Abstract
Prostate cancer carries an extraordinarily varied prognosis. Previously, most men presented with clinical symptoms often succumbed to their disease several years following treatment with hormonal manipulation. With the advent of prostate-specific antigen (PSA) testing, most men are now diagnosed with localized, well- to moderately differentiated disease. The most powerful predictor of long-term outcome is the Gleason score, followed by tumor volume. Over the past two decades, changes in the interpretation of Gleason patterns have resulted in the reclassification of many well-differentiated tumors as higher grade tumors. Men with well-differentiated disease have an excellent prognosis and often survive 10–20 years without intervention. Conversely, men with poorly differentiated disease often succumb to their cancer within a decade. PSA can estimate tumor volume, but poorly differentiated disease may not produce much PSA. We are unable to predict accurately the risk posed by a specific prostate cancer.
Historical Background
For the past 160 years, prostate cancer has challenged both clinicians and researchers. Thompson first described this disease in a monograph entitled The Enlarged Prostate in 1852 (1). Forty years later, Von Recklinghausen reported that prostate cancer could metastasize to bone even when there was only a small, local palpable lesion in the prostate (2). By the early 1900s, improvements in microscopy demonstrated that prostate cancer was a relatively common fatal cancer, which often produced systemic symptoms, such as bone pain and weight loss, and local symptoms, such as bladder outlet obstruction. Radon seed implants were the treatment of choice. At the turn of the last century, B.S. Barringer, a prominent New York City urologist, reported that only 36 of his first 352 patients receiving this treatment lived more than 5 years (3). Hugh Hampton Young suggested in 1905 that a careful digital rectal examination could identify changes in prostate gland texture that might lead to the early diagnosis of cancer and appropriate intervention (4).
The dismal prognosis associated with prostate cancer improved somewhat following the report by Huggins and Hodges in 1941 that prostate cancer was an endocrine-dependent tumor (5). By the 1950s, orchiectomy and/or diethylstilbesterol had become the standard of care for men who developed symptomatic disease. Although the average patient responded to androgen deprivation for about 3 years, many clinicians recognized that the disease often took many years to become clinically symptomatic.
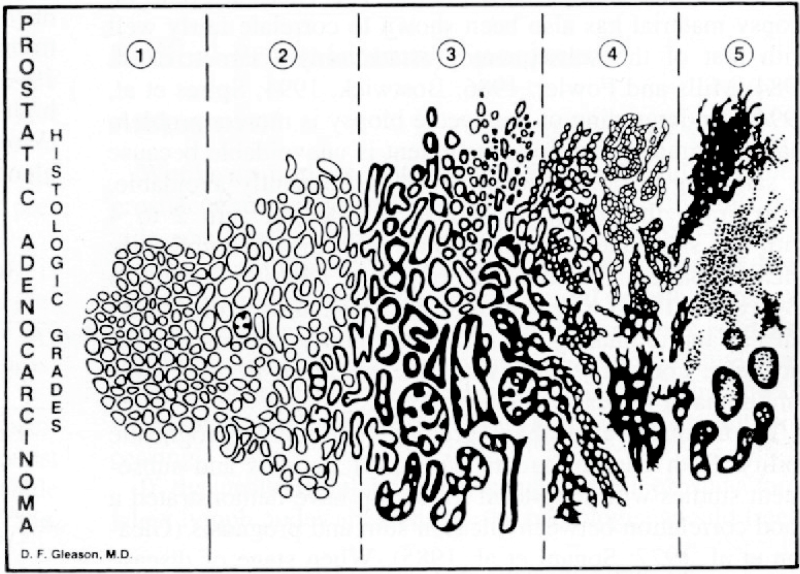
During the 1960s, the Veterans Administration Cooperative Urologic Research Group (VACURG) was organized to research the appropriate treatment of prostate cancer. Several trials were organized, but researchers had difficulty identifying men with localized disease. Most men presenting with prostate cancer had metastatic disease. The most significant legacy of these trials is the Gleason scoring system used to evaluate tumor histology (Figure 1). This was the first widely accepted standard to assess the risk posed by this disease. Originally, Gleason described nine clinical patterns of glandular proliferation (6). Several of these patterns were collapsed into the five patterns described in Gleason’s classic diagram that was validated using data from the VACURG trials. Although the original system has been modified, it still remains the most powerful predictor of clinical prognosis for this disease.
