Abstract
This chapter addresses issues relevant to prostate cancer overdiagnosis. Factors promoting the overdiagnosis of prostate cancer are reviewed. First is the existence of a relatively large, silent reservoir of this disease, as can be seen by evaluating autopsy studies and histologic step-sectioning results of prostates removed for other causes. The second main factor responsible for prostate cancer overdiagnosis is fairly widespread prostate-specific antigen and digital rectal examination-based screening, which has been fairly widely practiced in the United States for the past 20 years among heterogeneous groups of men. This has resulted in the identification of many men from this reservoir who otherwise may never have been diagnosed with symptomatic prostate cancer and is substantially responsible for the current annual incidence to mortality ratio for prostate cancer of approximately 6 to 1. Finally, the relatively indolent natural history and limited cancer-specific mortality as reported in a variety of contemporary randomized screening and treatment trials is reviewed. We attempt to quantitate the proportion of newly diagnosed prostate cancers that are overdiagnosed using various trial results and models. We explore the impact of prostate cancer overdiagnosis in terms of patient anxiety and the potential for overtreatment, with its attendant morbidity. We explore strategies to minimize overdiagnosis by targeting screening and biopsy only to men at high risk for aggressive prostate cancer and by considering the use of agents such as 5-alpha reductase inhibitors. Future prospects to prevent overtreatment, including better biopsy and molecular characterization of newly diagnosed cancer and the role of active surveillance, are discussed.
Intuitively, the early detection of cancer should result in a survival benefit if 1) earlier detection results in diagnosis of disease at an earlier, more curable stage, 2) effective treatment for the disease exists, and 3) the cancer would have ultimately resulted in death. In the case of prostate cancer, evidence for the first two factors seems relatively certain: There is stage migration seen in contemporary screen-detected prostate cancer (1), and radical prostatectomy is an effective therapy (2). However in this disease, mortality is more commonly the result of other competing risks (3,4), and many men both in the pre–prostate-specific antigen (PSA) era and in the contemporary PSA era are destined to die with a diagnosis of prostate cancer rather than from this cancer. This illustrates the concept of prostate cancer overdiagnosis.
Cancer overdiagnosis can be defined as the detection of cancer that would otherwise not become clinically manifest over a patient’s lifetime or not result in cancer-related death. This is a phenomenon that has been observed in a number of cancers including lung, breast, and prostate cancer (5,6). Three factors promoting the overdiagnosis of prostate cancer have been identified (6): the existence of a relatively large, silent disease reservoir, activities leading to the identification of patients within the reservoir, and an indolent natural history of limited cancer-specific mortality. This chapter explores these concepts as they relate to prostate cancer.
Silent Disease Reservoir
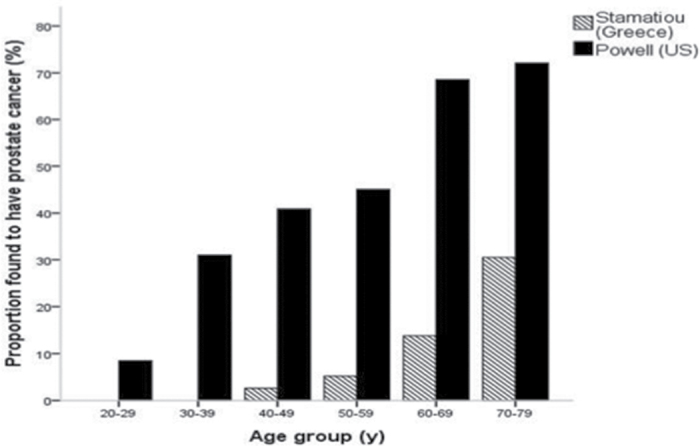
There are an estimated 240 890 new cases of prostate cancer diagnosed in 2011 (7), and over 2 million existing prostate cancer survivors in the United States have accumulated, given that annual incidence has exceeded current mortality by greater than 6 to 1 for the past decade or so. Further measures of the burden of this disease come from several series evaluating the incidental diagnosis of prostate cancer in autopsy studies of individuals who died from causes other than cancer (Figure 1) (8–10). In a series of 1056 men, Powell et al. found that the risk of an autopsy diagnosis of prostate cancer increased with advancing age (9). For men aged 60–79, the incidence of prostate cancer in step-sectioned autopsy specimens ranged from 68% to 77%. Similarly, an autopsy study from Greece revealed an age-dependent increase in prostate cancer incidence, with 14%–30% of specimens containing prostate cancer from men aged 60–79 (10). Remarkably, in these studies, disease was even found in men in their twenties (incidence 8%–11%), highlighting the long latency period from the development of prostate cancer to the eventual manifestation of symptoms for many men.
