Abstract
Active surveillance has evolved to become a standard of care for favorable-risk prostate cancer. This is a summary of the rationale, method, and results of active surveillance beginning in 1995 with the first prospective trial of this approach. This was a prospective, single-arm cohort study. Patients were managed with an initial expectant approach. Definitive intervention was offered to those patients with a prostate-specific antigen (PSA) doubling time of less than 3 years, Gleason score progression (to 4+3 or greater), or unequivocal clinical progression. Survival analysis and Cox proportional hazard model were applied to the data. Since November 1995, 450 patients have been managed with active surveillance. The cohort included men under 70 with favorable-risk disease and men of age more than 70 with favorable- or intermediate-risk cancer (Gleason score 3+4 or PSA 10–15). Median follow-up is 6.8 years (range 1–16 years). Overall survival is 78.6%. Ten-year prostate cancer actuarial survival is 97.2%. Five of 450 patients (1.1%) have died of prostate cancer. Thirty percent of patients have been reclassified as higher-risk patients and offered definitive therapy. The commonest indication for treatment was a PSA doubling time less than 3 years (48%) or Gleason upgrading (26%). Of 117 patients treated radically, the PSA failure rate was 50%. This represents 13% of the total cohort. Most PSA failures occurred early; at 2 years, 44% of the treated patients had PSA failure. The hazard ratio for non–prostate cancer mortality to prostate cancer mortality was 18.6 at 10 years. In conclusion, we observed a very low rate of prostate cancer mortality in an intermediate time frame. Among the one-third of patients who were reclassified as higher risk and retreated, PSA failure was relatively common. However, other-cause mortality accounted for almost all of the deaths. Further studies are warranted to improve the identification of patients who harbor more aggressive disease in spite of favorable clinical parameters at diagnosis [reproduced from Klotz (1) with permission from Wolters Kluwer Health].
The world of prostate cancer has changed dramatically in the last 20 years. Prostate-specific antigen (PSA) testing first became available in the mid-1980s and was not introduced in Canada until about 1988. In both the United States and Canada, PSA was almost immediately embraced as a biomarker for the early identification of patients at risk for prostate cancer. The result was a sharp increase in the incidence of prostate cancer. This initial spike in incidence was due to the diagnosis of the many prevalent, slow growing, previously undiagnosed cases in the population. Many of these patients had substantial volume of disease; had serial PSA screening been available, they would have been diagnosed otherwise years before.
With the passage of time, the prevalent cases were diagnosed and treated, and the median volume of prostate cancer in newly diagnosed patients began to fall. This process took about 5 years. By the mid-1990s, the “incident” cases began to predominate. Clinicians began to see a dramatic increase in the number of patients with minimal low-grade disease on biopsy. Other than stage T1a prostate cancer seen following a transurethral resection of the prostate, such patients had not previously been encountered in any significant numbers.
The members of my multidisciplinary genitourinary oncology group at Sunnybrook in Toronto were very cognizant of the high rate of prostate cancer found at autopsy and were also influenced by the excellent results of conservative management of T1a disease. Surprisingly, a widespread and relatively unremarked-upon consensus existed that T1a prostate cancer (<5% of chips showing Gleason 6 or less prostate cancer on transurethral resection of the prostate specimen) should not be treated. Yet this consensus was not applied to the diagnosis of T1c prostate cancer at all. Patients diagnosed with any prostate cancer, even microfocal low-grade disease, if this diagnosis occurred based on an elevated PSA and transrectal ultrasonography–guided biopsy, were offered radical therapy. Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) data showed that in the United States, 95% of such patients received radical treatment (2).
It seemed obvious to us that there was an incongruity between the conservative approach taken with great success for T1a disease, and the insistence on radical therapy for T1c disease. Many of the arguments by knowledgeable individuals seemed flawed. For example, “American men diagnosed with cancer demand treatment,” and yet, this had not been the case for T1a disease. “Some of these patients will progress and die of disease, even though their baseline parameters were favorable,” which is also true for T1a disease, which had a 15% progression rate at 10 years (3). “Patients managed with watchful waiting are deprived of an opportunity for cure,” but curative therapy offered after several years of observation of a slow-growing cancer seemed plausible. Thus, we reasoned, perhaps an initial conservative approach of expectant management, that using the natural history of the patient’s own disease, including PSA kinetics and serial biopsy to determine treatment, might go a long way toward reducing the overall morbidity of treatment. The concept of personalized therapy was also beginning to emerge, and further drove support for the concept.
In Canada, the newly formed Prostate Cancer Research Foundation announced their first project competition. We submitted an application and were funded $35 000 to begin a prospective trial of active surveillance. This was, in fact, the Foundation’s first grant.
