Abstract
The management of localized prostate cancer is controversial, and in the absence of comparative trials to inform best practice, choices are driven by personal beliefs with wide variation in practice patterns. Men with localized disease diagnosed today often undergo treatments that will not improve overall health outcomes, and active surveillance has emerged as one approach to reducing this overtreatment of prostate cancer. The selection of appropriate candidates for active surveillance should balance the risk of harm from prostate cancer without treatment, and a patient’s personal preferences for living with a cancer and the potential side effects of curative treatments. Although limitations exist in assessing the potential for a given prostate cancer to cause harm, the most common metrics used today consider cancer stage, prostate biopsy features, and prostate-specific antigen level together with the risk of death from nonprostate causes based on age and overall state of health.
Widespread prostate-specific antigen (PSA) testing for the early diagnosis of prostate cancer has been embraced as a strategy for reducing prostate cancer mortality in the United States. PSA-driven prostate biopsies uncover cancers that would have remained silent otherwise, and because most men who are diagnosed with prostate cancer undergo treatment, there is growing concern that overtreatment of prostate cancer is common. Active surveillance as an alternative to immediate curative intervention for carefully selected men could reduce the harms of invasive treatment of prostate cancer among men with nonaggressive disease (1). In the United States, active surveillance is an underutilized management option.
Active Surveillance for Prostate Cancer: An Underutilized Opportunity for Reducing Harm
The terms “watchful waiting,” “expectant management,” and “active surveillance” have been used interchangeably to indicate no immediate treatment for a newly diagnosed prostate cancer. However, the goal of watchful waiting is to avoid treatment in men with a limited life expectancy who have advanced disease—treatment being delayed until the development of metastatic disease with the intent to palliate, not cure (2). In contrast, active surveillance (expectant management) is an attempt to individualize management for men with localized disease who are fit for radical treatment—the intent to treat early for cure if the disease should progress. The underlying assumption with active surveillance is that a patient’s cancer can be as successfully treated with delayed intervention as with immediate intervention, and that by delaying intervention, quality adjusted life years will be improved. The most appropriate candidates for surveillance, the approach for monitoring men on surveillance, and the safety of active surveillance in terms of cancer mortality risk are being debated (1). However, despite strong evidence from randomized trials for the safety of surveillance for selected men (3,4), approximately 10% of patients in the United States are managed with surveillance (5) compared with three times that rate in European countries (6). Barriers to active surveillance could include patient and physician fear of losing the opportunity for cure, a litigious healthcare system, misaligned incentives that favor treatment, or a combination of these.
Current Paradigm of Active Surveillance
The current paradigm of active surveillance emphasizes risk stratification to identify men who are least likely to be harmed by prostate cancer in the absence of treatment. But in addition to tumor metrics (stage, grade, PSA) that are associated with the biological potential of the cancer (Table 1), consideration should be given to the risk of death from other causes dependent on age and overall health state. Furthermore, a man’s personal preferences for living with a cancer and living with the potential side effects of treatment should be weighed heavily in decision making. Available tumor metrics—biopsy extent of cancer and grade, cancer stage, PSA, and volume-adjusted PSA levels—correlate with the biological potential of the cancer when intermediate (pathological stage and grade, cancer recurrence after treatment) and longer term outcomes (cancer-specific and metastatic-free survival) are measured (7). Further, the patient metrics of age, health status, and personal preferences also correlate with health outcomes (8). For example, competing causes of death in older men or those in poor health can limit the biological potential of the cancer and the risk it will harm the host, and a patient’s preferences for living with cancer and/or side effects of treatment will impact quality-adjusted life expectancy (QALE).
Table 1.
Factors to consider in selection of men for active surveillance*
| Considerations | Metrics | |
|---|---|---|
| Risk of death from prostate cancer without treatment | Cancer grade, PSA, biopsy extent of cancer, PSA density, prostate volume | |
| Risk of death from cause other than prostate cancer | Patient age and overall health state | |
| Personal preferences | Living with cancer and potential side effects of treatment |
* PSA = prostate-specific antigen. PSA density is PSA divided by prostate volume.
The limitations of our current paradigm are an inability to accurately assess—on an individual basis—the presence or absence of a lethal cancer phenotype, individual life expectancy, a patient’s personal preferences for living with cancer or progression of the disease in an untreated state, and living with the potential side effects of treatment.
Tumor Metrics for Active Surveillance
Numerous studies have shown that the clinical stage, the extent and grade of cancer on a prostate biopsy, the PSA level, and volume-corrected PSA (PSA density) are all associated with the pathological extent of cancer and the probability of remaining free of disease after treatment (7).
A limitation of current paradigms that assess disease risk using biopsy grade is misclassification. For example, the strongest predictor of cancer-specific mortality with or without treatment of prostate cancer is grade (9,10). Thus, grade is used in all risk stratification tools. However, a pretreatment assessment of grade is associated with both over- and underestimation of risk—underestimation because studies have shown upgrading rates of around 25% at surgery for men who are thought to harbor favorable risk cancers on prostate biopsy (11), and overestimation because of grade inflation (12). Albertson et al. have demonstrated that cancers that are graded as high grade today (especially Gleason score 7) were classified as low-grade cancers previously (12). Thus, although grade is an important marker of risk, assessment of grade can be misleading as a measure of cancer lethality, especially for the most common grades (Gleason score 6 and 3 + 4) assigned today. This uncertainty with respect to risk assessment, in all likelihood, leads many physicians to recommend curative intervention because of the possibility of underestimation of risk.
Patient Metrics for Active Surveillance
Age and Overall Health Status.
Patient age and overall health that influence remaining years of life should play a major role in decision making regarding the option of active surveillance versus treatment. There is now high-level evidence from randomized trials demonstrating that for most men, even over 10–15 years after diagnosis, treatment of prostate cancer is unlikely to lead to additional years of life or reduce the chances of dying of prostate cancer when compared with no treatment (3,4). This is especially true for those men with low-risk disease in whom favorable features (PSA, biopsy grade, clinical stage) are associated with a long natural history in the absence of treatment. Thus, given the low likelihood of harm in the absence of treatment for older men with favorable-risk prostate cancer, personal preferences should be an important part of decision making.