Figure 1.
The Gleason scoring system.
The modern era of prostate cancer diagnosis and management began following the 1987 publication by Stamey et al. describing prostate-specific antigen (PSA) as a powerful new tumor marker for prostate cancer (7). This publication, along with Catalona’s 1991 report advocating PSA testing to screen for prostate cancer, dramatically altered the incidence of this disease (8). Since then, the number of new cases of prostate cancer has doubled in the United States (9). Furthermore, PSA testing changed how men presented with this disease. Prior to PSA testing, most men developed clinical symptoms such as back pain or bladder outlet obstruction; now, more than 80% of men present with clinically localized disease following a prostate biopsy performed because of an elevated PSA (9).
The Epidemiology of Prostate Cancer
Few cancers generate as much controversy surrounding screening, diagnosis, and treatment as prostate cancer. This controversy has escalated since PSA testing was embraced in the United States as a test to screen for this disease. As of 2009, investigators believe that in the United States over 55% of men aged 50 years and older are undergoing annual PSA testing and that over 75% have been tested at least once (10,11).
Prostate cancer is a major public health problem with an estimated 240 890 new diagnoses and 33 720 deaths in 2011 in the United States (9). From 1977 to 2005, the lifetime risk of prostate cancer diagnosis in the United States increased from 7.3% to 17% (12,13). During this same period, the lifetime risk of dying from prostate cancer fell from 3.0% to 2.4%. As a consequence of testing for PSA, prostate cancer incidence rates in the United States increased at an annual rate of over 16% until it peaked in 1992. Since then, the rate has declined somewhat, but the incidence rate is now almost double that seen in the early 1980s (6). Mortality from prostate cancer peaked in the United States in 1992 at almost 35 000 deaths but has since declined at an annual rate of 4% (9).
The natural history of prostate cancer is extraordinarily variable. Some men have aggressive disease that may benefit from early detection and intervention, but many others harbor cancers that grow slowly and never progress to clinical significance. Several key studies have helped shape our understanding of the natural history of this disease. Between 1989 and 2004, Johansson et al. published a series of four articles that documented the outcomes of untreated prostate cancer in a population-based cohort of patients diagnosed with prostate cancer in Sweden (14–17). No screening for prostate cancer took place during the period when this study population of 648 consecutive cases was assembled. Initially the authors found relatively low 5- and 10-year mortality rates among men with clinically localized disease and challenged the use of aggressive initial treatment for all patients with low-grade early-stage prostate cancer. Long-term follow-up of the study cohort, however, suggested a rise in prostate cancer mortality for those men surviving 15–20 years following diagnosis.
In 1994, Chodak et al. published a report describing the results of conservative management of clinically localized prostate cancer (18). Unlike the Johansson report, this study consisted of a pooled analysis of 828 case records from 6 nonrandomized studies published during the decade preceding the report. Patients with poorly differentiated cancers had a significantly higher 10-year cancer-specific mortality rate (66%) when compared with men who had well- or moderately differentiated cancers (13%). In addition, men with poorly differentiated tumors were much more likely to develop metastases when compared with men who were diagnosed with well-differentiated disease.