Thus, beginning in 1995, we initiated a prospective clinical trial to evaluate active surveillance, in which the decision to intervene was determined by PSA kinetics and/or histological progression. This strategy offered the attraction of individualizing therapy according to the biological behavior of cancer. Patients with a slowly growing malignancy would be spared the side effects of radical treatment, whereas those with more rapidly progressive cancer would still potentially benefit from curative therapy. Our initial cohort was first reported in 2002 (4–6) and again in 2010 (7,8). It is the most mature and one of the largest prospective cohorts in the world, now comprising approximately 700 patients.
Materials and Methods
A prospective, single-arm cohort study was initiated in November 1995 to assess the feasibility of an observation protocol with selective delayed intervention using PSA kinetics and/or histological progression as triggers for intervention. Favorable-risk patients were offered an initial surveillance approach. PSA was performed every 3 months for 2 years and then every 6 months in stable patients. A confirmatory biopsy was performed 6–12 months after the initial biopsy and then every 3–4 years until the patient reached 80. Patients were reclassified as higher risk and offered radical intervention for the following criteria:
1. A PSA doubling time (DT) of less than 3 years. For the first 4 years of the study, a PSA DT of 2 years was used as a trigger. However, this proved to be overly stringent, insofar as it identified only 10% of patients as high-risk patients. In 1999, the trigger was increased to 3 years. Approximately 20% of patients in the cohort were offered intervention for a PSA DT less than 3 years.
2. Histological upgrade on repeat prostate biopsy. Patients had an 8–14 core biopsy within a year after the initial biopsy (based on the “Vienna nomogram”) (9). This confirmatory biopsy was intended to identify higher-grade cancer that had been missed on the original biopsy. Where possible, particular attention was paid to the site of the previous biopsy and to the anterolateral horn. Subsequent biopsies were intended to identify biological progression and were performed every 3–4 years. Patients with a borderline PSA DT had biopsies more frequently at the discretion of the managing physicians.
3. Clinical progression: Development of an unequivocal palpable nodule during surveillance. Histology of the nodule was evaluated by directed biopsies. If the nodule was confirmed as evidence of cancer progression, patients were offered definitive therapy.
Between 1995 and 1999, the study was offered to all favorable-risk patients (Gleason 6 or less, PSA 10 or less) and to patients with age more than 70 with PSA up to 15 or Gleason up to 3+4 (8). Since January 2000, the study was restricted to favorable-risk patients only. This decision was based on more convincing evidence of a significant difference in natural history between Gleason 6 and 7 and an interest in studying a more homogeneous population.
The approach of active surveillance was initially considered experimental. The phase 2 study was launched as a formal trial, and it received approval by the Sunnybrook Research Ethics Board. From 1995 to 2002, informed consent was obtained from each participant. Beginning in 2003, the phase 2 study was terminated as a formal clinical trial, and active surveillance was offered as a treatment option to patients. The same data was collected prospectively on the patients choosing active surveillance as a treatment option as it had been under the aegis of the formal clinical trial.
PSA DT was calculated using the General Linear Mixed Model method. This has been described in a previous publication by the authors (6). All analysis was conducted using Statistical Analysis Software (SAS version 9.1 for Windows).
Results
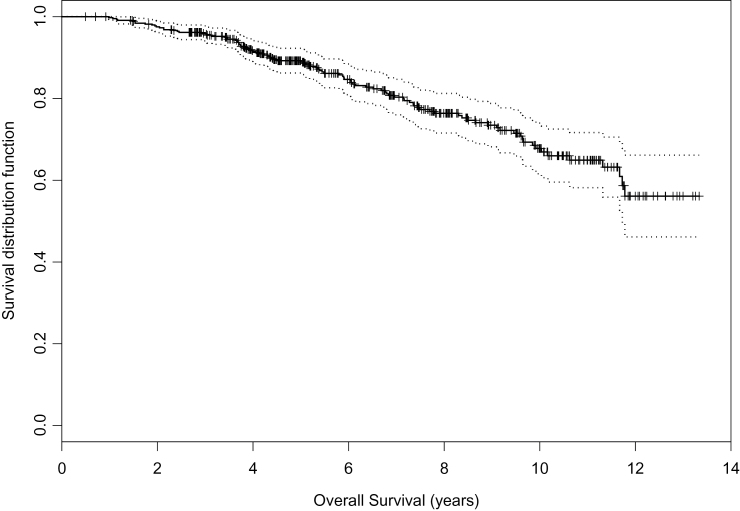
The cohort consists of 450 patients. Median age is 70.3. Median follow-up is 6.8 years. Eighty-three percent of patients were Gleason 6 or less, and 17% were Gleason 3+4=7. Eighty-five percent had PSA less than or equal to 10, and 12% had a PSA between 10 and 15. Seventy-one percent of patients were favorable risk by D’Amico criteria. The intermediate-risk patients either were more than 70 or had significant comorbidity. Among the 450 patients, 97 (21.6%) patients died and 353 are alive. Ten-year overall survival is 68% (CI 62% to 74%) (Figure 1). There was no difference in overall survival between patients who remained on surveillance and those who were reclassified and treated radically (P = .25).