Patient Preferences and Expectations.
When there is a lack of consensus regarding “best” practice for management of a disease, it is intuitive that the patient should play a large role in decision making. The treatment of localized prostate cancer is a good example of a problem for which shared decision making is paramount to realize a favorable outcome in terms of balancing benefit and harm.
Xu et al. (13) found that both physician recommendations and patient values and beliefs about prostate cancer influenced the management decision after a diagnosis of prostate cancer. However, a patient’s perception of the efficacy of treatment and its side effects derived mostly from the physician influenced a patient’s decision the most. Evaluating the decision-making process for active surveillance, Davison and Goldenberg found that two of three men played more than a passive role in decision making (14).
In two decision analyses, patient preferences strongly influenced QALE with active surveillance compared with curative intervention with radiation or surgery. Hayes et al. (15) found that for men aged 65 years and in average health, active surveillance was a reasonable management option for favorable-risk prostate cancer when the outcome of interest was QALE. However, results were sensitive to individual preferences for living with cancer and for side effects of treatment. Liu et al. (16) also found that for men with favorable-risk prostate cancer, QALE was higher for older men and/or those in poor health with surveillance compared with surgery. But, the outcome was dependent on patient preferences for living under surveillance and living with side effects of treatment. Patient expectations regarding the impact of treatment can be unrealistic in an era when unfounded claims often influence decisions (17), and unrealistic expectations can increase the probability of regret after treatment.
Schroeck et al. (18) reported that when compared with radical retropubic prostatectomy, men undergoing robot-assisted laparoscopic prostatectomy were three times more likely to be regretful and dissatisfied with their treatment. The authors attributed this to unrealistic expectations with a “new” innovative procedure. This study emphasizes the importance of patient expectations on outcome in the overall decision-making process.
Eligibility for Active Surveillance and the Effect of Patient Selection on Outcomes
The most commonly used criteria for assessing candidates for surveillance is that proposed by D’Amico et al. (19) and Epstein et al. (20) and recommended for use by the National Comprehensive Cancer Center (NCCN) (21) and the European Association of Urology (EUA) (22) (Table 2). Under this classification scheme, surveillance with the intention of cure, if needed, is considered most appropriate for those men with favorable (low to very low risk) to intermediate-risk prostate cancer. For men with intermediate-risk disease, surveillance is considered appropriate if the predicted life expectancy is below 10 years, and men with high-risk prostate cancer are not considered ideal candidates for surveillance because of the risk of disease progression without treatment.
Table 2.
Prostate cancer risk strata*
| Risk strata | Criteria† | Approximate proportion of newly diagnosed cases‡ |
|---|---|---|
| Favorable | 35% | |
| Very low | •T1c | |
| • Gleason score ≤6 | ||
| • PSA <10ng/mL | ||
| • Fewer than three biopsy cores positive, ≤50% cancer in any core | ||
| • PSA density <0.15ng/mL/cc | ||
| Low | • T1 or T2a | |
| • Gleason score ≤6 | ||
| • PSA <10ng/mL | ||
| Intermediate | • T2b–T2c or | 33% |
| • Gleason score 7 or | ||
| • PSA 10–20ng/mL | ||
| High | • T3a or | 32% |
| • Gleason score 8–10 or | ||
| • PSA >20ng/mL |
More recently, the NCCN described a “very low risk” category using the criteria originally reported by Epstein et al. (20) that uses prostate biopsy findings and the PSA corrected for prostate volume (PSA density) to predict the presence of small-volume (<0.5mL), low-grade (Gleason pattern 3 or lower) cancer (Table 2). Assuming that a cancer volume below 0.5mL that is low grade will behave in an indolent fashion for a prolonged period, and assuming that men who harbor these small-volume, low-grade cancers are ideal candidates for active surveillance, the “Epstein criteria” predicted small-volume disease with a sensitivity of 56% and specificity of 95% (Table 3). Because this model accurately predicts the presence of cancers that are not small volume and low grade (high specificity), to avoid the potential undertreatment of men with larger volume, higher grade cancers requires treating a large proportion of men with small-volume disease (low sensitivity). Among men with Gleason score 6 prostate cancer on a prostate biopsy, the criteria proposed by Epstein predict for a high likelihood of organ-confined disease and low likelihood of recurrence after surgical treatment (29).
Table 3.
Models for prediction of tumor volume <0.5mL and Gleason pattern 3 or less*
| Study | Criteria | Sensitivity | Specificity | |||
|---|---|---|---|---|---|---|
| Epstein et al. (20) | Biopsy extent, grade, PSA density | .56 | .95 | |||
| Kattan et al. (61) | Stage, PSA, biopsy extent, grade, prostate volume | .30 | .90 | |||
| Nakanishi et al. (62) | Age, PSA density, biopsy extent, grade | .77 | .55 | |||
| Steyerberg et al. (63) | Stage, PSA, biopsy extent, grade, prostate volume | .35 | .93 |
* PSA= prostate-specific antigen. PSA density is PSA divided by prostate volume. Biopsy extent is extent of cancer on prostate biopsy as estimated from length of biopsy core involved with cancer, number of cores with cancer, or percentage of the core involved with cancer.
Many other predictive models for “insignificant” disease have been published, and all use criteria that include combinations of clinical stage, pretreatment PSA, prostate volume, age, biopsy grade, and the extent of cancer on biopsy (Table 3). However, none has both high sensitivity and specificity, and all would require substantial overtreatment of small-volume, low-grade cancers to prevent undertreatment of those with larger volume, higher grade cancers. There is no general consensus on the “ideal” candidate for active surveillance, but most investigators have emphasized that those with low-risk features (low grade, low stage, low PSA, minimal disease on biopsy) are least likely to be harmed without treatment.
Observed Outcomes in the Absence of Treatment
Nonrandomized Observations.