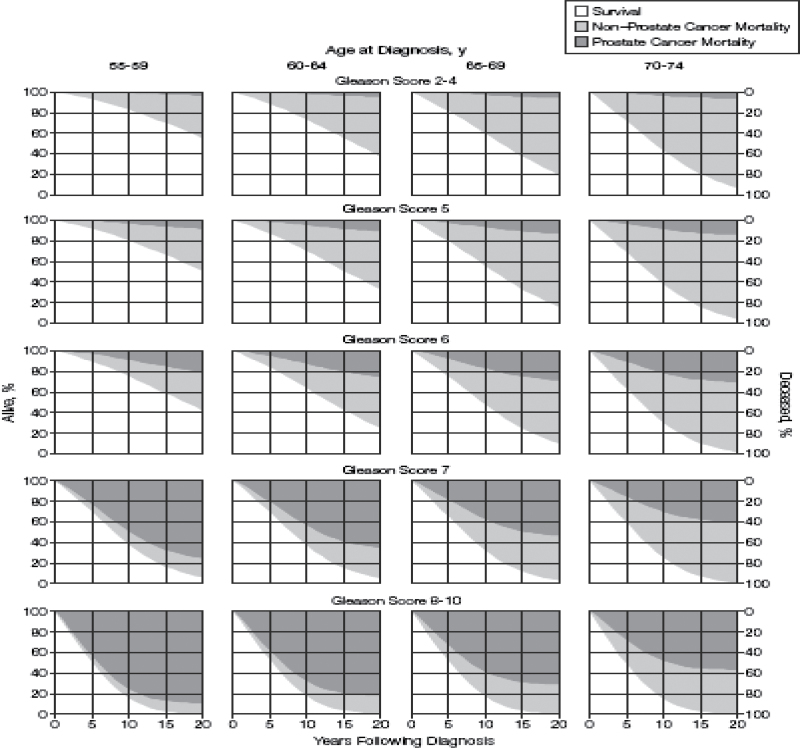
In 1998 and 2005, we reported long-term outcomes of a competing risk analysis of 767 men diagnosed between 1971 and 1984 who were managed expectantly for clinically localized prostate cancer (Figure 2) (19,20). Few men (4%–7%) with Gleason 2–4 tumors had progression leading to death from prostate cancer within 20 years of diagnosis. Men with Gleason 5 and 6 tumors experienced a somewhat higher 20-year risk of dying from prostate cancer when managed expectantly (6%–11% and 18%–30%, respectively). Men with Gleason scores 7 and 8–10 tumors were likely to die from prostate cancer within 20 years regardless of their age at diagnosis (42%–70% and 60%–87%, respectively). Very few of these men of any age survived more than 15 years.
Figure 2.
Twenty-year survival estimates of men diagnosed in the pre–prostate-specific antigen era. Reproduced from Albertsen et al. (20) with permission from American Medical Association.
More recently, Akre et al. conducted a registry-based nationwide review of men diagnosed with locally advanced prostate cancer (21). They limited their study to men with stage T2 (PSA 50–99ng/mL), stage T3, and stage T4 disease who had no signs of metastases. They found that the 8-year prostate cancer–specific mortality rate correlated with Gleason score at diagnosis and was 28% for men with Gleason score 2–6, 41% for Gleason 7, 52% for Gleason 8, and 64% for Gleason 9–10 disease. Even for men over 85 years of age, prostate cancer was a major cause of death when the Gleason score was 8 or above at diagnosis. Men with locally advanced prostate cancer and a PSA less than 4ng/mL were at particular risk of dying from prostate cancer.
Collectively, these studies reveal that men with high-grade prostate cancer (Gleason score ≥7) face a substantial risk of death from disease in the absence of treatment, whereas men with low-grade prostate cancer (Gleason score ≤6) can survive 10–20 years with their disease and often die from causes unrelated to prostate cancer. Furthermore, men whose disease was not identified by PSA screening are at significant risk of disease progression and death from prostate cancer, but this risk ranges from 20–80% depending upon the Gleason score and volume of the tumor at presentation.
