and structurally characterized. The observed product of CE(20:4) oxidation was determined to be CE(12(S)-HETE) (Supplementary ).
Oxidation of human lipoprotein CEs by macrophage 15-LO
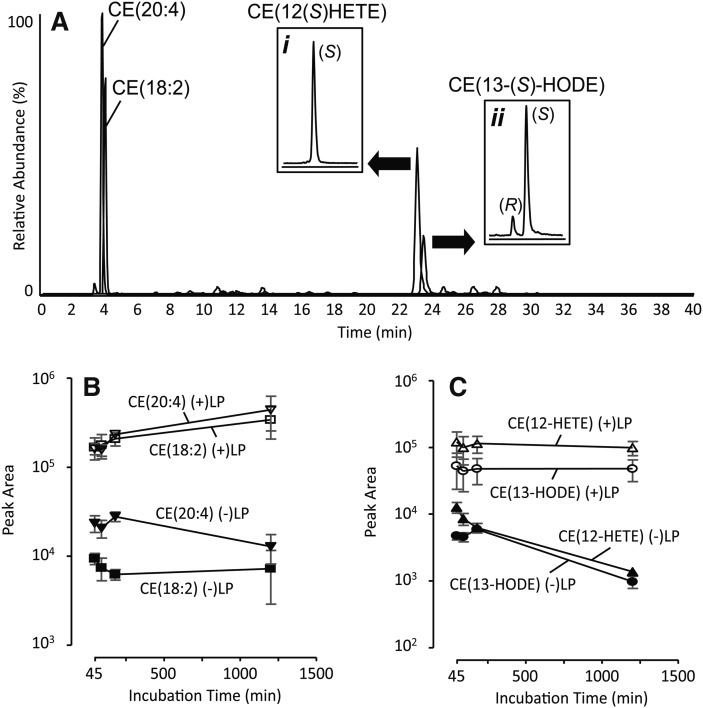
To determine if CE bound in LP particles could be oxidatively modified by murine macrophage 15-LO, human LP was coincubated with adherent macrophages for 45, 90, 180, or 1200 min. Neutral lipids were then extracted and analyzed by NP LC-MS/MS. The near absence of nonspecific CE oxidation products at 45 min (Fig. 2A), and even after 20 h (Supplemental Fig. 1B), indicated that very little auto-oxidation of CEs had occurred. This lack auto-oxidation was consistent with previous observations that plasma is an effective antioxidant (25). On the other hand, enzymatic oxidation products were readily apparent. Both CE(18:2) and CE(20:4) were specifically oxygenated by 15-LO forming CE(13(S)-HODE) and CE(12(S)-HETE), respectively (Fig. 2A). Oxygenation of these two polyunsaturated CEs by 15-LO was rather robust. Using previously generated calibration curves (17) to convert product/substrate peak area ratios to product/substrate relative abundance, maximal conversion of CE(18:2) to CE(13(S)-HODE) was determined to be 3.1 ± 1.3%, and conversion of CE(20:4) to CE(12(S)-HETE) was 9.6 ± 3.9% (mean ± SEM; n = 4; 20 h incubation).
Fig. 2.
NP LC-MS/MS analysis CEs and oxCEs after macrophage incubation with human lipoproteins (A). Insets in panel A show chiral separations after the indicated peaks were collected and rechromatographed. The graphs show average peak areas of common CEs (B) and oxCEs (C) after incubation with human lipoprotein, (+)LP, or control macrophages incubated with CE(d4-18:2)-containing LVs, (-)LP, (mean ± SEM; n = 4). The y-axis scales in panels B and C are logarithmic.
To assess CE uptake, we compared the peak areas of CEs and oxCEs from macrophages exposed to human LP with control macrophages exposed to synthetic LVs containing CE(d4-18:2). Macrophages exposed to human LP contained orders of magnitude more CE(18:2) and CE(20:4) (Fig. 2B). Although de novo CE synthesis cannot be ruled out, the remarkable increase in CE content was most likely due to uptake or perhaps to adhesion of human LP. The 15-LO-specific products CE(13(S)-HODE) and CE(12(S)-HETE) were also markedly increased (approximately 100-fold over control cells; Fig. 2C), strongly suggesting that a portion of the CE derived from the human LP had gained access to macrophage cytoplasm where it was oxygenated by 15-LO.