Albertsen et al. (30) were among the first investigators to chronicle the importance of competing causes of death on the natural history of favorable-risk prostate cancers in the pre-PSA era among untreated men. For men with low-grade cancers (Gleason score ≤6), the 20-year cancer-specific death rates without treatment ranged from 6% to 30% depending upon the Gleason score. These data almost certainly overestimate “true” cancer-specific mortality today for two reasons: the lead time afforded with PSA testing and grade migration. When compared with cancers detected without the use of PSA, PSA-detected cancers are thought to be diagnosed on average 6–12 years earlier depending upon patient age (31). Further, as pointed out by Albertsen et al. (12), many of the Gleason scores assigned as low grade in the past would be reclassified today as higher grade; thus the outcomes stratified by Gleason score in the past may have been overly pessimistic. In this regard, the lethality of PSA screen-detected cancers is overestimated by earlier pre-PSA era studies that evaluated untreated men.
There are no data on 15–20-year outcomes for untreated men with favorable-risk prostate cancer diagnosed in the modern “PSA era.” However, Lu-Yao et al. (32) evaluated 10-year outcomes of untreated men in the pre-PSA era (prior to 1992) compared with the PSA era (1992–2002). They reported that among those with moderately differentiated cancers (Gleason scores 5–7), cancer-specific mortality for untreated men aged 65–74 years after 10 years was 2%–6% for those diagnosed from 1992 to 2002 compared with 15%–23% for those diagnosed prior to 1992. In a competing risks model of hazard from prostate cancer mortality, Parker et al. (33) estimated the 15-year risk of prostate cancer mortality in the PSA era to be 0%–2% for men aged 55–74 years diagnosed with a Gleason score of 6 or below and managed conservatively. Thus, evidence suggests that when compared with prostate cancer diagnosed in the absence of PSA screening, prostate cancer diagnosed today has a longer natural history and is more likely to behave in an indolent fashion without treatment.
Observations From Randomized Trials.
The Scandinavian Prostate Cancer Group Study-4 (SPGS-4) randomized men from the pre-PSA era with localized prostate cancer to surgery versus observation with a median follow-up of 13 years (3). Unlike a contemporary cohort, these men had non–screen-detected prostate cancers, over three in four with palpable disease, almost 50% with PSA levels over 10ng/mL, and one in three with Gleason score of 7 or above (34). The cumulative incidence of death from prostate cancer among those undergoing observation was 21% overall and for those with low-risk disease (Gleason score ≤6 and PSA <10ng/mL) was 11% (Table 4). The cumulative incidence of metastatic disease was 33% in the observation arm. As compared with observation, radical prostatectomy significantly improved overall, cancer-specific, and metastatic-free survival, but benefits appeared limited to those under age 65 years in a subset analysis. Further, a subset analysis of those with low-risk disease suggested that when compared with observation, surgically treated patients had a significant 11% absolute reduction in the risk of metastatic disease, which is a benefit limited to those under age 65 years.
Table 4.
Outcomes in observation arms of randomized trials comparing surgery with observation for localized prostate cancer*
| Study | Mean age, y | Median follow-up, y | Study era (years of enrollment) | Cumulative incidence of prostate cancer, % | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Death | Metastases | |||||||||||||||
| All† | Low‡ | Intermediate‡ | High‡ | |||||||||||||
| SPGS-4 (3) | 65 | 13 | 1989–1999 | 21 | 11 | 33 | ||||||||||
| PIVOT (4) | 67 | 10 | 1994–2002 | 8 | 3 | 11 | 18 | |||||||||
* SPGS-4 = Scandinavian Prostate Cancer Group Study-4; PIVOT = Prostate cancer Intervention Versus Observation Trial.
† Observation arm of trial.
‡ In PIVOT, low-, intermediate-, high-risk strata based on prostate-specific antigen, stage, and grade; in SPGS-4, strata are based on prostate-specific antigen and grade.
In contrast to the SPGS-4, a more contemporary analysis of surgery versus observation in the pre-PSA era has been presented in oral format in a number of settings in 2011 (4). Unlike the SPGS-4, in the Prostate cancer Intervention Versus Observation Trial (PIVOT), 50% of men had nonpalpable prostate cancers and 40% were considered to be at low risk. The cumulative incidence of prostate cancer death in the observation arm was almost three times lower in PIVOT compared with the SPGS-4 (8% vs 21%), albeit with shorter follow-up in PIVOT. Prostate cancer mortality varied considerably by tumor metrics. For example, deaths by risk category were 3%, 11%, and 18% for low-, intermediate-, and high-risk disease, respectively, in the observation arm (Table 4). Although cancer-specific survival was not different for men with low-risk disease treated surgically compared with those observed, the absolute risk reduction in prostate cancer death with surgery compared with observation for men with a PSA above 10ng/mL and high-risk disease was 7% and 8%, respectively, similar to that seen in the SPGS-4 of 6%.
It is likely that the intermediate-risk category in PIVOT from the pre-PSA era was similar to the low-risk category in the SPGS-4 from the pre-PSA era, explaining the similarity in the cumulative incidence of prostate cancer deaths of 11% for both groups (Table 4). The contemporary outcomes of untreated men in PIVOT with low-risk disease are similar to those of Lu-Yao et al. (32) who reported a cancer-specific mortality at 10 years for untreated men aged 65–74 years with moderately differentiated prostate cancers (Gleason scores 5–7) of 2%–6%.
These data from the observation arms of randomized trials and observational cohorts suggest that the balance of benefit and harm would favor no immediate treatment for a man diagnosed today with low-risk prostate cancer and a life expectancy less than 10–15 years and would favor treatment for those with intermediate- and high-risk disease with a life expectancy exceeding 10 years. However, the outcomes for patients who were randomized to watchful waiting could be very different than the outcomes for men in active surveillance being monitored with curative intent today.
Observed and Predicted Outcomes With Active Surveillance
There are no comparative studies of immediate intervention versus active surveillance for contemporary men diagnosed with early prostate cancer today. Assuming that the goal of active surveillance—in contrast to watchful waiting—is to initiate treatment during a window of curability for those with disease progression or reclassification, it remains to be determined whether or not delayed intervention negatively or positively affects health outcomes when compared with immediate intervention. To date, single-arm studies and observational cohort studies have reported the relatively short-term outcomes through a decade or less for men choosing active surveillance.
Retrospective Analyses.