Pathological Considerations When Interpreting the Risk Posed by Prostate Cancer
Prostate cancer is unique in that many men harbor multiple foci of indolent disease. Autopsy studies have shown that small foci of prostate cancer are common even among men under 50 years of age (22). Estimates suggest that anywhere from 14% to 70% of men in their sixties and 31%–83% of men in their seventies have evidence of disease. A 2003 publication from the Prostate Cancer Prevention Trial confirmed the high prevalence of low-grade prostate cancer (23). Researchers designing this trial estimated the prevalence of prostate cancer to be 6% and powered the trial to detect a 25% reduction in incidence. After seven years of follow-up, prostate cancer was detected in 24.4% of men in the control arm and 18.4% in the treatment arm. These substantially higher rates were the result of a decision to biopsy as many men as possible in each arm regardless of their clinical findings or PSA levels. The trial demonstrated that high-grade prostate cancers were present even among men with serum PSA values less than 4.0ng/mL. Equally important was the observation that most of the cancers detected were low-grade Gleason 3 + 3 = 6 tumors. Most of these incidental cancers would never have been discovered if men had not undergone a transrectal ultrasound and biopsy.
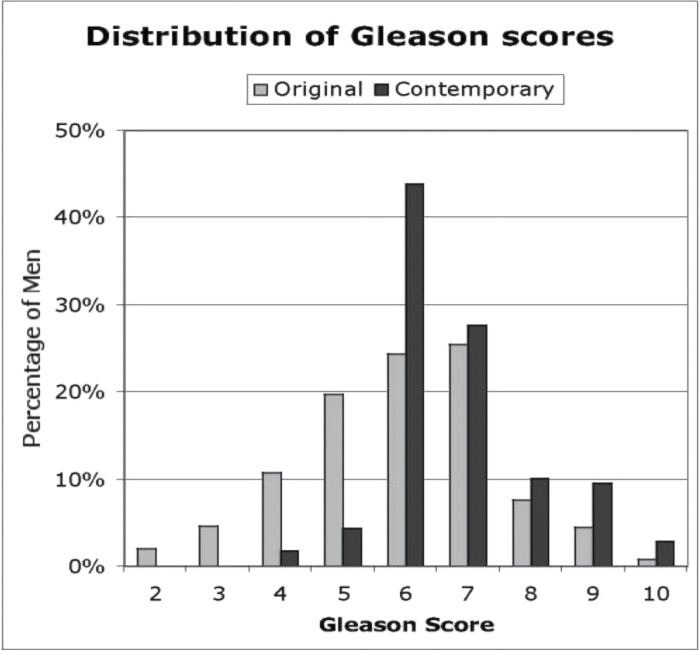
Understanding the threat posed by prostate cancer has also been confounded by pathologists’ changing interpretation of the Gleason scoring system. When Gleason originally developed his scoring system based on low-power analysis of glandular architecture, men were commonly diagnosed with prostate cancer following a transurethral resection, an open prostatectomy performed to treat obstructive urinary symptoms or a needle biopsy with an 18-gauge needle (6). Before 2000, most pathologists used all five patterns described by Gleason. Since then, they have become increasingly hesitant to use Gleason patterns 1 and 2. This stems in part from a report by Epstein who commented that Gleason score assignments following radical prostatectomy were frequently higher for those men who had biopsies assigned Gleason scores less than 3 + 3 = 6 (24). Furthermore, changes in the interpretation of Gleason patterns have reclassified some features from the original pattern 3 to pattern 4 (25). Thus, many low-grade tumors previously recorded as Gleason score 2–5 are now classified as Gleason score 6, and many Gleason score 6 tumors are now classified as Gleason score 7. Reclassification during the past two decades has been so extensive that clinical outcomes are significantly improved if historical classifications are replaced by contemporary classifications (Figure 3) (26). Death from prostate cancer appears to be rare among men with contemporary low- and moderate-grade T1c prostate cancers during the first 10 years following diagnosis (27).
Figure 3.
Change in the interpretation of Gleason score patterns. Results recorded in 1990 compared with results recorded of the same slides in 2007. Reproduced from Albertsen et al. (26).