Figure 1.
Overall survival. Reproduced from Klotz et al. (8) with permission from the American Society of Clinical Oncology.
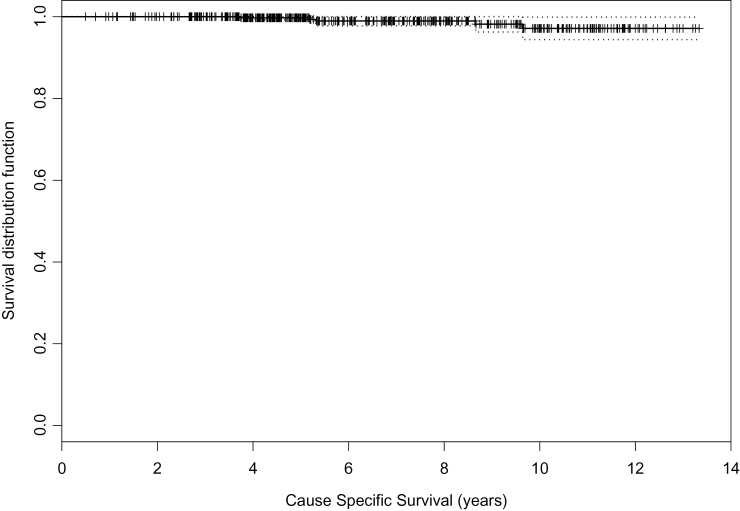
Cause-specific survival (CSS) is shown in Figure 2. The 5- and 10-year CSS is 99.7% and 97.2%. All prostate cancer mortalities (6) occurred in men who had been reclassified as higher risk and were offered radical treatment. These deaths occurred at 3.7, 5.2, 5.3, 8.7, and 9.6 years after diagnosis.
Figure 2.
Cause-specific survival. Reproduced from Klotz et al. (8) with permission from the American Society of Clinical Oncology.
All five patients had a PSA DT less than 2 years triggering a recommendation of radical therapy as per protocol. Radical intervention was undertaken in three of the five patients (radiation in two cases and prostatectomy in one). Two refused treatment. One of the radiation patients was Gleason 7 at baseline. Of the two radically treated patients who were Gleason 6, one had a radical prostatectomy within a year of diagnosis. His PSA continued to rise after surgery, with no nadir observed. He recurred with metastatic disease within 1 year of treatment, which progressed rapidly to hormone refractory disease and death.
One patient, age 74 at diagnosis, was upgraded to Gleason 8 on confirmatory biopsy at 20 months after diagnosis. His PSA DT at that point was 1.3 years. He received radiation therapy and adjuvant androgen deprivation therapy about 2 years after study entry. At 6 years, he was found to have liver and bone metastases and was treated with androgen deprivation therapy. At 7 years, he developed hormone refractory disease and received Taxotere. He expired 9 years and 8 months after entering the study.
Thus the cohort of 450 men contains only a single patient who was treated after a relatively prolonged period of observation (>2 years) and subsequently progressed to metastatic disease and death.