As a proxy for longer term outcomes, investigators have compared the pathological outcomes and biochemical recurrence–free rates of men on active surveillance undergoing delayed surgery with matched cohorts that would have qualified for surveillance but chose immediate surgical intervention (35–37). These studies are an attempt to address whether delayed intervention compromises the chance of cure. Recognizing that various criteria were used for enrollment into active surveillance in these studies, a delay in surgery from 18 months to 31 months after diagnosis did not seem to compromise the chances for cure when cancer grade, pathological stage, and biochemical recurrence were used as intermediate outcomes in comparisons of immediate versus delayed surgery. But the comparisons would be biased against surveillance because most men undergoing delayed intervention after a time on surveillance have some trigger for intervention when compared with those undergoing immediate intervention, for example, a grade change on prostate biopsy or a PSA rise.
Stattin et al. (38) compared the outcomes from a cancer registry of men who were aged 61–65 years, had low-risk prostate cancers, and were either managed with surveillance or curative intervention. The 10-year prostate cancer–specific mortality was below 3% for men with low-risk disease managed initially with surveillance. The absolute difference in prostate cancer–specific mortality at 10 years for those managed initially with surveillance compared with surgery was 2%. Thus, the authors concluded that surveillance may be a suitable option for many men with low-risk prostate cancer, which is a stance consistent with recent guidelines (23).
In an evaluation of over 3000 men in the Health Professionals Follow-up Study (mean age 68 years), 10% of men overall deferred treatment and 51% remained untreated at 8 years (39). On average, 4 years elapsed prior to treatment for those treated. There were no differences in the rates of metastatic disease or prostate cancer deaths when comparing those who deferred treatment with those who were treated initially at a median follow-up of 8–9 years. Similar to PIVOT, the prognostic risk category (low, intermediate, high) was strongly predictive of the lethal phenotype. When compared to those with low-risk disease, those with intermediate- and high-risk disease were three- and sixfold more likely to die of prostate cancer, respectively. For those men with low-risk prostate cancer, metastases occurred in 7 of 139 (5%) and 33 of 1252 (3%) men in the deferred and treatment groups, respectively; death from prostate cancer occurred in 3 of 139 (2%) and 9 of 1252 (1%) men in the deferred and treatment groups, respectively.
The retrospective data above suggest that men with low-risk disease who have a 10- to 15-year life expectancy or less should consider surveillance as a primary management option rather than immediate intervention. Prospective data also support this conclusion.
Prospective Analyses.
The longest running contemporary study of active surveillance for low- to intermediate-risk disease has been chronicled by Klotz et al. (40), who followed 450 men (median age 70 years) for a median of 7 years in a longitudinal cohort single-arm study beginning in 1995. Seventeen percent of the subjects had a Gleason score of 3 + 4, 83% were Gleason 6 or less, and 71% were considered low risk by D’Amico criteria. Thus, one in three men had intermediate-risk disease. The 10-year actuarial prostate cancer–specific survival was 97%. Five deaths have occurred in this cohort between 4 and 10 years after diagnosis, and all deaths occurred in men who were reclassified as higher risk disease during follow-up. By comparison, in the SPGS-4 (34), untreated men over age 65 years had a 12-year prostate cancer–specific survival of approximately 88% that did not differ from those that underwent surgical treatment. One could argue that prostate cancer deaths in the Klotz et al. study (40) occurred in men who had advanced disease to begin with, and thus surveillance did not compromise length of life (41). An alternative explanation is that deaths occurred in men who were not favorable candidates for surveillance, and that earlier intervention would have prolonged life. Nevertheless, there is a definite, but as yet unquantifiable, long-term risk to men who choose surveillance as a management option for low-risk disease, regardless of how indolent the disease appears on a prostate biopsy.
The Johns Hopkins Program of Active Surveillance (42) began in 1995 as an open enrollment longitudinal cohort study. Strict criteria for enrollment were followed longitudinally; 80% met all criteria for very low-risk prostate cancer described by Epstein et al. (20) (Table 2). Twenty percent of the men met all criteria for low-risk prostate cancer (Table 2) except the PSA criterion of less than 10ng/mL, because when PSA density was below 0.15ng/mL/cc, men with PSA above 10ng/mL were considered appropriate candidates for surveillance if biopsy and stage criteria were met. Further, no patients in this program had Gleason score above 6 (pattern 4 or 5) that was considered an absolute trigger for intervention.
Since 1995, 986 have been enrolled and Tosoian et al. (42) recently updated the findings from the Johns Hopkins active surveillance program reporting on 769 men with a median follow-up of 2.7 years (range 0.01–15). Overall, 255 men (33.2%) underwent curative intervention at a median of 2.2 years (range 0.6–10.2) after diagnosis, and 74% of these men were treated because of reclassification on a prostate biopsy. The median survival free of curative intervention was 6.5 years (range 0.0–15.0) after diagnosis; at 2-, 5-, and 10-year follow-ups, there were 81%, 59%, and 41% of men, respectively, free of curative intervention.
When comparing men who were treated or not (Table 5), there were significant differences in variables at diagnosis, including PSA, percentage of free PSA, PSA density, and year of cancer diagnosis. All of these variables have previously been shown to be predictors of biopsy reclassification and appear to be associated with a disease that is either larger in volume or higher grade than indicated by the diagnostic biopsy (43,44). Year of cancer diagnosis is most likely related to reclassification, because in the early years of the program fewer biopsy samples were taken when compared with later years in the program. This suggests the possibility of undersampling of the cancer by biopsy in earlier years when compared with later years.
Table 5.
Study group demographics for Johns Hopkins active surveillance program*
| Variables at diagnosis | Treated (n = 255) | Not treated (n = 514) | P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||||||
| Age, y | 65 | 5.6 | 66 | 6.2 | .309 | |||||
| PSA, ng/mL | 5.7 | 2.9 | 5.0 | 3.0 | .003 | |||||
| % free PSA | 16.6 | 6.8 | 19.4 | 7.6 | .024 | |||||
| PSA density, ng/mL/cc | 0.12 | 0.07 | 0.10 | 0.06 | <.001 | |||||
| No. of cores positive for cancer | 1.3 | 0.6 | 1.2 | 0.5 | .126 | |||||
| Maximum % core involvement with cancer | 10.1 | 15.5 | 8.5 | 12.3 | .266 | |||||
| Year of cancer | 2002 | 3.8 | 2005 | 3.3 | <.001 | |||||
* PSA = prostate-specific antigen; adapted from Tosoian et al. (42).