The Risk Posed by Contemporary Prostate Cancers
Extensive annual testing for PSA in the United States has dramatically altered the type of patients presenting with newly diagnosed disease. Draisma et al. estimate that PSA testing has advanced the date of diagnosis by approximately 10 years for men aged 50 years and approximately 5 years for those aged 70 years (28). Equally important is the recognition that PSA testing leads to the discovery of indolent disease never destined to become clinically significant. Draisma et al. estimate that 20% of all cancers diagnosed at age 50 years and about 50% of all cancers diagnosed at age 70 years are clinically unimportant. These estimates support the findings of Sakr et al. who used autopsy studies to show that the incidence of small volume, low-grade cancers increased by 10% per decade such that a man aged 60 years has about a 60% chance of harboring a small indolent prostate cancer (22).
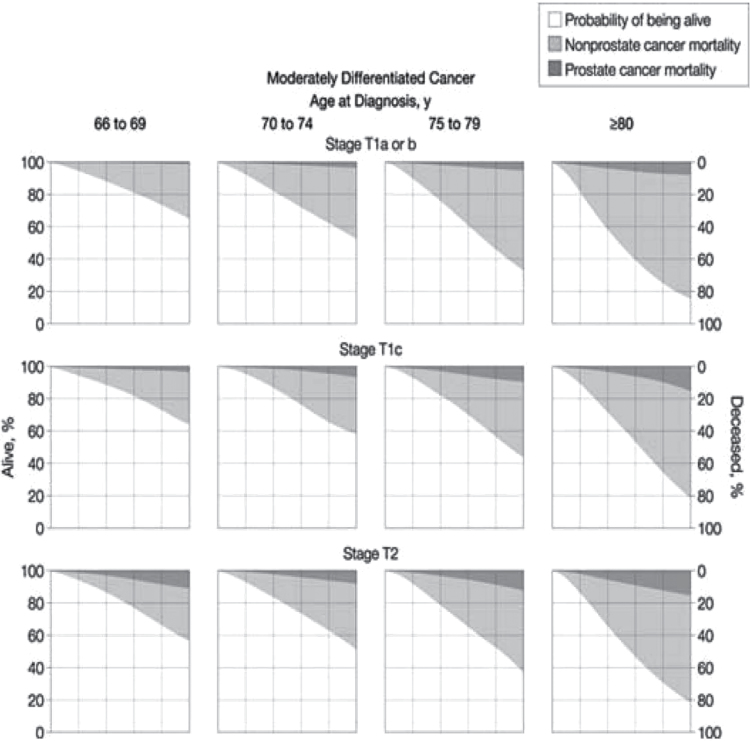
To adjust for the lead time introduced by PSA testing and the changes in the interpretation of the Gleason scoring system, Lu-Yao et al. used data available from the Surveillance, Epidemiology, and End Results program of the National Cancer Institute and data available through Medicare claims analysis to calculate the competing risks of men diagnosed with localized prostate cancer after age 65 years and managed conservatively. These results are presented in Figure 4. In a subsequent publication, Albertsen et al. used the same dataset to explore the impact of comorbidity on the probability of dying from prostate cancer (29). These 10-year survival curves reflect the current best estimates of the risk posed by prostate cancer in contemporary practice in the United States.
Figure 4.
Contemporary 10-year survival estimates for men with Gleason 5–7 disease. Reproduced from Lu-Gao et al. (27) with permission from American Medical Association.
Clinical Outcomes Associated With Contemporary Prostate Cancer Screening Trials
Results from two long-awaited randomized trials on PSA screening were published in 2009: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial conducted by the National Cancer Institute and the European Randomized Study of Screening for Prostate Cancer (ERSPC) supported by grants from Europe Against Cancer and multiple European agencies and health authorities (30,31). Both trials have strengths and weaknesses. The PLCO trial followed a common protocol and was conducted at 10 study centers located within the United States. The ERSPC study was a collection of seven PSA screening trials that employed different study designs, screening tests, screening intervals, different ages of patient at entry, and choices of controls. Both of these trials are ongoing. The results reported in 2009 represent interim analyses. Several more years of follow-up will be needed before the full impact of PSA testing can be determined.