Reasons for Discontinuing the Surveillance Protocol
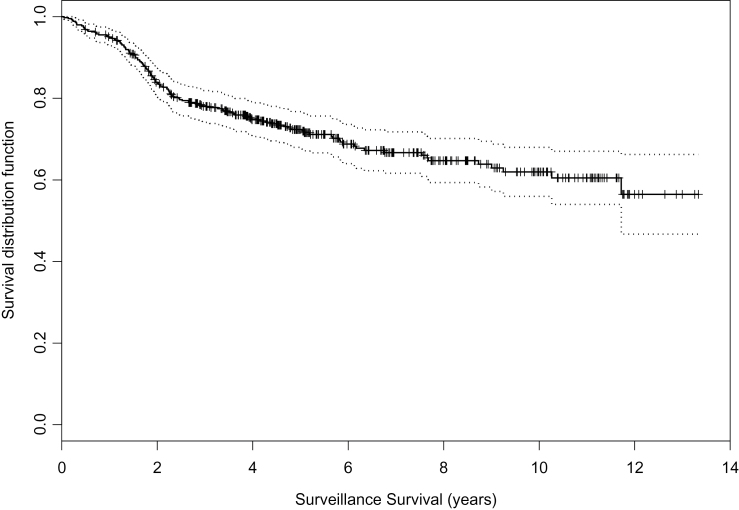
The reasons for discontinuing surveillance are summarized in Table 1. The choice of radiotherapy, surgery, or androgen deprivation therapy was based on age, general health, and patient preference. Figure 3 shows the time to stopping surveillance with 95% confidence intervals for the 450 patients. At 2, 5, and 10 years, the likelihood that a patient remained on surveillance was 84%, 72%, and 62%, respectively. One hundred thirty-five (30%) have been treated definitively. One hundred twenty-five patients were treated radically, 90 with radiotherapy and 35 with surgery. An additional 10 patients received androgen deprivation therapy alone. PSA failure was defined as a PSA more than 0.2 for surgery patients and PSA Nadir+2 for radiation patients. Of 117 patients on whom posttreatment PSAs are available, 59 (50.4%) have had PSA failure. This represents 13% of the overall cohort. The 5-year recurrence-free survival is 47%. Median time to PSA failure is 48 months. PSA-free survival at 5 years was 62% in the patients treated with surgery and 43% in patients treated with radiation. Log-rank test shows no difference finding between patients with surgery treatment and patients with radiation (P = .12).
Table 1.
Reasons for intervention on surveillance*
| Reason for treatment | N | % of those treated (n=135) | % of total cohort (n=450) |
|---|---|---|---|
| Short PSA doubling time | 65 | 48 | 14 |
| Grade progression | 36 | 27 | 8 |
| T1 to T2 progression | 6 | 4 | 1 |
| Volume progression | 4 | 3 | 0.9 |
| Ureteral obstruction | 2 | 2 | 0.4 |
| Patient preference | 14 | 10 | 3 |
| Unknown | 8 | 6 | 2 |
| Total | 135 | 100% | 30% |
* PSA = prostate-specific antigen. Reproduced from Klotz et al. (8) with permission from the American Society of Clinical Oncology.
Figure 3.
Likelihood of remaining alive and on surveillance. Reproduced from Klotz et al. (8) with permission from the American Society of Clinical Oncology.
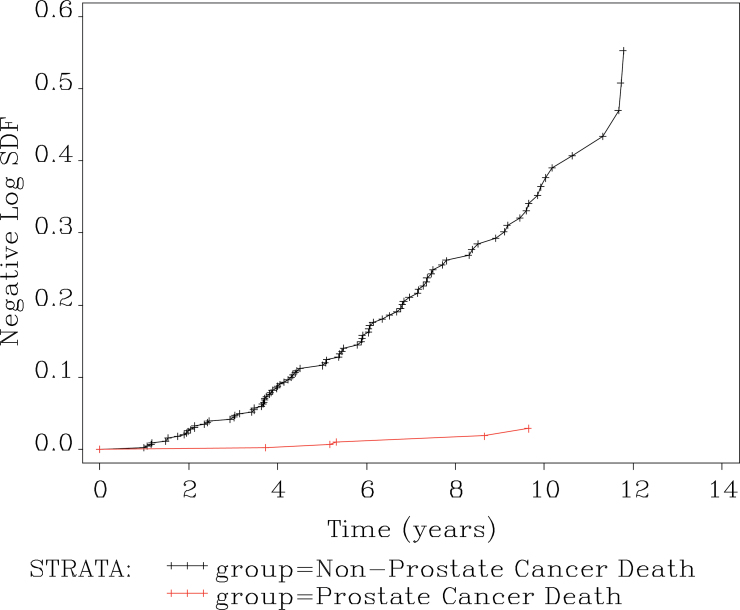
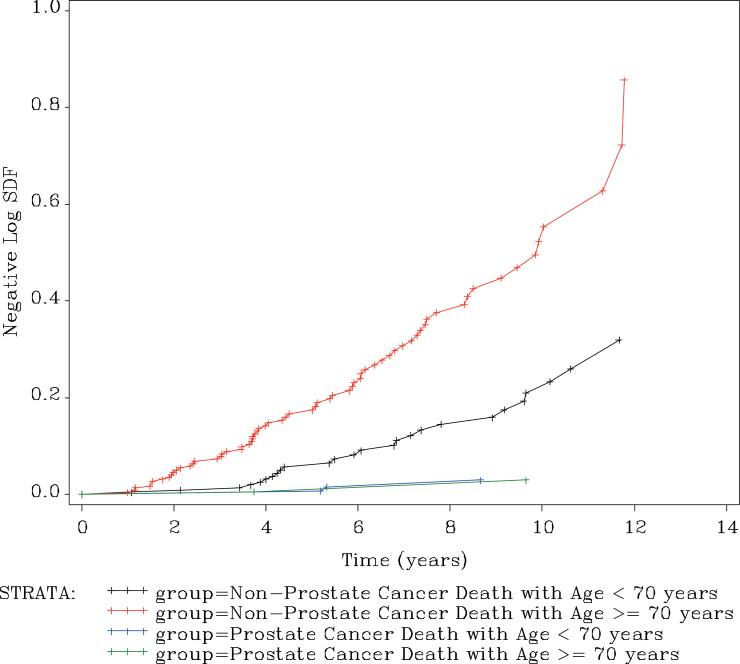
Figure 4 shows the hazard ratio for non–prostate cancer mortality vs prostate cancer mortality over time. Using Cox proportional hazard model, the hazard ratio between two groups was 18.6 with 95% confidence intervals of 7.6 to 45.7. The hazard ratio is assumed to be constant, so it is independent of time.