Undersampling of a cancer in terms of grade, or “true” grade progression, would appear to be the greatest risk of active surveillance in terms of cancer-specific death, given the direct relationship between grade and prostate cancer–specific death among untreated men (30). Upgrading on a surveillance prostate biopsy occurred in 4 per 100 person-years of follow-up in the Johns Hopkins surveillance program with an actuarial rate of 30% at 10 years of follow-up (Table 6). The extent to which these cancers are missed initially or actually dedifferentiate over time is not known. However, our analyses suggest that, in most, finding a higher grade cancer on biopsy represents initial undersampling and not dedifferentiation in the first years of surveillance (45).
Table 6.
Outcomes among men in Johns Hopkins active surveillance program*
| Event | No. of men | % of men | Rate per 100 person-years | |||
|---|---|---|---|---|---|---|
| Curative intervention† | 256 | 33.3 | 9.7 | |||
| Biopsy reclassification‡ | 235 | 30.6 | 8.9 | |||
| Upgrading to Gleason score ≥7 | 106 | 13.8 | 4.0 | |||
| Exit from program (nondeath)§ | 338 | 44.0 | 12.8 | |||
| Death, nonprostate‖ | 14 | 1.8 | 0.5 |
* Adapted from Tosoian et al. (42); includes 769 patients with 2635.91 person-years of follow-up.
† Radical prostatectomy or radiation therapy.
‡ Any Gleason pattern 4 or 5, any Gleason score ≥7, more than two cores containing cancer, or >50% involvement of any core with cancer.
§ Exit from program included all patients who underwent curative intervention, withdrew, or were lost to follow-up.
‖ Death included mortality from any cause; there were no deaths in this cohort as a result of prostate cancer.
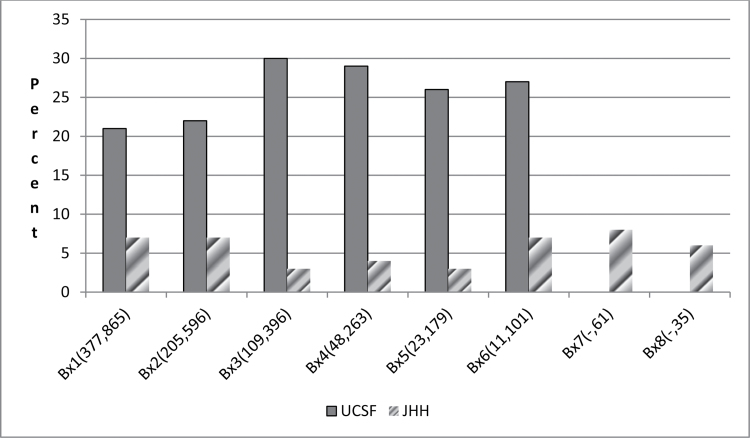
If the object of surveillance—at least for most men who have a life expectancy of 10 years or more—is to select a low-grade cancer in which the rate of reclassification to a higher grade is unlikely, then upgrading rates at prostate biopsy while on surveillance could be used as a proxy for the success of selection criteria. Recognizing that about 25% of men with a Gleason score 6 prostate cancer on prostate biopsy will be upgraded at radical prostatectomy (11), a comparison between the upgrading rates for two surveillance programs (Johns Hopkins and University of California San Francisco [UCSF]) (42,46) with very different selection criteria are informative (Figure 1). Note the higher rates of upgrading at each surveillance biopsy in the UCSF program when compared with the Johns Hopkins program despite the entry Gleason score of 6 for 94% of men at UCSF and 100% of the men at Johns Hopkins. We believe this may be due to the fact that PSA density—a strong predictor of adverse pathological features—is not used at UCSF but is a critical selection criterion at Johns Hopkins and/or the fact that many men in the UCSF program were identified retrospectively from a large database rather than recruited prospectively into a longitudinal cohort program using predefined selection criteria. However, the use of more restrictive criteria to recruit men for active surveillance would result in a greater degree of overtreatment, whereas the use of less restrictive criteria risks a higher likelihood of undertreatment—a tradeoff that is difficult to balance using current paradigms. In addition to upgrading on surveillance prostate biopsies, freedom from disease after delayed intervention on surveillance is another outcome of interest for men on surveillance.
Figure 1.
Percent of men upgraded to Gleason score above 6 on surveillance biopsies after diagnosis. Surveillance biopsy (Bx) number shown on x-axis; numbers in parentheses represent absolute number of men at University of California San Francisco (UCSF) and Johns Hopkins (JHH), respectively, undergoing a biopsy. Ninety-six percent of men at UCSF and 100% of men at JHH had Gleason score 6 at initial diagnosis. Data from UCSF adapted from Porten et al. (46), and from JHH adapted from Tosoian et al. (42).
Freedom from disease after curative intervention on surveillance could be influenced by initial selection criteria used for surveillance and triggers for intervention while on surveillance. There are clear distinctions between the selection criteria used at Johns Hopkins and other surveillance programs. The selection of men who are defined as at very low risk (20) most likely influences intermediate outcomes. Furthermore, in the Johns Hopkins program, PSA kinetics is not used as a trigger for intervention because it has been shown to be a poor predictor of high-grade cancer (47). In contrast, PSA doubling time triggered intervention in more than one in three men in the Prostate cancer Research International: Active Surveillance (PRIAS) study (48) and nearly one in two men in the Toronto experience (40). It could be differences in both selection and triggers for intervention that explains a lower biochemical recurrence after treatment in the Johns Hopkins program when compared with reports from van den Bergh (48) and Klotz (40).