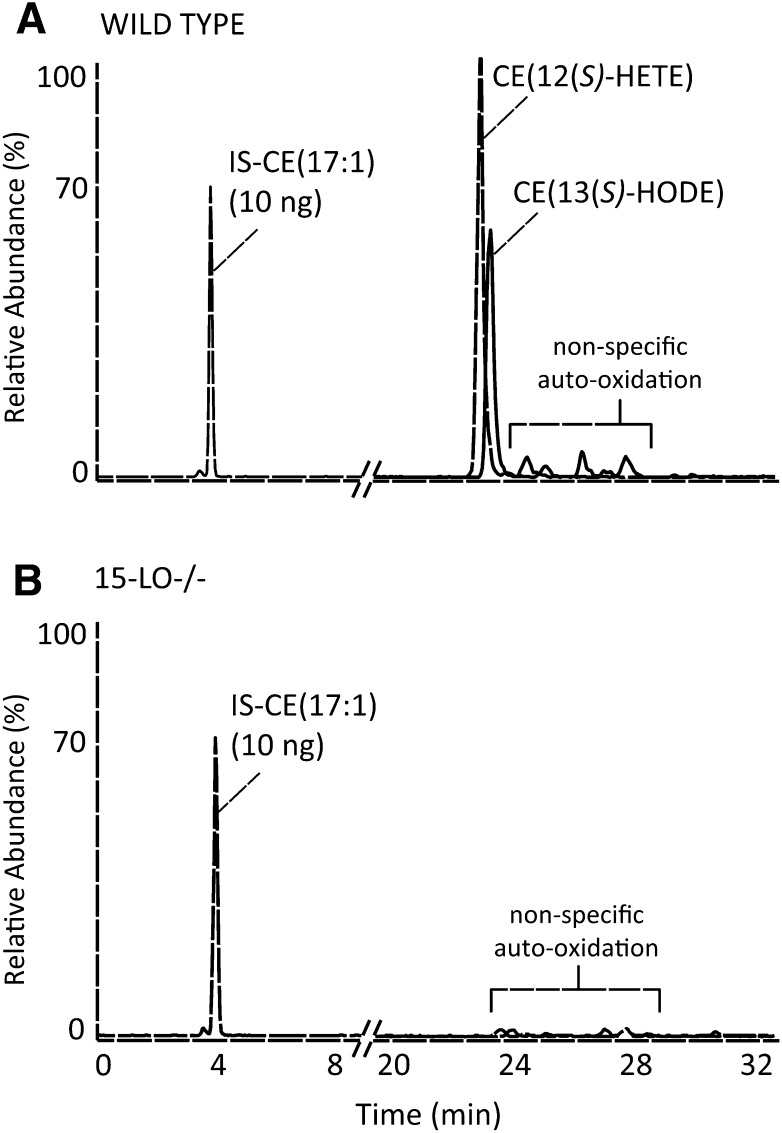
To determine unambiguously if 15-LO was responsible for oxygenation of CE(18:2) and CE(20:4), human LP was incubated with murine peritoneal macrophages collected from wild-type mice or 15-LO-deficient mice (n = 2). After 3-h incubations, cellular lipids were extracted and analyzed by NP LC-MS/MS. Wild-type macrophages contained significant amounts of CE(13(S)-HODE) and CE(12(S)-HETE) (Fig. 3A), yet there was no evidence of any significant oxidation products after incubation with macro phages lacking 15-LO (Fig. 3B). These results support the hypothesis that murine intracellular 15-LO oxygenates CE(18:2) and CE(20:4) to the stereospecific products identified.
Fig. 3.
NP LC-MS/MS analysis of oxCEs after incubation of human lipoproteins with murine peritoneal macrophages collected from wild-type (A) or 15-LO-deficient (B) mice. The chromatograms are normalized such that the internal standard peak height is set to 70% relative abundance; 10 ng of the internal standard, CE(17:1), was added before lipid extraction.
Freshly harvested peritoneal macrophages contain 15-LO-specific oxCEs
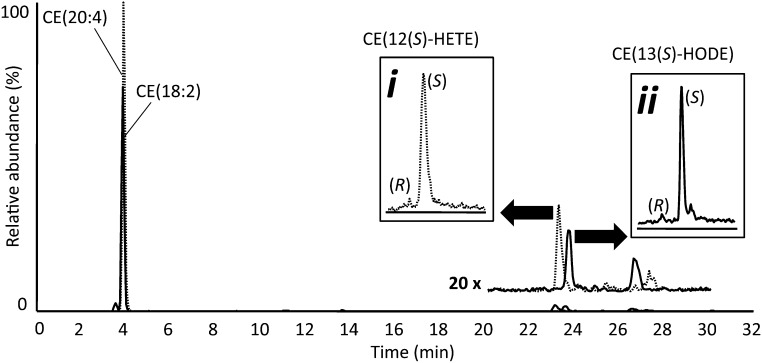
To determine if murine resident peritoneal macrophages contained 15-LO-specific oxCEs in vivo, experiments were carried out to minimize macrophage activation and quench enzymatic activity upon harvesting. Lavage fluid (Ca/Mg-free HBSS) collected from wild-type mice (n = 4) was mixed with one volume of ice-cold MeOH containing SnCl2 (2 mM) immediately after collection from the peritoneal cavity. Lipids were then extracted and analyzed by NP LC-MS/MS. The CE profile was dominated by the polyunsaturated species CE(18:2) and CE(20:4). 15-LO-specific oxidation products of CE(18:2) and CE(20:4) were consistently present at low levels (Fig. 4). When these products were isolated and rechromatographed on a chiral column, they showed high enantiomeric excess of the S configuration, confirming enzymatic oxygenation (Fig. 4, insets). These results indicated that small amounts of 15-LO-specific CE oxidation products were present in freshly harvested murine peritoneal lavages.
Fig. 4.
NP LC-MS/MS analysis of CEs and oxCEs extracted from freshly harvested murine peritoneal macrophages. The portion of the chromatogram from 20 to 30 min is reproduced above the original trace at 20-fold amplification. Insets show chiral separations after the indicated peaks were collected and rechromatographed.