The PLCO trial was initiated in 1993 and recruited 76 693 men at 10 study centers within the United States before closing to accrual in 2001 (30). Men were randomly assigned to receive annual PSA tests for 6 years and digital rectal examinations for 4 years (n = 38 343) or were assigned to usual care (n = 38 350). Study coordinators notified primary care givers whenever a study participant was found to have a PSA greater than or equal to the threshold of 4.0ng/mL.
Compliance with the screening protocol was 85% for PSA testing and 86% for digital rectal examination. Unfortunately, the rate of PSA testing in the control arm was 40% in the first year and increased to 52% by the sixth year. Compliance was monitored through random surveys. Surprisingly, after 7–10 years of follow-up, only 94 prostate cancer deaths were recorded: 50 in the screening arm and 44 in the control arm.
The report of the ERSPC trial represents a combined analysis of seven separate PSA screening trials conducted from 1991 to 2003 in the following countries: the Netherlands, Belgium, Sweden, Finland, Italy, Spain, and Switzerland (31). All study centers included a core age group of men 55–69 years of age who became the subject of the report (n = 162 387). The recruitment and randomization procedures differed among countries. In Finland, Sweden, and Italy, trial subjects were identified from population registries and underwent randomization before written informed consent was provided. In the Netherlands, Belgium, Switzerland, and Spain, the target population was identified from population lists, but only those who agreed to participate were randomized. Finland contributed about 50% of the study subjects (n = 80 379) and the Netherlands about 20% (n = 34 833).
The screening protocol used in the trials varied both by site and by calendar year. In the early 1990s, the Dutch and Belgium sites relied on a combination of digital rectal examination, transrectal ultrasonography, and PSA tests. Later, most centers relied on a PSA test alone except in Finland and Italy, where rectal examinations were used to identify men for biopsy who had marginally normal values (ie, PSA values between 2.5 and 3.9ng/mL). Initially most sites used a cut point of 4.0ng/mL, but this was lowered to 3.0ng/mL as the study progressed. Most countries used a 4-year screening interval. Sweden screened men every 2 years and Belgium screened men every 4–7 years because of funding problems. Unlike the PLCO trial, all of the screening protocols mandated a subsequent transrectal ultrasound and biopsy. Most centers used a sextant biopsy protocol, but this was later amended in several centers to include 10–12 cores.
After a median follow-up of 9 years, the cumulative incidence of prostate cancer was 8.2% in the screening group and 4.8% in the control group. An analysis by Gleason score demonstrated that the incidence of high-grade cancer (Gleason score ≥7) was comparable between the groups (2.1% vs 2.2%). Most of the excess prostate cancers diagnosed by PSA testing were Gleason 6 tumors. Although the study showed a clinically significant relative reduction in prostate cancer mortality of 20%, the absolute risk difference was just 0.71 deaths per 1000 men. Approximately 0.294% of the men died in the screening arm and 0.365% died in the control arm. One prostate cancer death was averted for every 1410 men screened and 48 prostate cancers diagnosed.
More recently, Hugosson et al. published the 14-year outcomes among men enrolled in the Swedish arm of the ERSPC trial (32). This study differed from the other participating centers in that screening was conducted every 2 years. Furthermore, the number of prostate cancers diagnosed was slightly higher than in the other centers. A sensitivity analysis of the original ERSPC study demonstrated that exclusion of the Swedish data would have rendered the trial results statistically insignificant. Hugosson et al. reported that after 14 years, mortality from prostate cancer was reduced by 56%. When the data are scrutinized more closely, screening in the Swedish trial resulted in identifying slightly more high-grade tumors (Gleason score ≥7) than in the control group (5.7% vs 5.0%). The majority of additional cancers identified were low-grade tumors. Again the absolute numbers of men who died from prostate cancer after 14 years of follow-up was modest: 0.784% in the control group and 0.442% in the screened group. These numbers confirm that the risk of dying from screen-detected prostate cancer is extremely low after 14 years although this number is likely to rise during the next decade.