The Cancer Intervention and Surveillance Modeling Network (CISNET) have contributed to our understanding of the natural history of screen-detected prostate cancers and the potential for benefit and harm with screening (49). Investigators from CISNET recently modeled the outcomes of cancer-specific overall survival and time remaining without treatment for men who qualified for the Johns Hopkins active surveillance program (J. Xia, B. J. Trock, M. R. Cooperberg, et al., unpublished data). The model compared outcomes for immediate surgical intervention versus active surveillance. Using robust datasets, the inputs into the model were the time to biopsy reclassification by grade (ie, upgrading on prostate biopsy above Gleason score 6) using the longitudinal data from the Johns Hopkins active surveillance program (42), time to recurrence after surgical treatment using data from a large disease registry (50), and time from recurrence to death from prostate cancer or another cause using data from the Johns Hopkins surgical series (51). Data from the CISNET model suggested that cancer-specific death was 2% for patients treated immediately with surgery compared with 4% for those in active surveillance, some of whom underwent delayed surgery. The average increase in life expectancy with surgery was 3 months, and on average, men on surveillance lived 7 years longer without treatment. The findings are in agreement with short-term outcomes that were reviewed previously. These data suggest that death from prostate cancer will be a low-risk event among men in a surveillance program who were identified based on the criteria defining very low-risk disease (Table 2).
Many centers are gaining experience with active surveillance for low-risk prostate cancers, although enrollment criteria and triggers for intervention differ (52). In a multi-institutional study (53), which included the University of Miami, University of British Columbia, Memorial Sloan-Kettering Cancer Center, and the Cleveland Clinic, 262 patients with low-risk disease were on surveillance for a median of 29 months. The 5-year probability of remaining on surveillance was 75%, which is very consistent across studies.
In the authors’ opinion, men with more than an estimated 15-year life expectancy and low-risk disease should be told that active surveillance is an investigational approach that may result in a lost opportunity for disease control. For those men who are older with associated comorbidities that limit the remaining years of life, active surveillance should be considered the optimum management for low-risk disease (21). For men with very low-risk prostate cancer, the author agrees with the recommendation of the NCCN; men with a life expectancy of less than 20 years should consider active surveillance to be the preferred management option (21).
The overwhelming evidence that overtreatment is a substantial problem suggests that a new paradigm may be needed to improve the balance of benefit and harm of invasive prostate cancer treatments.
New Paradigm for Active Surveillance
First, patient and physician education regarding active surveillance is crucial, especially at the primary care level where men often have trusting relationships with a physician that can help them with management decisions. A primary care physician is often the best person to help the confused patient navigate the prostate cancer bazaar (54), where the recommendation may oftentimes be based more on physician beliefs and practice patterns (55) rather than evidence based. A website (56) has been created as part of a collaboration between Johns Hopkins and Cedars-Sinai Medical Center in California, supported by the Prostate Cancer Foundation, to increase awareness of and education regarding active surveillance. Ultimately, the website will be interactive for patients and physicians allowing entry of patient and tumor metrics. Other tools that are envisioned as part of a web based-program would be instruments to help estimate remaining years of life and to help patients explore their personal preferences, such as living with cancer and avoiding side effects of treatment. In this regard, a decision support tool that accounts for tumor and patient metrics could help patients understand the tradeoffs that should be an important aspect of a management decision for prostate cancer (16).
Second, given the limitations of accurately assessing the presence or absence of a lethal cancer phenotype on an individual basis, improved markers of tumor biology would be a large step forward. A number of investigators are exploring gene signatures or expression profiles—similar to the Oncocyte DX gene expression assay for assessing breast cancer risk—for prostate cancer, but these are in their infancy at present (57,58). Furthermore, small specimens, undersampling of the biologically important cancer, and tumor heterogeneity hinder the assessment of the cancer phenotype from a biopsy. The limitation of tumor sampling on biopsy emphasizes the need for improved imaging as part of any advance in identifying appropriate candidates for surveillance and monitoring patients on active surveillance.
Third, improvements in imaging are needed in order to assess the presence and significance of prostate cancer. Whereas some studies suggest that multiparametric magnetic resonance imaging (MRI) may be helpful for detection and assessment of prostate cancer (59–62), no data at present are persuasive enough to incorporate MRI as a standard for determining the appropriate surveillance candidate or for follow-up of these patients as a trigger for intervention. Improvements in functional imaging, and the use of molecular probes for imaging, could help individualize the approach to cancer management (63).
Conclusions
A major cause of overtreatment in the United States is the intervention for favorable-risk prostate cancers in men for whom treatment is unlikely to improve any health outcome. One approach to reducing overtreatment is active surveillance—selection of those men who are not likely to be harmed from cancer in the absence of treatment and monitoring with the intention to cure should the disease change over time. There is no universal consensus with regard to the “ideal” candidate for surveillance and on the optimal methods for monitoring. In large part, this is due to the use of proxies for the lethal phenotype (PSA, stage, grade) that may or may not reflect tumor biology for any given individual. The authors believe that a new paradigm is necessary to reduce barriers to surveillance in the United States.