Acyl-remodeling of oxidized CEs
Studies of further metabolism, specifically hydrolysis and reacylation of isotopically labeled CE-derived fatty acyl groups, were carried out by incubating adherent macrophages with CE(d4-18:2) LVs for 20 h. To assess hydrolysis of d4-oxCEs, the medium and the cells were collected together and free fatty acids were isolated by solid-phase extraction before analysis by RP LC-MS/MS. These analyses revealed the presence of free d4-9-HODE and d4-13-HODE (Supplementary Fig. IV), suggesting that oxidized CE(d4-18:2) was hydrolytically cleaved during incubation with macrophages, a finding consistent with previous studies (11). Alternatively, CE(d4-18:2) may have been hydrolyzed before oxygenation. In separate experiments, to determine if oxidized acyl chains could be esterified into macrophage PLs, the incorporation of [1-14C]13-HpODE was determined by TLC. These experiments, detailed in the supplementary materials, indicated this substrate was indeed incorporated into macrophage PLs, mainly PC (Supplementary Fig. V). These findings were consistent with two previous studies demonstrating preferential incorporation of 13-HODE into PC of epithelial cells (26, 27).
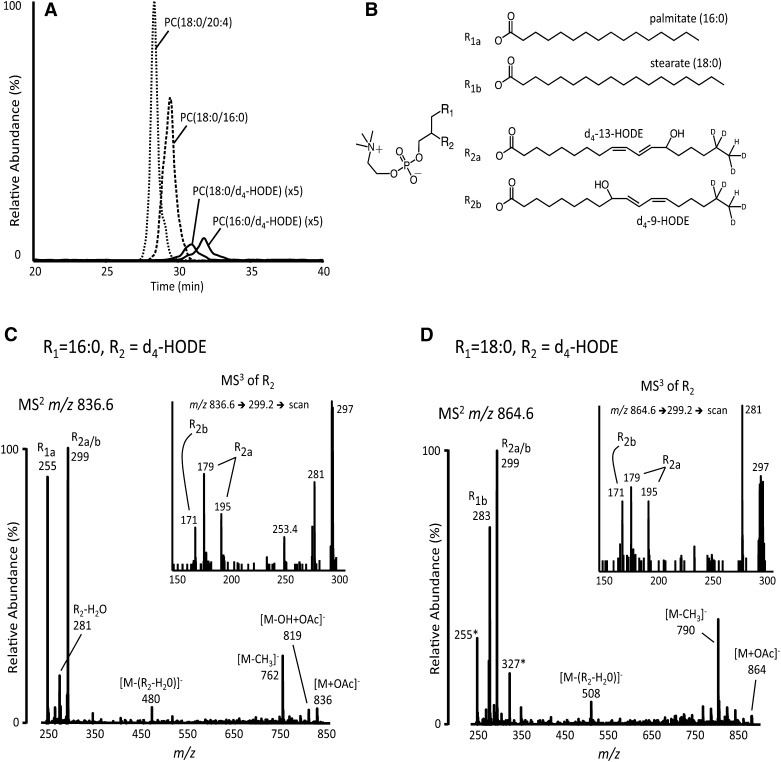
The results of these two experiments suggested that oxidation, hydrolysis, and reacylation of CE-derived fatty acids might lead to formation of oxPC in murine peritoneal macrophages. In additional experiments, CE(d4-18:2) LVs were incubated with macrophages for 20 h. The medium was then removed, the cells were washed, and cellular phospholipids were extracted before analysis by NP LC-MS/MS. Using specific MRM transitions corresponding to PC(18:0/d4-HODE) and PC(16:0/d4-HODE), these molecular species were observed to elute at 31 and 32 min, respectively, just after the elution of unoxidized PCs [e.g., PC(18:0/20:4) and PC(16:0/18:0) at 28 and 29 min], as would be expected for silica-based separation (Fig. 5A). Incorporation of d4-HODE into other PL classes (e.g., PE, PS, PI, or PG) was not detected. To further characterize these phospholipid metabolites of CEs, MS2 and MS3 spectra were acquired as d4-HODE-containing PCs eluted from the HPLC (Fig. 5C,D). The resulting product ion spectra were consistent with the structures proposed in Fig. 5B. Specifically, MS2 product ion spectra of PC acetate adducts, [M+OAc]−, contained the expected carboxylate anions, including the ions at m/z 299 corresponding to d4-HODE as well other product ions typically formed from collisionally induced dissociation of PC-acetate adducts, such as [M-H]− and [M-CH3]− (Fig. 5C,D). When the product ion at m/z 299 was isolated in the linear ion trap of this hybrid instrument and collisionally activated (i.e., MS3), the diagnostic products ions of d4-9-HODE and d4-13-HODE (i.e., m/z 171, 179, and 195) were observed (Fig. 5C,D, insets).
Fig. 5.