Figure 4.
Cumulative hazard ratio for non–prostate cancer to prostate cancer mortality. Reproduced from Klotz et al. (8) with permission from the American Society of Clinical Oncology.
Figure 5 shows this hazard ratio stratified by age more than or equal to or less than 70. For men of age more than or equal to 70, the hazard ratio for non–prostate cancer mortality to prostate cancer mortality is 33.3 with 95% confidence intervals of 8.2 to 136. In men of age less than 70, the hazard ratio is 8.76 with 95% confidence intervals of 2.65 to 28.9. As expected, the risk of non–prostate cancer mortality is higher in older men. However, even in the men under 70, the risk of non–prostate cancer mortality was far higher than that of prostate cancer mortality.
Figure 5.
Cumulative hazard ratio for mortality by cause and age, stratified around age 70. Reproduced from Klotz et al. (8) with permission from the American Society of Clinical Oncology.
Among 450 patients in the study, there were 85 patients (18.9%) who were intermediate risk at baseline, defined as PSA more than 15, Gleason score of 7, or stage T3. Forty-nine patients (11%) remained untreated, and 36 patients (8%) were eventually treated. Among the 49 untreated patients, no patient has disease progression. Among the 36 who were treated, only one has progressed to metastatic disease and death.
Discussion
Active surveillance offers the appealing prospect of personalized therapy based on predictable favorable natural history in the majority of patients with low-risk prostate cancer and selective delayed definitive therapy for the minority who are reclassified as high risk over time.
The overall survival is 78.6%, and the overall prostate cancer–specific survival is 99%. The 10-year actuarial cancer-specific survival is 97%. The hazard ratio for non–prostate to prostate cancer mortality is 18.6.
There are seven published active surveillance series, including this one (Table 2) (10–15). These vary in size and duration of follow-up. All have largely restricted this approach to favorable-risk patients. The precise method of follow-up and trigger for intervention varies. However, all rely on PSA kinetics and repeat biopsy to identify a subset of patients for definitive therapy. The cohort constitutes approximately 3000 patients in total. The median age is 68. Median follow-up is 43 months. Approximately 150 patients in the six series have been followed for more than 10 years. The overall survival in the cohort is 90%, and the disease-specific survival is 99.7%. About one-third of patients have been treated definitively. These figures clearly reflect the modest duration of follow-up relative to the long natural history of favorable-risk prostate cancer in these studies. Nonetheless, the ratio of non–prostate cancer to prostate cancer mortality is about 30:1, which is an impressive figure. (A 30:1 ratio of risk of other-cause mortality to prostate cancer mortality also approximates the lifetime risk of dying of prostate cancer in undiagnosed men.)
Table 2.
Summary of seven active surveillance series*
| Author, ref. | N | Median follow-up, mo. | pT3 in RP pts | OS | CSS |
|---|---|---|---|---|---|
| Van As (15) | 326 | 22 | 8/18 (44%) | 98 | 100 |
| Carter (10) | 407 | 41 | 10/49 (20%) | 98 | 100 |
| PRIAS (14) | 533–1000 | 48 | 4/24 (17%) | 90 | 99 |
| Soloway (11) | 99 | 45 | 0/2 | 100 | 100 |
| Roemeling (12) | 278 | 41 | 89 | 100 | |
| Khatami (13) | 270 | 63 | Not stated | 100 | |
| Klotz (8) | 452 | 73 | 14/24 (58%) | 82 | 97 @ 10 y |
| Total | 2130–3000 | 43 | 90 | 99.7 |
* CSS = cause-specific survival; OS = overall survival; PRIAS = Prostate Cancer Research International Active Surveillance; pts = patients; RP = radical prostatectomy. Adapted from Klotz et al. (8) with permission from the American Society of Clinical Oncology.