Figure 1.
Prevalence of autopsy diagnosed prostate cancer in men dying from other causes (9,10).
Additional estimates of latent disease prevalence come from the evaluation cystoprostatectomy specimens. Based on multiple series, 27%–60% of prostates were found to harbor clinically unsuspected prostate cancer (11–14). Notably, 18%–53% of these prostates had histologically significant disease based on varying criteria (most commonly a tumor volume ≥0.5 cc, Gleason grade >6, positive surgical margin, or non–organ-confined disease).
Detection of Silent Prostate Cancer
Enhanced detection of this latent disease reservoir was facilitated by the introduction of PSA as a screening test in the 1980s (15), resulting in increased prostate cancer detection over preexisting methods (16). This resulted in many asymptomatic men being diagnosed with prostate cancer, corresponding to the observed marked increase in prostate cancer incidence shortly after the widespread adoption of PSA tests in the early 1990s (7).
Comparing a man’s current lifetime risk of prostate cancer (7,16) (approximately 17%) with his approximate 3% risk of prostate cancer mortality provides one measure of the magnitude of overdiagnosis in this disease (an incidence to mortality ratio of 6:1), as up to 20% of men in screening programs may be diagnosed with prostate cancer. Other estimates of the degree of overdiagnosis of prostate cancer have varied depending on the underlying population being studied and screening methodology used. Several large randomized trials of prostate cancer screening (17–19) can shed additional light on the magnitude of overdiagnosis by comparing the two trial arms.
In The Prostate, Lung, Colorectal, and Ovarian Cancer (PLCO) trial, a study evaluating the survival benefit of screening, a 22% increased incidence of prostate cancer was seen in the screening group despite considerable contamination in the usual care group (52% of men in the usual care group received a PSA test at some point during the study) (17). There was a 58% increase in the observed incidence of prostate cancer in the screened arm compared with the incidence expected in a contemporary US population (based on Surveillance, Epidemiology, and End Results [SEER] data) and an approximately 200% increase compared with men in the pre-PSA era (20), thereby demonstrating the potential for significant overdiagnosis with annual screening for 6 years as performed in PLCO. Follow-up of PLCO through 13 years shows a persistent 12% excess in prostate cancer incidence in the intervention arm (21). Continuous annual screening (versus just 6 years of annual screening as in the PLCO) may result in additional overdiagnosis.
The European Randomized Study of Screening for Prostate Cancer (ERSPC) (18), which evaluated PSA testing every 4 years, found a statistically significant 20% relative risk reduction in prostate cancer mortality at 9 years with screening in a predefined “core group” of men aged 55–69. However, the absolute risk reduction in prostate cancer mortality was only 0.071%, thus resulting in both large numbers needed to screen (n = 1410) and numbers needed to treat (n = 48) to prevent 1 prostate cancer death. In ERSPC, the prostate cancer incidence rate ratio between the screened arm and the usual care arm was 1.71 (95% confidence interval [CI] = 1.32–2.33).
Prostate cancer mortality was reduced by 44% in the Göteborg trial (19) (of which a subset was included in the analysis of the ERSPC trial). Similar to PLCO and ERSPC, the incidence rate ratio between the 2 arms of this trial was 1.64 (95% CI = 1.5–1.8). The numbers needed to screen (n = 293) and the numbers needed to treat (n = 12) to prevent one prostate cancer death were still high (19).
The results from these studies prompted concerns of unacceptably high rates of overdiagnosis (22) and potential for overtreatment with PSA screening with a relatively small impact on mortality. Based on data from the Göteborg trial (19), screening 1000 men for 14 years would prevent only 5 prostate cancer deaths (decrease from nine men to four men) and result in the diagnosis of approximately 120 men (23).