Characterization of deuterated, oxidized PC species (d4-oxPC) formed by macrophages after 20 h incubations with CE(d4-18:2). A: The chromatograms show the elution of two common PC species (dashed traces) and two isotopically labeled, oxidized PC species (solid traces). The solid traces are amplified 5-fold. B: Structures of the two most abundant d4-HODE-containing PC species. Relative sn positions and double bond geometries were not determined. Product ion spectra (MS2) of PC(16:0/d4-HODE) (C) or PC(18:0/d4-HODE) (D) acetate adducts, [M+OAc]−, were acquired on-line. MS3 spectra of d4-HODE carboxylate anions, m/z 299, also acquired on-line, are shown in insets. Product ions are labeled with the fatty acyl components indicate in panel B. In panel D, carboxylate product ions from isobaric PC(16:0/22:6), also m/z 864, are indicated with asterisks.
Remodeling of oxCE is a robust pathway of oxidized phosphocholine formation
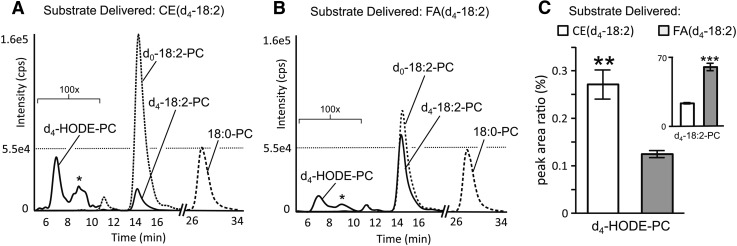
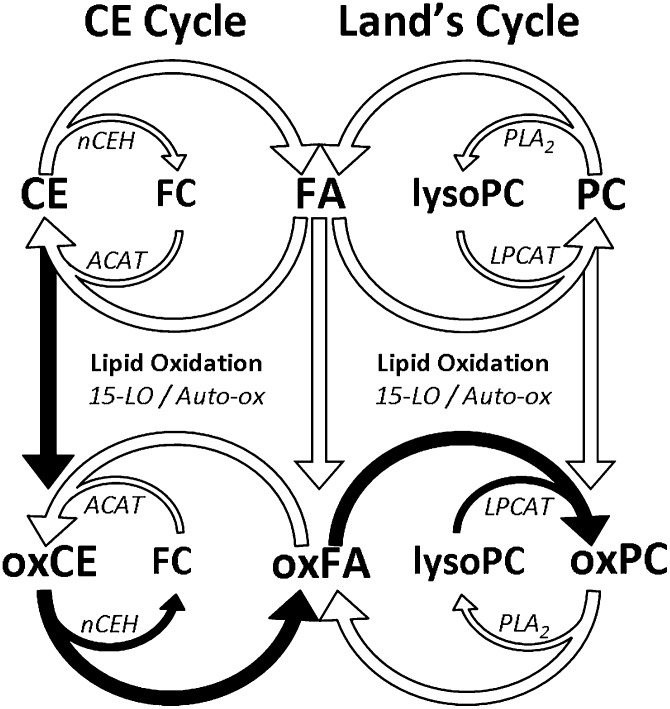
The formation of d4-oxPC from CE(d4-18:2) substrate suggested that oxidation and remodeling of CEs might form oxPC in macrophages. However, the complex network of cellular acyl remodeling pathways could provide alternate routes of d4-oxPC formation, which did not involve remodeling of oxidized CE(d4-18:2), such as oxidation of preformed PC(16:0/d4-18:2) or oxidation of free d4-18:2 and subsequent esterification into PC. To determine if d4-oxCE remodeling contributed significantly to d4-oxPC formation or if the above-mentioned alternate routes were responsible for the observed d4-oxPC, murine peritoneal macrophages were incubated in parallel with LVs containing CE(d4-18:2) or an equivalent molar amount of free d4-18:2. After incubation, production of PC(16:0/d4-HODE) was compared by RP LC-MS/MS analysis of cellular phospholipids. Macrophage incubation with free d4-18:2 resulted in significant incorporation of this substrate into PC, with over 40% of the total PC(16:0/18:2) being deuterated (Fig. 6B). Macrophages incubated with CE(d4-18:2) formed far less PC(16:0/d4-18:2), with approximately 12% of the total PC(16:0/18:2) being deuterated (Fig. 6A). However, macrophage incubation with CE(d4-18:2) resulted in significantly more PC(16:0/d4-HODE) than did incubation with free d4-18:2 (Fig. 6C). This result is even more remarkable considering that the insoluble LVs containing CE(d4-18:2) have greatly reduced access to the adhered macrophages than the soluble d4-18:2. These results suggested that metabolism of oxidized CE(d4-18:2) (Fig. 7, filled arrows) was a robust pathway for d4-oxPC production when compared with oxidation and acylation of free d4-18:2 or oxygenation of preformed PC(d4-18:2) (Fig. 7, open arrows).
Fig. 6.
Oxidation and remodeling of CE(d4-18:2) produces more PC(d4-HODE) than oxidation and esterification of d4-18:2 or oxidation of preformed PC(d4-18:2). Chromatograms show RP LC-MS/MS analysis of palmitoyl-containing PC species, esterified with the indicated acyl component, after 20 h macrophage incubations with CE(d4-18:2) (A) or free d4-18:2 (B). Peaks marked with asterisks are PC(d4-EpOME) isomers. C: The relative abundances of PC(d4-HODE) and PC(d4-18:2) (inset) were determined by the peak area ratio of deuterated analyte to PC(16:0/18:0). Values are shown as means ± SEM (n = 3). ** P < 0.005; *** P < 0.0005.