References
- 1. Cooperberg MR, Carroll PR, Klotz L. Active surveillance for prostate cancer: progress and promise. J Clin Oncol. 2011;29(27):3669–3676 [DOI] [PubMed] [Google Scholar]
- 2. Parker C. Active surveillance: towards a new paradigm in the management of early prostate cancer. Lancet Oncol. 2004;5(2):101–106 [DOI] [PubMed] [Google Scholar]
- 3. Bill-Axelson A, Holmberg L, Ruutu M, et al. ; SPCG-4 Investigators. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2011;364(18):1708–1717 [DOI] [PubMed] [Google Scholar]
- 4. Wilt TJ, Brawer MK, Barry MJ, et al. The Prostate cancer Intervention Versus Observation Trial: VA/NCI/AHRQ Cooperative Studies Program #407 (PIVOT): design and baseline results of a randomized controlled trial comparing radical prostatectomy to watchful waiting for men with clinically localized prostate cancer. Contemp Clin Trials. 2009;30(1):81–87 [DOI] [PubMed] [Google Scholar]
- 5. Andriole GL, Crawford ED, Grubb RL, III, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360(13):1310–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hugosson J, Carlsson S, Aus G, et al. Mortality results from the Goteborg randomised population-based prostate-cancer screening trial. Lancet Oncol. 2010;11(8):725–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lughezzani G, Briganti A, Karakiewicz PI, et al. Predictive and prognostic models in radical prostatectomy candidates: a critical analysis of the literature [published online ahead of print August 6, 2010]. Eur Urol. 2010;58(5):687–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hayes JH, Ollendorf DA, Pearson SD, et al. Active surveillance compared with initial treatment for men with low-risk prostate cancer: a decision analysis. JAMA. 2010;304(21):2373–2380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eggener SE, Scardino PT, Walsh PC, et al. Predicting 15-year prostate cancer specific mortality after radical prostatectomy. J Urol. 2011;185(3):869–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Albertsen PC, Hanley JA, Gleason DF, Barry MJ. Competing risk analysis of men aged 55 to 74 years at diagnosis managed conservatively for clinically localized prostate cancer. JAMA. 1998;280(11):975–980 [DOI] [PubMed] [Google Scholar]
- 11. van den Bergh RC, Steyerberg EW, Khatami A, et al. Is delayed radical prostatectomy in men with low-risk screen-detected prostate cancer associated with a higher risk of unfavorable outcomes? Cancer. 2011;116(5):1281–1290 [DOI] [PubMed] [Google Scholar]
- 12. Albertsen PC, Hanley JA, Barrows GH, et al. Prostate cancer and the Will Rogers phenomenon. J Natl Cancer Inst. 2005;97(17):1248–1253 [DOI] [PubMed] [Google Scholar]
- 13. Xu J, Dailey RK, Eggly S, Neale AV, Schwartz KL. Men’s perspectives on selecting their prostate cancer treatment. J Natl Med Assoc. 2011;103(6):468–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Davison BJ, Goldenberg SL. Patient acceptance of active surveillance as a treatment option for low-risk prostate cancer. BJU Int. 2011;108(11):1787–1793 [DOI] [PubMed] [Google Scholar]
- 15. Hayes JH, Ollendorf DA, Pearson SD, et al. Active surveillance compared with initial treatment for men with low-risk prostate cancer: a decision analysis. JAMA. 2010;304(21):2373–2380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu DLH, Frick KD, Carter HB. Active surveillance vs surgery in low-risk prostate cancer: a clinical decision analysis. J Urol. 2012;187(4):1241–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eastham JA. Robotic-assisted prostatectomy: is there truth in advertising? Eur Urol. 2008;54(4):720–722 [DOI] [PubMed] [Google Scholar]
- 18. Schroeck FR, Krupski TL, Sun L, et al. Satisfaction and regret after open retropubic or robot-assisted laparoscopic radical prostatectomy. Eur Urol. 2008;54(4):785–793 [DOI] [PubMed] [Google Scholar]
- 19. D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969–974 [DOI] [PubMed] [Google Scholar]
- 20. Epstein JI, Walsh PC, Carmichael M, Brendler CB. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. 1994;271(5):368–374 [PubMed] [Google Scholar]
- 21. Mohler JL. The 2010 NCCN clinical practice guidelines in oncology on prostate cancer. J Natl Compr Canc Netw. 2010;8(2):145 [DOI] [PubMed] [Google Scholar]
- 22. Heidenreich A, Bellmunt J, Bolla M, et al. EAU guidelines on prostate cancer. Part I: screening, diagnosis, and treatment of clinically localised disease. Actas Urol Esp. 2011;35(9):501–514 [DOI] [PubMed] [Google Scholar]
- 23. Mohler J, Bahnson RR, Boston B, et al. NCCN clinical practice guidelines in oncology: prostate cancer. J Natl Compr Canc Netw. 2010;8(2):162–200 [DOI] [PubMed] [Google Scholar]
- 24. Shao YH, Albertsen PC, Roberts CB, et al. Risk profiles and treatment patterns among men diagnosed as having prostate cancer and a prostate-specific antigen level below 4.0ng/ml. Arch Intern Med. 2010;170(14):1256–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Porten SP, Richardson DA, Odisho AY, McAninch JW, Carroll PR, Cooperberg MR. Disproportionate presentation of high risk prostate cancer in a safety net health system. J Urol. 2010;184(5):1931–1936 [DOI] [PubMed] [Google Scholar]
- 26. Kattan MW, Eastham JA, Wheeler TM, et al. Counseling men with prostate cancer: a nomogram for predicting the presence of small, moderately differentiated, confined tumors. J Urol. 2003;170(5):1792–1797 [DOI] [PubMed] [Google Scholar]
- 27. Nakanishi H, Wang X, Ochiai A, et al. A nomogram for predicting low-volume/low-grade prostate cancer: a tool in selecting patients for active surveillance. Cancer. 2007;110(11):2441–2447 [DOI] [PubMed] [Google Scholar]
- 28. Steyerberg EW, Roobol MJ, Kattan MW, van der Kwast TH, de Koning HJ, Schroder FH. Prediction of indolent prostate cancer: validation and updating of a prognostic nomogram. J Urol. 2007;177(1):107–112; discussion 112 [DOI] [PubMed] [Google Scholar]
- 29. Lee MC, Dong F, Stephenson AJ, Jones JS, Magi-Galluzzi C, Klein EA. The Epstein criteria predict for organ-confined but not insignificant disease and a high likelihood of cure at radical prostatectomy. Eur Urol. 2010;58(1):90–95 [DOI] [PubMed] [Google Scholar]
- 30. Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA. 2005;293(17):2095–2101 [DOI] [PubMed] [Google Scholar]
- 31. Draisma G, Boer R, Otto SJ, et al. Lead times and overdetection due to prostate-specific antigen screening: estimates from the European Randomized Study of Screening for Prostate Cancer. J Natl Cancer Inst. 2003;95(12):868–878 [DOI] [PubMed] [Google Scholar]