Summary
Prostate cancer is an enigmatic disease. Historically, it was viewed as a disease of elderly men that often progressed slowly over many years. Clinical symptoms usually resulted from metastatic disease to bone or from urinary obstruction. The advent of testing for PSA dramatically altered the incidence and presentation of this disease. Men diagnosed with contemporary prostate cancer are much more likely to be middle aged and have disease localized to the prostate. Despite the advent of multiple genetic and molecular probes, the most powerful predictor of disease progression is tumor histology. Men with contemporary Gleason score 6 disease will often survive two decades or more without disease progression, whereas men with Gleason score 8–10 disease will often die from prostate cancer despite therapeutic intervention within 15 years of diagnosis. Screening for PSA remains controversial. Men with PSA values between 4 and 20 may harbor clinically significant prostate cancer but may also only have an enlarged, benign prostate. Men with PSA values over 20ng/mL are much more likely to develop clinically significant disease that will progress during their lifetime, whereas men with clinical evidence of prostate cancer and a PSA less than 4ng/mL often have a particularly poor prognosis.
References
- 1. Thompson H. The Enlarged Prostate. London: John Churchill Company; 1852. [Google Scholar]
- 2. Levin I, Sittenfield MJ. On the mechanism of the formation of metastases in malignant tumors: an experimental study. J Exp Med. 1911;14(2):148–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barringer BS. Radium in the treatment of carcinoma of the bladder and prostate. JAMA. 1917;68(17):1227–1230 [Google Scholar]
- 4. Young HH. Early diagnosis and radical cure of carcinoma of the prostate. Bull Johns Hopkins Hosp. 1905;16:314–321 [Google Scholar]
- 5. Huggins C, Hodges CV. Studies on prostatic cancer II: the effects of castration on advanced carcinoma of the prostate gland. Arch Surg. 1941;43(2):209–223 [Google Scholar]
- 6. Gleason DF, Mellinger GT. Predication of prognosis for prostatic adenocarcinoma by combined histologic grading and clinical staging. J Urol. 1974;111(1):58–64 [DOI] [PubMed] [Google Scholar]
- 7. Stamey TA, Yang N, Hay AR, McNeil JE, Freiha FS, Redwine E. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med. 1987;317(15):909–916 [DOI] [PubMed] [Google Scholar]
- 8. Catalona WJ, Smith DS, Ratliff TL, et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. 1991;324(17):1156–1161 [DOI] [PubMed] [Google Scholar]
- 9. Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212–236 [DOI] [PubMed] [Google Scholar]
- 10. Smith RA, Cokkinides Brawley OW. Cancer screening in the United States, 2008: a review of current American Cancer Society guidelines and cancer screening issues. CA Cancer J Clin. 2008;58(3):161–179 [DOI] [PubMed] [Google Scholar]
- 11. Ross LE, Berkowitz Z, Ekwueme DU. Use of the prostate specific antigen test among US men: findings from the 2005 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2008;17(3): 636–644 [DOI] [PubMed] [Google Scholar]
- 12. Ries LAG, Melbert D, Krapcho M. et al. , eds. SEER Cancer Statistics Review, 1975–2005. Surveillance, Epidemiology, and End Results. Bethesda, MD: National Cancer Institute; 2008. [Google Scholar]
- 13. Merrill RM, Weed DL. Measuring the health burden of cancer in the United States through lifetime and age-conditional risk estimates. Ann Epidemiol. 2001(8);11:547–533 [DOI] [PubMed] [Google Scholar]
- 14. Johansson JE, Adami HO, Andersson SO, Bergstrom R, Krusemo UB, Kraaz W. Natural history of localized prostatic cancer. A population-based study in 223 untreated patients. Lancet. 1989;1(8642):799–803 [DOI] [PubMed] [Google Scholar]