Fig. 7.
Simplified schematic representation of the proposed interactions between the CE cycle and the Lands cycle acyl remodeling pathways. The proposed route of oxPC formation through oxCE remodeling is indicated by filled arrows. Enzymes mediating hydrolysis and esterification events are represented by abbreviated names as follows: ACAT, acetyl-CoA acyltransferase; LPCAT, lysophosphatidylcholine acyl transferase; nCEH, neutral cholesterol hydrolase; PLA2, phospholipase A2.
DISCUSSION
Direct oxidation of multiple CE substrates by macrophage 15-LO
Previous studies demonstrating direct oxygenation of CE(18:2) were performed using recombinant 15-LO and therefore did not address enzymatic oxidation of CE substrate in the intact cell (5, 28, 29). Separate studies, examining CE oxidation in intact cells (e.g., murine peritoneal macrophages, fibroblasts, and human monocytes), demonstrated that 15-LO expression increased oxidized CE content; however, direct evidence of enzymatic CE oxidation (i.e., chiral oxCEs) was not shown (9, 10, 30). Because 15-LO is capable of oxidizing substrates other than CE, and considering that hydroperoxy species can initiate radical propagation, the oxidation of CE observed may have been an indirect effect of the lipoxygenase. Furthermore, 15-LO is an intercellular enzyme, and therefore its interaction with substrate originating outside the cytoplasm is not straightforward. To determine whether CEs, both endogenous and from the extracellular environment, could be directly modified by 15-LO within intact cells, isotopically labeled CE(d4-18:2) LVs were incubated with murine peritoneal macrophages. This allowed metabolism of CE(d4-18:2) to be analyzed independent of endogenous CEs, which are present in lipid bodies within murine peritoneal macrophages. These experiments revealed that CE(d4-18:2) taken up from the medium was directly oxygenated by intracellular 15-LO as evidenced by the deuterated, chiral oxidation product, CE(d4-13(S)-HODE) (Fig. 1A).
Nondeuterated CEs were also oxidized by 15-LO during macrophage incubations, indicating that CEs in endogenous lipid bodies were substrates for enzymatic oxidation. As previously reported using recombinant 15-LO, CE(13-S)-HODE) was the product of CE(18:2) oxygenation. Additionally, it was observed in the macrophage that CE(20:4) was oxidized, specifically forming CE(12-S)-HETE) in an even more efficient reaction than the oxidation of CE(18:2). Because the 15-LO null macrophages produced neither product, this single enzyme was likely responsible for the oxidation of both CE(18:2) and CE(20:4) (Fig. 3). Although these results demonstrated that 15-LO has dual CE substrate specificity, there was no evidence for the dual regiospecificity normally observed during peroxidation of free fatty acids. For example, 15-LO exclusively formed CE(12-HETE) from CE(20:4) (Supplementary Fig. IIIB) despite the fact that this mouse enzyme produces 12- and 15-HETE from free arachidonate (31). This curious finding suggests that binding interactions of the CE acyl chain within the 15-LO catalytic pocket are remotely influenced by the presence of the lipophilic cholesterol moiety. Cholesteryl esters are highly insoluble in aqueous solutions and are almost exclusively found inside lipid bodies (or synthetic variants) or the membranes engaged in their synthesis. In order to oxidize CEs, 15-LO must associate with the amphipathic portion of these macromolecular structures, which may induce conformational changes in the enzyme enhancing regiospecificity. Alternatively, CE within lipid bodies may be presented to 15-LO in a different manner than the association of free fatty acids.
To examine whether oxidation of extracellular CE would extend to substrate bound in actual LP particles, adherent macrophages were incubated in medium containing human LP. Marked increases in cellular CE content after LP incubation indicated that the macrophages had likely taken up significant amounts of LP, that LP had adhered to the outer surfaces of the macrophages, or both (Fig. 2B). Comparable increases in 15-LO-specific oxCEs, nearly 100-fold over control macrophages (Fig. 2C), suggested that some of the CE derived from human LP had accessed the intracellular compartment, where 15-LO directly oxidized the newly taken up CE substrate. These oxCEs were readily formed without the need for potent experimental stimuli (e.g., macrophage stimulation), consistent with the activity of 15-LO, which requires minimal activation (32). Therefore, the concomitant increases in the level of available substrate and 15-LO-specific CE oxidation products suggested that 15-LO CE oxidase activity is partly regulated by simple substrate availability. These observations further implicate direct 15-LO catalyzed CE oxidation as an initiating event during in vitro LDL oxidation. However, because evidence of chiral products eroded over time, this phenomenon may be difficult to track in vivo using oxidized product stereochemistry. The occurrence of chiral oxCEs in freshly harvested murine peritoneal macrophages (Fig. 4) strongly suggests that 15-LO-catalyzed CE oxidation occurs in the animal and therefore may play a role in CE metabolism in vivo, but it remains to be determined whether this pathway is relevant to LDL oxidation and atherogenesis within the arterial wall.