- 32. Lu-Yao GL, Albertsen PC, Moore DF, et al. Outcomes of localized prostate cancer following conservative management. JAMA. 2009;302(11):1202–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Parker C, Muston D, Melia J, Moss S, Dearnaley D. A model of the natural history of screen-detected prostate cancer, and the effect of radical treatment on overall survival. Br J Cancer. 2006;94(10):1361–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bill-Axelson A, Holmberg L, Filen F, et al. ; Scandinavian Prostate Cancer Group Study Number 4. Radical prostatectomy versus watchful waiting in localized prostate cancer: the Scandinavian prostate cancer group-4 randomized trial. J Natl Cancer Inst. 2008;100(16):1144–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Warlick C, Trock BJ, Landis P, Epstein JI, Carter HB. Delayed versus immediate surgical intervention and prostate cancer outcome. J Natl Cancer Inst. 2006;98(5):355–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dall’Era MA, Cowan JE, Simko J, et al. Surgical management after active surveillance for low-risk prostate cancer: pathological outcomes compared with men undergoing immediate treatment. BJU Int. 2011;107(8):1232–1237 [DOI] [PubMed] [Google Scholar]
- 37. van den Bergh RC, Steyerberg EW, Khatami A, et al. Is delayed radical prostatectomy in men with low-risk screen-detected prostate cancer associated with a higher risk of unfavorable outcomes? Cancer. 2010;116(5):1281–1290 [DOI] [PubMed] [Google Scholar]
- 38. Stattin P, Holmberg E, Johansson JE, Holmberg L, Adolfsson J, Hugosson J. Outcomes in localized prostate cancer: National Prostate Cancer Register of Sweden follow-up study. J Natl Cancer Inst. 2010;102(13):950–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shappley WV, III, Kenfield SA, Kasperzyk JL, et al. Prospective study of determinants and outcomes of deferred treatment or watchful waiting among men with prostate cancer in a nationwide cohort. J Clin Oncol. 2009;27(30):4980–4985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Klotz L, Zhang L, Lam A, Nam R, Mamedov A, Loblaw A. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010;28(1):126–131 [DOI] [PubMed] [Google Scholar]
- 41. Krakowsky Y, Loblaw A, Klotz L. Prostate cancer death of men treated with initial active surveillance: clinical and biochemical characteristics. J Urol. 2010;184(1):131–135 [DOI] [PubMed] [Google Scholar]
- 42. Tosoian JJ, Trock BJ, Landis P, et al. Active surveillance program for prostate cancer: an update of the Johns Hopkins experience. J Clin Oncol. 2011;29(16):2185–2190 [DOI] [PubMed] [Google Scholar]
- 43. Carter HB, Kettermann A, Warlick C, et al. Expectant management of prostate cancer with curative intent: an update of the Johns Hopkins experience. J Urol. 2007;178(6):2359–2364; discussion 2364–2355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tseng KS, Landis P, Epstein JI, Trock BJ, Carter HB. Risk stratification of men choosing surveillance for low risk prostate cancer. J Urol. 2010;183(5):1779–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Epstein JI, Walsh PC, Carter HB. Dedifferentiation of prostate cancer grade with time in men followed expectantly for stage T1c disease. J Urol. 2001;166(5):1688–1691 [PubMed] [Google Scholar]
- 46. Porten SP, Whitson JM, Cowan JE, et al. Changes in prostate cancer grade on serial biopsy in men undergoing active surveillance. J Clin Oncol. 2011;29(20):2795–2800 [DOI] [PubMed] [Google Scholar]
- 47. Ross AE, Loeb S, Landis P, et al. Prostate-specific antigen kinetics during follow-up are an unreliable trigger for intervention in a prostate cancer surveillance program. J Clin Oncol. 2010;28(17):2810–2816 [DOI] [PubMed] [Google Scholar]
- 48. van den Bergh RC, Vasarainen H, van der Poel HG, et al. Short-term outcomes of the prospective multicentre ‘Prostate Cancer Research International: Active Surveillance’ study. BJU Int. 2010;105(7):956–962 [DOI] [PubMed] [Google Scholar]
- 49. Etzioni R, Tsodikov A, Mariotto A, et al. Quantifying the role of PSA screening in the US prostate cancer mortality decline. Cancer Causes Control. 2008;19(2):175–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Porten SP, Cooperberg MR, Konety BR, Carroll PR. The example of CaPSURE: lessons learned from a national disease registry. World J Urol. 2011;29(3):265–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Eggener SE, Scardino PT, Walsh PC, et al. Predicting 15-year prostate cancer specific mortality after radical prostatectomy. J Urol. 2011;185(3):869–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cooperberg MR, Carroll PR, Klotz L. Active surveillance for prostate cancer: progress and promise. J Clin Oncol. 2011;29(27):3669–3676 [DOI] [PubMed] [Google Scholar]
- 53. Eggener SE, Mueller A, Berglund RK, et al. A multi-institutional evaluation of active surveillance for low risk prostate cancer. J Urol. 2009;181(4):1635–1641; discussion 1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Barry MJ. The prostate cancer treatment bazaar: comment on “Physician visits prior to treatment for clinically localized prostate cancer.”. Arch Intern Med. 2010;170(5):450–452 [DOI] [PubMed] [Google Scholar]
- 55. Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010;28(7):1117–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. National Proactive Surveillance Network Web site http://www.npsn.net Accessed July 13, 2012.
- 57. Cuzick J, Swanson GP, Fisher G, et al. Prognostic value of an RNA expression signature derived from cell cycle proliferation genes in patients with prostate cancer: a retrospective study. Lancet Oncol. 2011;12(3):245–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ding Z, Wu CJ, Chu GC, et al. SMAD4-dependent barrier constrains prostate cancer growth and metastatic progression. Nature. 2011;470(7333):269–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shukla-Dave A, Hricak H, Kattan MW, et al. The utility of magnetic resonance imaging and spectroscopy for predicting insignificant prostate cancer: an initial analysis. BJU Int. 2007;99(4):786–793 [DOI] [PubMed] [Google Scholar]
- 60. Shukla-Dave A, Hricak H, Akin O, et al. Preoperative nomograms incorporating magnetic resonance imaging and spectroscopy for prediction of insignificant prostate cancer. BJU Int. 2011;109(9):1315–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fradet V, Kurhanewicz J, Cowan JE, et al. Prostate cancer managed with active surveillance: role of anatomic MR imaging and MR spectroscopic imaging. Radiology. 2010;256(1):176–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Turkbey B, Mani H, Shah V, et al. Multiparametric 3T prostate magnetic resonance imaging to detect cancer: histopathological correlation using prostatectomy specimens processed in customized magnetic Resonance imaging based molds. J Urol. 2011;186(5):1818–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bouchelouche K, Tagawa ST, Goldsmith SJ, Turkbey B, Capala J, Choyke P. PET/CT imaging and radioimmunotherapy of prostate cancer. Semin Nucl Med. 2011;41(1):29–44 [DOI] [PMC free article] [PubMed] [Google Scholar]