Other measures of the degree of overdiagnosis in prostate cancer have been published (6,24–27). Using ERSPC data, Welch and Black estimated that 60% of such screen-detected cancers were overdiagnosed (6). Draisma et al. (24) assessed overdiagnosis in US men aged 54–80 years in 1985–2000 by using three different mathematical models applied to incidence data from the SEER program. Estimates of overdiagnosis ranged from 23% to 42% for the population studied. When these models were applied to estimates from the Rotterdam section of the ERSPC, 66% of screen-detected cancers were felt to be overdiagnosed (24). Previous simulation analysis has shown that the age of diagnosis heavily influences the degree of overdiagnosis, with estimates ranging from 27% of men aged 55 years to 56% of men aged 75 years (22). These results are particularly relevant, given the findings of Drazeret et al. (28), which demonstrate that substantial PSA testing occurs in men in the United States aged 65–79 years (approximately 45%), and those of Albertsen et al. (29), which showed that 31% of elderly men with limited life expectancy are still screened for prostate cancer in a contemporary population.
Racial differences also influence estimates of overdiagnosis. Etzioni et al. (25) modeled the effect of racial differences on prostate cancer overdiagnosis using SEER registry data from 1988 through 1998. Overdiagnosis was found to be more common in black men (44%) than in white men (29%). The authors also estimated that the use of PSA would identify up to 37% of autopsy cancers in black men and 15% in white men (25). Telesca et al. (26) also identified racial differences in overdiagnosis, finding that 34.4% and 22.7% of screen-detected cancers are overdiagnosed in black and white men, respectively.
Based on a more strict definition of clinically significant cancer, with significant cancers defined as those resulting in cancer-specific death, McGregor et al. (27) reported an overdiagnosis rate of 84% due to screening. This estimate is understandably higher than others, given the limited prostate cancer mortality seen in men with prostate cancer (3,4) and especially in those men with other comorbidities (29). The impact of comorbidity on a man’s chance of dying from prostate cancer has been well described (29). Despite having aggressive cancer (clinical T1c and Gleason grade 8–10), men with comorbidities were up to five times as likely to die of other causes. As expected, competing causes of mortality were even more influential in patients with intermediate-to-low-risk disease (clinical T1c and Gleason grade 5–7) (29).
Indolent Natural History of Prostate Cancer
The majority of screen-detected prostate cancers are asymptomatic and clinically localized, with 94% of newly diagnosed man having T1 or T2 disease based on SEER data from 2004 to 2005 (1). These localized tumors have been shown to have favorable, long-term oncologic outcomes. Lu-Yao et al. (4) evaluated outcomes of men diagnosed with localized disease, who were initially managed conservatively. The 10-year prostate cancer–specific mortality was 9.1% and 25.6% for moderately differentiated (Gleason grade 5–7) and poorly differentiated (Gleason grade 8–10) cancers, respectively (4). Similar estimates stratified by comorbidity were seen by Albertsen et al. (29).
The observation arms of two randomized studies (2,30) provide further insight into the natural history of this disease. The Scandinavian Prostate Cancer Group 4 Trial randomized 695 men with clinically detected cancer to watchful waiting or radical prostatectomy in 1998–1999. After a median follow-up of 12.8 years, 81 of the 201 deaths in the watchful waiting group (n = 348) were due to prostate cancer resulting in a 15-year prostate cancer– specific mortality of 20.7%, in a population of men who were diagnosed prior to the wide acceptance of PSA screening (2). Lower cancer-specific mortality was also observed in the observation arm (n = 367) of the Prostate Cancer Intervention Versus Observation Trial (PIVOT), which enrolled men with screen-detected cancers in the “early” PSA era, accounting for only 31 out of a total of 183 deaths, at a median 10-year follow-up (30).
Impact of Overdiagnosis
PSA screening itself and a resultant diagnosis of prostate cancer have been found to have detrimental effects on mental health, including anxiety and depression (31). The US Preventive Services Task Force concluded that false positives associated with screening result in adverse psychological effects; however, the magnitude of these has not been quantified (32). A measurable impact of the diagnosis of prostate cancer was seen in a population-based analysis, which revealed a significant increase in cardiovascular events (relative risk [RR] = 1.3, 95% CI = 1.3–1.3) and suicide (RR = 2.6, 95% CI = 2.1–3.0) within the first year following a diagnosis, with an even more pronounced effect noted in the first week after diagnosis (33).