Interactions of Acyl remodeling pathways generate oxPC from oxCE
Considering that cellular cholesterol is in dynamic equilibrium between free and esterified forms and that there is evidence that oxCEs are included in this cycle (33, 34), we hypothesized that remodeling of abundant oxCEs in intact macrophages could lead to the generation of other oxidized lipid species, particularly oxPLs. The detection of free d4-HODE isomers after macrophage incubation with CE(d4-18:2) supported the hypothesis that oxCEs are substrates for CE hydrolases. This reaction itself may have biological implications; indeed, the hydrolysis products of both 15-LO-specific oxCEs, 13(S)-HODE and 12(S)-HETE, are ligands for peroxisome proliferator-activated receptor-γ and the newly defined G protein-coupled 12(S)-HETE receptor, respectively (35, 36). Although the hydrolysis of oxCEs has previously been reported, the fate of the newly freed oxidized acyl components has not been examined.
Similar to the CE cycle, the Lands cycle describes the liberation and esterification of fatty acyl moieties into phospholipid pools (Fig. 7) (37). The incorporation of [1-14C]13-HpODE into PC of peritoneal macrophages suggested that oxylipins were viable substrates for the Lands cycle in macrophages; however, whether oxidized acyl species derived from hydrolytic actions of the CE cycle could be substrates for incorporation into PC through the Lands cycle was unclear. The detection of d4-oxPC species [e.g., PC(16:0/d4-HODE)] in macrophages after CE(d4-18:2) incubation (Fig. 5) provided the initial evidence that acyl components of hydrolyzed d4-oxCEs might be transferred to PC via the Lands cycle forming d4-oxPC.
We speculate that oxidation and remodeling of cellular CE might efficiently form oxPC for two reasons. First, CEs are particularly good targets for oxidation, as evidenced by high levels of oxCE in human atheromata and the ease of experimentally forming oxCEs reported here and elsewhere (17). Indeed, the primary CE species [i.e., CE(18:2) and CE(20:4)] are both substrates for 15-LO (38) and susceptible to free-radical mediated oxidation. Second, the required enzymatic machinery for CE conversion to oxPC appears to be localized to cytoplasmic lipid bodies. Both 15-LO and neutral cholesterol hydrolase associate with lipid bodies (38, 39), and oxCEs have been shown to be preferred substrates for hydrolysis (11). Additionally, the newly described lysophosphatidylcholine acyltransferases 1 and 2 are also localized to lipid bodies where they catalyze acylation of lysophosphatidylcholine at the monolayer (40). The results described here demonstrate that remodeling of oxCE indeed contributes to PC oxidation and suggests that the presence of abundant oxCE may influence overall PC oxidation levels in the macrophage. Because oxCEs can accumulate significantly under certain circumstances (e.g., in the atheromatous plaque), their degradation and remodeling into biologically active compounds such as oxPC, even to a partial extent, may have biological implications. Indeed, this work suggests that a portion of the atherogenic oxPC, which has been the focus of considerable attention (15), might originate from 15-LO-initiated oxidation of susceptible CEs and subsequent metabolism of these unique species.
Supplementary Material
Footnotes
Abbreviations:
- CE
- cholesteryl ester
- CE(18:2)
- cholesteryl linoleate
- CE(d4-18:2)
- cholesteryl 17,17,18,18-d4-linoleate
- CE(20:4)
- cholesteryl arachidonate
- CE(22:6)
- cholesteryl docosahexaenoate
- CE(13-(S)-HpODE)
- cholesteryl 13(S)-hydroperoxy-9,11-octadecadienoic acid
- CE(13-(S)-HODE)
- cholesteryl 13(S)-hydroxy-9,11-octadecadienoic acid
- CE(12-(S)-HETE)
- cholesteryl 12(S)-hydroxy-5,8,10,14-eicosatetraenoic acid
- d4-18:2
- 17,17,18,18-d4-linoleic acid
- 15-LO
- 15-lipoxygenase
- PL
- phospholipid
- PC
- phosphatidylcholine
- LV
- synthetic lipid vesicle
- LP
- human lipoprotein
- MRM
- multiple reaction monitoring
- NP
- normal phase
- RP
- reverse phase
This work was supported in part by the LIPIDMAPS Large Scale Collaborative grant (GM069338) from the General Medical Sciences Institute of the National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of five figures and one table.and supplementary methods and results.