Seventy-one percent of the patients in the study fulfilled the D’Amico criteria for favorable risk. The remainder either had Gleason 3+4 cancer or a PSA more than 10. These patients were of age more than 70 or had significant comorbidity. In spite of a significant proportion of intermediate-risk patients in the cohort, the 10-year prostate cancer mortality is low. This supports the concept that in a screened population, even intermediate-risk prostate cancer in men of age more than 70 may present a relatively low risk of prostate cancer mortality.
Most “watchful waiting” series (of observation with no treatment option) were initiated in the pre-PSA era and represent a nonscreened cohort. A major feature of widespread PSA screening has been stage and volume migration. Thus those watchful waiting patients would be expected to have significantly higher volume of cancer than a typical screen-detected favorable-risk patient and a worse prognosis with conservative management.
A key concern in advocating for a policy of active surveillance is the risk that a patient, who appears favorable at diagnosis, will progress to incurability during the period of observation and suffer avoidable prostate cancer mortality as a result of the delay in definitive treatment. The low mortality of favorable-risk prostate cancer suggests that this event is likely to be unusual. However, critics of the surveillance approach point to the Swedish observation series, which was reported by Johannson (16,17). This consisted of 223 patients managed with watchful waiting (no salvage option). Median follow-up, remarkably, was 21 years. CSS was 79% at 15 years and 54% at 20 years. The annual risk of prostate cancer mortality was calculated at 15/1000 during the first 15 years, but 44/1000 after 15 years, based on 49 patients followed-up for more than 15 years. The implication is that, in younger patients with a long life expectancy, prostate cancer mortality will rise to unacceptable levels eventually.
However, in that cohort, the prostate cancer mortality among the patients with grade 1 disease was 8% at 7 years. In contrast, the 7-year cancer-specific mortality in our surveillance cohort is 1.1%. This suggests that the relative risk for prostate cancer mortality in a nonscreened, pre-PSA, watchful waiting cohort (with no salvage option) is as much as seven times higher than a contemporary, screen-detected group managed with surveillance with selective delayed intervention. Thus the increase in prostate cancer mortality seen after 15 years of follow-up in Johannsen’s unscreened watchful waiting series may not be recapitulated in a screened, active surveillance cohort.
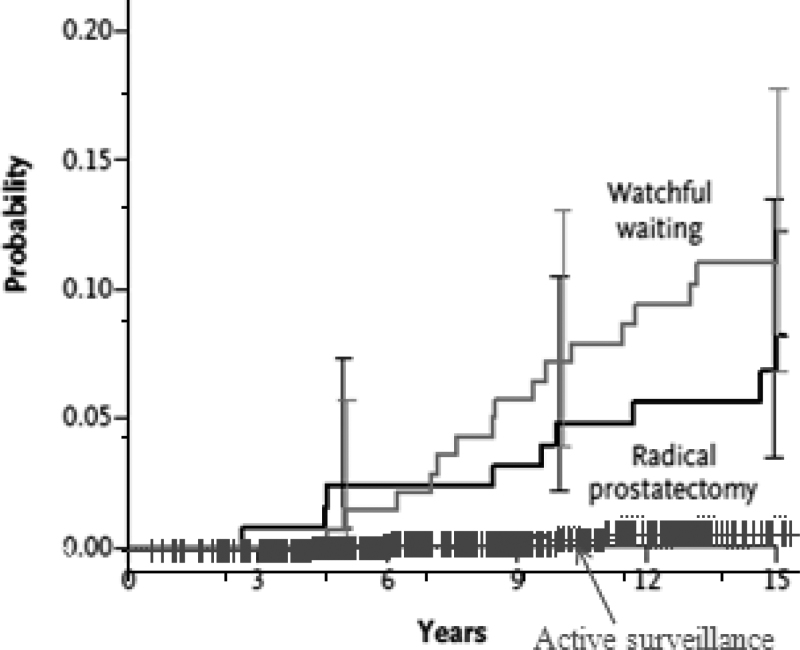
Further evidence for the powerful impact of the difference in natural history between screen-detected and non–screen-detected disease is apparent in comparing the outcome of “favorable risk” prostate cancer in the pivotal Scandinavian trial comparing radical prostatectomy to watchful waiting. In the third publication from this trial, prostate cancer mortality at 15 years was 12% in the watchful waiting group and 6% in the surgery group, which is a significant improvement. At 10 years, it was 9% and 5%, respectively. This lower mortality in the group in whom all patients received surgery is considerably higher than the prostate cancer mortality in the Toronto active surveillance group (3% at 10 years) (Figure 6) (18). This is almost certainly due to the baseline difference in extent and nature of disease. The Scandinavian trial consisted of unscreened patients diagnosed with sextant biopsies or aspiration cytology, with a median PSA of 13. This “low-risk” group in fact constituted a considerably higher-risk group than a contemporary cohort of serially screened Gleason 6 patients. This is the only reasonable explanation for the apparent decreased mortality in a surveillance cohort compared with a group of whom all were treated surgically.