While overdiagnosis per se is detrimental, an additional major concern is the resultant overtreatment of screen-detected tumors. In the United States, most men with such cancers receive aggressive treatment: 91% of men in PLCO (17) and 92.5% of men in The Cancer of the Prostate Strategic Urological Research Endeavor (CaPSURE) (34) were treated. Welch and Albertsen estimated that 80% of the 1.3 million extra cancer diagnosed in the first 20 years of PSA screening were aggressively treated (35). Such treatment is unlikely to yield a survival benefit in those with indolent disease (30) or in those men older than 65 years (2), but it can result in a considerable decrease in quality of life as a result of potentially persistent urinary, sexual, and bowel dysfunction (36). Active surveillance of such low-risk disease could potentially mitigate some of this overtreatment and has shown favorable outcomes in carefully selected patients (37,38). However, of all men diagnosed between 1999 and 2004 who were eligible for active surveillance in the CaPSURE database, only 9% were managed with active surveillance (39). Following the increased acceptance of active surveillance, the overtreatment of patients with low-risk disease appears to be improving with time (34).
Overtreatment largely occurs because physicians cannot definitively distinguish indolent from potentially aggressive disease (40). Nomograms, models, and various online tools can evaluate clinical variables and prognosticate risk, but their applicability at an individual level is unclear. Improved biopsy techniques using three-dimensional mapping of the prostate (41,42) have increased the diagnostic yield of biopsy and provide more accurate assessment of tumor volume and grade, both of which have considerable impact on patient management. Progress is also being made with molecular markers on biopsy specimens (43). Continued progress with such strategies should assist with better risk prognostication and improve selection of more appropriate risk-based therapies for patients, such as active surveillance.
Efforts have also been made to better identify aggressive disease preoperatively by using PSA isoforms. Although some groups have demonstrated the improved diagnostic yield of PSA isoforms, such as percent free PSA (ie, PSA that is unbound to plasma proteins), intact PSA (a form of free PSA that has not been internally cleaved), and human kallikrein-related peptidase (hk2) (a serine protease that shares 80% sequence homology with PSA) in predicting biopsy outcome (44–46), their utility in predicting aggressive disease and biochemical recurrence is less clear. Some series have found that a lower percent free PSA correlates with aggressive pathology at radical prostatectomy (extracapsular extension and seminal vesicle invasion) (47,48) and biochemical recurrence (48,49) following radical prostatectomy, whereas others have refuted both of these observations (50,51). Additionally, although the use of a four kallikrein panel including free, intact, and total PSA as well as hk2 improves biopsy outcome prediction and thus can potentially reduce the number of biopsies performed (52), such a panel does not sufficiently distinguish indolent from aggressive disease and thus at this time is unlikely to influence treatment decisions at the individual patient level.
Prospects to Reduce Overdiagnosis
One strategy to decrease overdiagnosis is to selectively apply screening and/or biopsy only to those individuals at highest risk of prostate cancer mortality. These men can potentially be identified on the basis of certain specific risk factors (53). Examples of such risk factors include a family history of prostate cancer (54,55), which can predict an increased risk of prostate cancer (hazard ratio [HR] = 23 for men before age 65 years with 3 affected brothers), and African American race (7,53,56,57), which can predict increased prostate cancer mortality (mortality rate increased 2.4 times in African Americans compared with Caucasians).