REFERENCES
- 1.Quehenberger O., Armando A. M., Brown A. H., Milne S. B., Myers D. S., Merrill A. H., Bandyopadhyay S., Jones K. N., Kelly S., Shaner R. L., et al. 2010. Lipidomics reveals a remarkable diversity of lipids in human plasma. J. Lipid Res. 51: 3299–3305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartz R., Li W-H., Venables B., Zehmer J. K., Roth M. R., Welti R., Anderson R. G. W., Liu P., Chapman K. D. 2007. Lipidomics reveals that adiposomes store ether lipids and mediate phospholipid traffic. J. Lipid Res. 48: 837–847 [DOI] [PubMed] [Google Scholar]
- 3.Brown M. S., Goldstein J. L., Krieger M., Ho Y. K., Anderson R. G. 1979. Reversible accumulation of cholesteryl esters in macrophages incubated with acetylated lipoproteins. J. Cell Biol. 82: 597–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghosh S., Zhao B., Bie J., Song J. 2010. Macrophage cholesteryl ester mobilization and atherosclerosis. Vascul. Pharmacol. 52: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belkner J., Stender H., Kühn H. 1998. The rabbit 15-lipoxygenase preferentially oxygenates LDL cholesterol esters, and this reaction does not require vitamin E. J. Biol. Chem. 273: 23225–23232 [DOI] [PubMed] [Google Scholar]
- 6.Funk C. D., Cyrus T. 2001. 12/15-lipoxygenase, oxidative modification of LDL and atherogenesis. Trends Cardiovasc. Med. 11: 116–124 [DOI] [PubMed] [Google Scholar]
- 7.Heinecke J. W. 1998. Oxidants and antioxidants in the pathogenesis of atherosclerosis: implications for the oxidized low density lipoprotein hypothesis. Atherosclerosis. 141: 1–15 [DOI] [PubMed] [Google Scholar]
- 8.Ezaki M., Witztum J. L., Steinberg D. 1995. Lipoperoxides in LDL incubated with fibroblasts that overexpress 15-lipoxygenase. J. Lipid Res. 36: 1996–2004 [PubMed] [Google Scholar]
- 9.Folcik V. A., Cathcart M. K. 1994. Predominance of esterified hydroperoxy-linoleic acid in human monocyte-oxidized LDL. J. Lipid Res. 35: 1570–1582 [PubMed] [Google Scholar]
- 10.Rankin S. M., Parthasarathy S., Steinberg D. 1991. Evidence for a dominant role of lipoxygenase(s) in the oxidation of LDL by mouse peritoneal macrophages. J. Lipid Res. 32: 449–456 [PubMed] [Google Scholar]
- 11.Belkner J., Stender H., Holzhütter H. G., Holm C., Kühn H. 2000. Macrophage cholesteryl ester hydrolases and hormone-sensitive lipase prefer specifically oxidized cholesteryl esters as substrates over their non-oxidized counterparts. Biochem. J. 352: 125–133 [PMC free article] [PubMed] [Google Scholar]
- 12.Belkner J., Chaitidis P., Stender H., Gerth C., Kuban R. J., Yoshimoto T., Kuhn H. 2005. Expression of 12/15-lipoxygenase attenuates intracellular lipid deposition during in vitro foam cell formation. Arterioscler. Thromb. Vasc. Biol. 25: 797–802 [DOI] [PubMed] [Google Scholar]
- 13.Kuhn H., Belkner J., Wiesner R., Brash A. R. 1990. Oxygenation of biological membranes by the pure reticulocyte lipoxygenase. J. Biol. Chem. 265: 18351–18361 [PubMed] [Google Scholar]
- 14.Hazen S. L. 2008. Oxidized phospholipids as endogenous pattern recognition ligands in innate immunity. J. Biol. Chem. 283: 15527–15531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Itabe H. 1998. Oxidized phospholipids as a new landmark in atherosclerosis. Prog. Lipid Res. 37: 181–207 [DOI] [PubMed] [Google Scholar]
- 16.Suarna C., Dean R. T., Southwell-Keeley P. T., Moore D. E., Stocker R. 1997. Separation and characterization of cholesteryl oxo- and hydroxy-linoleate isolated from human atherosclerotic plaque. Free Radic. Res. 27: 397–408 [DOI] [PubMed] [Google Scholar]
- 17.Hutchins P. M., Moore E. E., Murphy R. C. 2011. Electrospray MS/MS reveals extensive and nonspecific oxidation of cholesterol esters in human peripheral vascular lesions. J. Lipid Res. 52: 2070–2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luthria D. L., Sprecher H. 1995. Metabolism of deuterium-labeled linoleic, 6,9,12-octadecatrienoic, 8,11,14-eicosatrienoic, and arachidonic acids in the rat. J. Lipid Res. 36: 1897–1904 [PubMed] [Google Scholar]
- 19.Hutchins P. M., Barkley R. M., Murphy R. C. 2008. Separation of cellular nonpolar neutral lipids by normal-phase chromatography and analysis by electrospray ionization mass spectrometry. J. Lipid Res. 49: 804–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chapman K. D., Trelease R. N. 1991. Acquisition of membrane lipids by differentiating glyoxysomes: role of lipid bodies. J. Cell Biol. 115: 995–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zarini S., Gijón M. A., Folco G., Murphy R. C. 2006. Effect of arachidonic acid reacylation on leukotriene biosynthesis in human neutrophils stimulated with granulocyte-macrophage colony-stimulating factor and formyl-methionyl-leucyl-phenylalanine. J. Biol. Chem. 281: 10134–10142 [DOI] [PubMed] [Google Scholar]