Figure 6.
Comparison of prostate cancer mortality in the two arms of the Scandinavian randomized trials and the Toronto surveillance cohort. Reproduced from Klotz et al. (18) with permission from the Massachusetts Medical Society.
A comparison of prostate cancer mortality among men treated conservatively demonstrated that for stage T1 disease this has diminished over the last 20 years from 16%–23% to 2%–5% (18).
The PSA progression rate among the 125 patients offered definitive local therapy is 53% at 5 years. There was no significant difference between patients treated with surgery or radiation.
We believe this PSA failure rate should be interpreted in the following context:
1. Among the “stable” cohort of untreated patients, none have progressed clinically, either to metastatic locally advanced disease. Thus the appropriate denominator for PSA failure is the 450 patients in the total cohort. Therefore, the real PSA failure rate in the cohort is 59/450, or 13%. This is comparable to PSA failure rates for radical prostatectomy or radiation for favorable-risk patients.
2. PSA DT less than 3 years and Gleason upgrading clearly identify a group of high-risk patients, in spite of favorable prognostic criteria at diagnosis.
3. Approximately two-thirds of the patients were treated with primary radiation treatment. The dose used for most of these patients, who were largely treated in the late 1990s, was 64 Gy. This is now considered to be a subtherapeutic dose and may have accounted for the poor durable PSA response.
4. PSA failure does not mean death from prostate cancer (20). In the Hopkins cohort, the 15-year prostate cancer mortality in men with Gleason 6 and 7 with PSA recurrence before 3 years was 19%. Extrapolating this to the current cohort gives an overall cancer mortality of 3% at 15 years postrecurrence (19% × 13%). Further, the prostatectomy patients have, in most cases, been managed with early salvage radiation therapy, and the expectation is that half or more will have a complete response. (A detailed description of the outcome of the treated patients will be the subject of a subsequent manuscript.)
5. The mortality rate, with a median follow-up of over 6.8 years, is extremely low (1%), and the ratio of other-cause to prostate cancer mortality is 18.6:1. Although the rate of cancer mortality may rise with longer follow-up, so will the rate of non–prostate cancer mortality. We believe that the ratio of other-cause mortality to prostate cancer mortality is likely to remain stable over time. Longer follow-up will be required to determine the impact of the PSA failure rate in this cohort on disease-specific survival.
Current Approach
We have relied upon PSA kinetics as a trigger for definitive intervention, with reasonably good results. However, as is described elsewhere in this book, PSA kinetics does not seem to reliably predict for aggressive disease in the setting of favorable-risk prostate cancer. There are likely many reasons for this, including the confounding effects of biological variation, prostatitis, and enlarged prostate. Further, multiparametric MRI has recently emerged as an accurate diagnostic tool for the identification of larger-volume cancer. Indeed, it appears to be the perfect partner for surveillance, insofar as it is insensitive for small-volume disease and has a high positive predictive value for disease of volume more than 1 cc, the rough threshold for clinically significant disease.
Accordingly our current practice, along with serial PSA and biopsies, is to request a multiparametric MRI in patients with poor PSA kinetics (ie, DT < 3 years). The finding of an unequivocal large lesion is sufficient to drive either radical intervention or a targeted biopsy, depending on the clinical circumstances. Thus we no longer use PSA kinetics alone as a driver for radical treatment. This approach will require further validation.
Conclusions
Active surveillance for favorable-risk prostate cancer for men of all ages, and intermediate-risk disease in men of age more than 70, is feasible and appears safe in the 10–15-year timeframe. This strategy provides the benefit of an individualized approach based on the demonstrated risk of clinical or biochemical progression with time. In this cohort, the likelihood of dying of other causes was 18.6 times greater than the likelihood of prostate cancer death. Uncertainty remains regarding the long-term impact of delayed treatment in men reclassified as higher risk after a period of observation and repeat biopsy.
This article has been reprinted from Klotz (21) with permission from Springer. A version of this article that was previously published by Klotz et al. (8) was reproduced with permission from the American Society of Clinical Oncology.