Numerous groups have evaluated the utility of serum PSA as predictor of future aggressive disease. Lilja et al. (58) evaluated the predictive ability of a baseline PSA in 21 277 men aged 33–50 years from the Malmö Preventive Project. At a median follow-up of 23 years, a strong association between baseline PSA in these relatively young men, who likely did not have much benign prostatic hyperplasia to confound their PSA levels, and subsequent advanced cancer was observed (area under the curve 0.75). The authors concluded that low-risk men (those with a baseline PSA below the median of 0.65) could be screened less often, which may help to decrease the diagnosis of indolent cancers. Vickers et al. (52) also reported on the predictive ability of a PSA value at age 60 using 1167 men from the Malmö Preventive Project. Men with a PSA of less than or equal to 1ng/ml were unlikely to develop clinically significant prostate cancer over 25 years of follow-up (0.5% risk of metastasis, 0.2% risk of death from prostate cancer) (52). Data from the Rotterdam arm of the ERSPC revealed a 0.15% incidence of prostate cancer mortality at 11.5 years in men aged 55–74 years with an initial PSA of less than or equal to 3ng/ml (59). In this group of men, further significantly reduced cancer-specific mortality was seen in those with an initial PSA of less than or equal to 1ng/ml (59). Recently Williams et al. developed an Early Detection Research Network (EDRN)–derived prediction model and validated it in the Prostate Cancer Prevention Trial placebo group, which uses clinical factors to selectively detect only aggressive cancers (60).
An alternative approach to decrease overdiagnosis would be to improve the diagnostic performance of PSA as a screening test. PSA is not prostate cancer–specific (61,62); because it generally increases with age, many men undergo prostate biopsy and are discovered to have incidental cancer merely because of progression of their benign prostatic hyperplasia. Use of 5-alpha reductase inhibitors can increase the specificity of PSA for aggressive prostate cancer by limiting the confounding contributions of PSA produced by benign prostatic hyperplasia (63–65). Moreover, 5-alpha reductase inhibitors reduce a man’s chance of being diagnosed with low-grade cancer by 25%–30% (66,67), as well as potentially reducing biopsy progression of men on active surveillance (68). However, the exact role of these medications remains undefined, given concerns that these medications may predispose men to developing high-grade prostate cancer (69).
Restricting screening to men without comorbidity may also be of benefit in potentially improving survival outcomes (70) and decreasing overdiagnosis. Men with comorbidities have been shown to have, in general, a reduced risk of prostate cancer–specific mortality and an increased overall mortality (29,71). Daskivichet al. (71) demonstrated a twofold increase in other-cause mortality with each point increase in Charlson comorbidity score in men with nonmetastatic prostate cancer.
Conclusions
The confluence of three previously described factors (6)—a large latent pool, activities leading to diagnosis (screening), and a long natural history—results in substantial overdiagnosis of prostate cancer. Although some degree of overdiagnosis must be accepted in any disease detected with screening, the overdiagnosis rate currently seems excessively high. There are some plausible strategies to help reduce the overdiagnosis of prostate cancer, for example, selectively targeting “high-risk” groups and young men with limited or no comorbidity. Ongoing efforts are required to improve prognostication and allow for appropriate risk-based treatment so that costly overtreatment, perhaps the most significant sequela of overdiagnosis, can be avoided.
Funding
There was no funding for this work.
References
- 1. Shao YH, Demissie K, Shih W, et al. Contemporary risk profile of prostate cancer in the United States. J Natl Cancer Inst. 2009; 101(18):1280–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bill-Axelson A, Holmberg L, Ruutu M, et al. ; SPCG-4 Investigators Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2011; 364(18):1708–1717 [DOI] [PubMed] [Google Scholar]
- 3. Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA. 2005; 293(17):2095–2101 [DOI] [PubMed] [Google Scholar]
- 4. Lu-Yao GL, Albertsen PC, Moore DF, et al. Outcomes of localized prostate cancer following conservative management. JAMA. 2009; 302(11):1202–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Black WC. Overdiagnosis: an underrecognized cause of confusion and harm in cancer screening. J Natl Cancer Inst. 2000; 92(16):1280–1282 [DOI] [PubMed] [Google Scholar]