- 22.Qiu Z. H., de Carvalho M. S., Leslie C. C. 1993. Regulation of phospholipase A2 activation by phosphorylation in mouse peritoneal macrophages. J. Biol. Chem. 268: 24506–24513 [PubMed] [Google Scholar]
- 23.Gijón M. A., Zarini S., Murphy R. C. 2007. Biosynthesis of eicosanoids and transcellular metabolism of leukotrienes in murine bone marrow cells. J. Lipid Res. 48: 716–725 [DOI] [PubMed] [Google Scholar]
- 24.Riekhof W. R., Wu J., Gijón M. A., Zarini S., Murphy R. C., Voelker D. R. 2007. Lysophosphatidylcholine metabolism in Saccharomyces cerevisiae. J. Biol. Chem. 282: 36853–36861 [DOI] [PubMed] [Google Scholar]
- 25.Steinbrecher U. P., Parthasarathy S., Leake D. S., Witztum J. L., Steinberg D. 1984. Modification of low density lipoprotein by endothelial cells involves lipid peroxidation and degradation of low density lipoprotein phospholipids. Proc. Natl. Acad. Sci. USA. 81: 3883–3887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fang X., Kaduce T. L., Spector A. A. 1999. 13-(S)-Hydroxyoctadecadienoic acid (13-HODE) incorporation and conversion to novel products by endothelial cells. J. Lipid Res. 40: 699–707 [PubMed] [Google Scholar]
- 27.Cho Y., Ziboh V. A. 1994. Incorporation of 13-hydroxyoctadecadienoic acid (13-HODE) into epidermal ceramides and phospholipids: phospholipase C-catalyzed release of novel 13-HODE-containing diacylglycerol. J. Lipid Res. 35: 255–262 [PubMed] [Google Scholar]
- 28.Belkner J., Wiesner R., Kühn H., Lankin V. Z. 1991. The oxygenation of cholesterol esters by the reticulocyte lipoxygenase. FEBS Lett. 279: 110–114 [DOI] [PubMed] [Google Scholar]
- 29.Belkner J., Wiesner R., Rathman J., Barnett J., Sigal E., Kühn H. 1993. Oxygenation of lipoproteins by mammalian lipoxygenases. Eur. J. Biochem. 213: 251–261 [DOI] [PubMed] [Google Scholar]
- 30.Sun D., Funk C. D. 1996. Disruption of 12/15-lipoxygenase expression in peritoneal macrophages. Enhanced utilization of the 5-lipoxygenase pathway and diminished oxidation of low density lipoprotein. J. Biol. Chem. 271: 24055–24062 [PubMed] [Google Scholar]
- 31.Colin D. F. 1996. The molecular biology of mammalian lipoxygenases and the quest for eicosanoid functions using lipoxygenase-deficient mice. Biochim. Biophys. Acta 1304: 65–84 [DOI] [PubMed] [Google Scholar]
- 32.Walther M., Wiesner R., Kuhn H. 2004. Investigations into calcium-dependent membrane association of 15-lipoxygenase-1. Mechanistic roles of surface-exposed hydrophobic amino acids and calcium. J. Biol. Chem. 279: 3717–3725 [DOI] [PubMed] [Google Scholar]
- 33.Brown M. S., Ho Y. K., Goldstein J. L. 1980. The cholesteryl ester cycle in macrophage foam cells. Continual hydrolysis and re-esterification of cytoplasmic cholesteryl esters. J. Biol. Chem. 255: 9344–9352 [PubMed] [Google Scholar]
- 34.Mathur S. N., Albright E., Field F. J. 1988. Incorporation of lipoxygenase products into cholesteryl esters by acyl-CoA:cholesterol acyltransferase in cholesterol-rich macrophages. Biochem. J. 256: 807–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagy L., Tontonoz P., Alvarez J. G., Chen H., Evans R. M. 1998. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell. 93: 229–240 [DOI] [PubMed] [Google Scholar]
- 36.Guo Y., Zhang W., Giroux C., Cai Y., Ekambaram P., Dilly A-K., Hsu A., Zhou S., Maddipati K. R., Liu J., et al. 2011. Identification of the orphan G protein-coupled receptor GPR31 as a receptor for 12-(S)-hydroxyeicosatetraenoic acid. J. Biol. Chem. 286: 33832–33840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lands W. E. 1958. Metabolism of glycerolipides; a comparison of lecithin and triglyceride synthesis. J. Biol. Chem. 231: 883–888 [PubMed] [Google Scholar]
- 38.Weibel G. L., Joshi M. R., Wei C., Bates S. R., Blair I. A., Rothblat G. H. 2009. 15(S)-Lipoxygenase-1 associates with neutral lipid droplets in macrophage foam cells: evidence of lipid droplet metabolism. J. Lipid Res. 50: 2371–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao B., Fisher B. J., St Clair R. W., Rudel L. L., Ghosh S. 2005. Redistribution of macrophage cholesteryl ester hydrolase from cytoplasm to lipid droplets upon lipid loading. J. Lipid Res. 46: 2114–2121 [DOI] [PubMed] [Google Scholar]
- 40.Moessinger C., Kuerschner L., Spandl J., Shevchenko A., Thiele C. 2011. Human lysophosphatidylcholine acyltransferases 1 and 2 are located in lipid droplets where they catalyze the formation of phosphatidylcholine. J. Biol. Chem. 286: 21330–21339 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.