References
- 1. Klotz L. Active surveillance: the Canadian experience. Curr Opin Urol. 2012; 22(3):222–230 [DOI] [PubMed] [Google Scholar]
- 2. Cooperberg MR, Broering JM, Carroll PR. Time trends in treatment of localized prostate cancer. J Clin Oncol. 2010; 28(7):1117–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Matzkin H, Patel JP, Altwein JE, Soloway MS. Stage T1A carcinoma of prostate. Urology. 1994; 43(1):11–21 [DOI] [PubMed] [Google Scholar]
- 4. Choo R, Klotz L, Danjoux C, et al. Feasibility study: watchful waiting for localized low to intermediate grade prostate carcinoma with selective delayed intervention based on prostate specific antigen, histological and/or clinical progression. J Urol. 2002; 167(4):1664–1669 [PubMed] [Google Scholar]
- 5. Klotz L. Active surveillance for prostate cancer: for whom? J Clin Oncol. 2005; 23(32):8165–8169 [DOI] [PubMed] [Google Scholar]
- 6. Zhang L, Loblaw A, Klotz L. Modeling prostate specific antigen kinetics in patients on active surveillance. J Urol. 2006; 176(4, pt 1):1392–7; discussion 1397 [DOI] [PubMed] [Google Scholar]
- 7. Krakowsky Y, Loblaw A, Klotz L. Prostate cancer death of men treated with initial active surveillance: clinical and biochemical characteristics. J Urol. 2010; 184(1):131–135 [DOI] [PubMed] [Google Scholar]
- 8. Klotz L, Zhang L, Lam A, Nam R, Mamedov A, Loblaw A. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010; 28(1):126–131 [DOI] [PubMed] [Google Scholar]
- 9. Remzi M, Fong YK, Dobrovits M, et al. The Vienna nomogram: validation of a novel biopsy strategy defining the optimal number of cores based on patient age and total prostate volume. J Urol. 2005; 174(4, pt 1):1256–12–60; discussion 1260 [DOI] [PubMed] [Google Scholar]
- 10. Carter HB, Kettermann A, Warlick C, et al. Expectant management of prostate cancer with curative intent: an update of the Johns Hopkins experience. J Urol. 2007; 178(6):2359–23–64; discussion 2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Soloway MS, Soloway CT, Williams S, Ayyathurai R, Kava B, Manoharan M. Active surveillance; a reasonable management alternative for patients with prostate cancer: the Miami experience. BJU Int. 2008; 101(2):165–169 [DOI] [PubMed] [Google Scholar]
- 12. Roemeling S, Roobol MJ, de Vries SH, et al. Active surveillance for prostate cancers detected in three subsequent rounds of a screening trial: characteristics, PSA doubling times, and outcome. Eur Urol. 2007; 51(5):1244–12–50 [DOI] [PubMed] [Google Scholar]
- 13. Khatami A, Ali K, Aus G, et al. PSA doubling time predicts the outcome after active surveillance in screening-detected prostate cancer: results from the European randomized study of screening for prostate cancer, Sweden section. Int J Cancer. 2007; 120(1):170–174 [DOI] [PubMed] [Google Scholar]
- 14. van den Bergh RC, Vasarainen H, van der Poel HG, et al. Short-term outcomes of the prospective multicentre ‘Prostate Cancer Research International: Active Surveillance’ study. BJU Int. 2010; 105(7):956–962 [DOI] [PubMed] [Google Scholar]
- 15. van As NJ, Norman AR, Thomas K, et al. Predicting the probability of deferred radical treatment for localised prostate cancer managed by active surveillance. Eur Urol. 2008; 54(6):1297–1305 [DOI] [PubMed] [Google Scholar]
- 16. Johansson JE. Expectant management of early stage prostatic cancer: Swedish experience. J Urol. 1994; 152(5, pt 2):1753–1756 [DOI] [PubMed] [Google Scholar]
- 17. Johansson JE, Andrén O, Andersson SO, et al. Natural history of early, localized prostate cancer. JAMA. 2004; 291(22):2713–2719 [DOI] [PubMed] [Google Scholar]
- 18. Klotz L, Thompson I. Early prostate cancer–treat or watch? N Engl J Med. 2011; 365(6):569 [DOI] [PubMed] [Google Scholar]
- 19. Lu-Yao GL, Albertsen PC, Moore DF, et al. Outcomes of localized prostate cancer following conservative management. JAMA. 2009; 302(11): 1202–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005; 294(4):433–439 [DOI] [PubMed] [Google Scholar]
- 21. Klotz L. Active surveillance: the Canadian experience.In: Klotz L, ed. Active Surveillance for Localized Prostate Cancer: A New Paradigm for Clinical Management. New York, NY: Humana Press; 2012:95–105 http://www.springerlink.com/content/978-1-61779-911-2/#section=1086238&page=4&locus=0 Accessed October 19, 2012. [Google Scholar]