- 15. Johansson JE, Adami HO, Andersson SO, Bergrstrom R, Holmberg L, Krusemo UB. High 10 year survival rate in patients with early, untreated prostatic cancer. JAMA. 1992;267(16):2191–2196 [PubMed] [Google Scholar]
- 16. Johansson JE, Holmberg JS, Bergrstrom R, Adami HO. Fifteen year survival in prostate cancer. A prospective, population-based study in Sweden. JAMA. 1997;277(6):467–471 [PubMed] [Google Scholar]
- 17. Johansson JE, Andren O, Andersson SO, et al. Natural history of early, localized prostate cancer. JAMA. 2004;291(22):2713–2719 [DOI] [PubMed] [Google Scholar]
- 18. Chodak GW, Thisted RA, Gerber GS, et al. Results of conservative management of clinically localized prostate cancer. N Engl J Med. 1994;330(4): 242–248 [DOI] [PubMed] [Google Scholar]
- 19. Albertsen PC, Hanley JA, Gleason DF, Barry MJ. Competing risk analysis of men aged 55 to 74 years at diagnosis managed conservatively for clinically localized prostate cancer. JAMA. 1998;280(11): 975–980 [DOI] [PubMed] [Google Scholar]
- 20. Albertsen PC, Hanley JA, Fine J. 20 year outcomes following conservative management of clinically localized prostate cancer. JAMA. 2005;293(17):2095–2101 [DOI] [PubMed] [Google Scholar]
- 21. Akre O, Garmo H, Adolfsson J, Lambe M, Bratt O, Stattin P. Mortality among men with locally advanced prostate cancer managed with noncurative intent: a nationwide study in PCBaSe Sweden. Eur Urol. 2011;60(3):554–563 [DOI] [PubMed] [Google Scholar]
- 22. Sakr WA, Haas GP, Cassin BF, Pontes JE, Crissman JD. The frequency of carcinoma and intraepithelial neoplasia of the prostate in young male patients. J Urol. 1993;150(2, pt 1):379–385 [DOI] [PubMed] [Google Scholar]
- 23. Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349(3):215–224 [DOI] [PubMed] [Google Scholar]
- 24. Epstein JI. Gleason score 2-4 adenocarcinoma of the prostate on needle biopsy. Am J Surg Pathol. 2000;24(4)477–478 [DOI] [PubMed] [Google Scholar]
- 25. Berney DM, Fisher G, Kattan MW, et al. Major shifts in the treatment and prognosis of prostate cancer due to changes in pathologic diagnosis and grading. BJU Int. 2007;100(6):1240–1244 [DOI] [PubMed] [Google Scholar]
- 26. Albertsen PC, Hanley JA, Barrows GH, et al. Prostate cancer and the Will Rogers phenomenon. J Natl Cancer Inst. 2005;97(17):1248–1253 [DOI] [PubMed] [Google Scholar]
- 27. Lu-Yao GL, Albertsen PC, Moore DF, et al. Outcomes of localized prostate cancer following conservative management. JAMA. 2009;302(11):1202–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Albertsen PC, Moore DF, Shih W, Lin Y, Li H, Lu-Yao GL. Impact of comorbidity on survival among men with localized prostate cancer. J Clin Oncol. 2011;29(10):1335–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Draisma G, Boer R, Otto SJ, et al. Lead times and over detection due to prostate-specific antigen screening: estimates from the European Randomized Study of Screening for Prostate Cancer. J Natl Cancer Inst. 2003;95(12):868–878 [DOI] [PubMed] [Google Scholar]
- 30. Andriole GL, Grubb RL, Buys SS, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360(13)1310–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schroeder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality in a randomized European study. N Engl J Med. 2009;360(13):1320–1328 [DOI] [PubMed] [Google Scholar]
- 32. Hugosson J, Carlsson S, Aus G, et al. Mortality results from the Goteborg randomized population-based prostate-cancer screening trial. Lancet Oncol. 2010;11(8):725–732 [DOI] [PMC free article] [PubMed] [Google Scholar]