- 6. Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010; 102(9):605–613 [DOI] [PubMed] [Google Scholar]
- 7. Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011; 61(4):212–236 [DOI] [PubMed] [Google Scholar]
- 8. Sakr WA, Grignon DJ, Haas GP, Heilbrun LK, Pontes JE, Crissman JD. Age and racial distribution of prostatic intraepithelial neoplasia. Eur Urol. 1996; 30(2):138–144 [DOI] [PubMed] [Google Scholar]
- 9. Powell IJ, Bock CH, Ruterbusch JJ, Sakr W. Evidence supports a faster growth rate and/or earlier transformation to clinically significant prostate cancer in black than in white American men, and influences racial progression and mortality disparity. J Urol. 2010; 183(5):1792–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stamatiou K, Alevizos A, Agapitos E, Sofras F. Incidence of impalpable carcinoma of the prostate and of non-malignant and precarcinomatous lesions in Greek male population: an autopsy study. Prostate. 2006; 66(12):1319–1328 [DOI] [PubMed] [Google Scholar]
- 11. Abdelhady M, Abusamra A, Pautler SE, Chin JL, Izawa JI. Clinically significant prostate cancer found incidentally in radical cystoprostatectomy specimens. BJU Int. 2007; 99(2):326–329 [DOI] [PubMed] [Google Scholar]
- 12. Winkler MH, Livni N, Mannion EM, Hrouda D, Christmas T. Characteristics of incidental prostatic adenocarcinoma in contemporary radical cystoprostatectomy specimens. BJU Int. 2007; 99(3):554–558 [DOI] [PubMed] [Google Scholar]
- 13. Pettus JA, Al-Ahmadie H, Barocas DA, et al. Risk assessment of prostatic pathology in patients undergoing radical cystoprostatectomy. Eur Urol. 2008; 53(2):370–375 [DOI] [PubMed] [Google Scholar]
- 14. Gakis G, Schilling D, Bedke J, Sievert KD, Stenzl A. Incidental prostate cancer at radical cystoprostatectomy: implications for apex-sparing surgery. BJU Int. 2010; 105(4):468–471 [DOI] [PubMed] [Google Scholar]
- 15. Stamey TA, Yang N, Hay AR, McNeal JE, Freiha FS, Redwine E. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med. 1987; 317(15):909–916 [DOI] [PubMed] [Google Scholar]
- 16. Catalona WJ, Smith DS, Ratliff TL, et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. 1991; 324(17):1156–1161 [DOI] [PubMed] [Google Scholar]
- 17. Andriole GL, Crawford ED, Grubb RL, III, et al. ; PLCO Project Team Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009; 360(13):1310–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schröder FH, Hugosson J, Roobol MJ, et al. ; ERSPC Investigators Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009; 360(13):1320–1328 [DOI] [PubMed] [Google Scholar]
- 19. Hugosson J, Carlsson S, Aus G, et al. Mortality results from the Göteborg randomised population-based prostate-cancer screening trial. Lancet Oncol. 2010; 11(8):725–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pinsky PF, Blacka A, Kramer BS, Miller A, Prorok PC, Berg C. Assessing contamination and compliance in the prostate component of the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial. Clin Trials. 2010; 7(4):303–311 [DOI] [PubMed] [Google Scholar]
- 21. Andriole GL, Crawford ED, Grubb RL, III, et al. ; PLCO Project Team Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012; 104(2):125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Draisma G, Boer R, Otto SJ, et al. Lead times and overdetection due to prostate-specific antigen screening: estimates from the European Randomized Study of Screening for Prostate Cancer. J Natl Cancer Inst. 2003; 95(12):868–878 [DOI] [PubMed] [Google Scholar]
- 23. Carroll PR, Whitson JM, Cooperberg MR. Serum prostate-specific antigen for the early detection of prostate cancer: always, never, or only sometimes? J Clin Oncol. 2011; 29(4):345–347 [DOI] [PubMed] [Google Scholar]
- 24. Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009; 101(6):374–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Etzioni R, Penson DF, Legler JM, et al. Overdiagnosis due to prostate-specific antigen screening: lessons from U.S. prostate cancer incidence trends. J Natl Cancer Inst. 2002; 94(13):981–990 [DOI] [PubMed] [Google Scholar]
- 26. Telesca D, Etzioni R, Gulati R. Estimating lead time and overdiagnosis associated with PSA screening from prostate cancer incidence trends. Biometrics. 2008; 64(1):10–19 [DOI] [PubMed] [Google Scholar]
- 27. McGregor M, Hanley JA, Boivin JF, McLean RG. Screening for prostate cancer: estimating the magnitude of overdetection. CMAJ. 1998; 159(11):1368–1372 [PMC free article] [PubMed] [Google Scholar]
- 28. Drazer MW, Huo D, Schonberg MA, Razmaria A, Eggener SE. Population-based patterns and predictors of prostate-specific antigen screening among older men in the United States. J Clin Oncol. 2011; 29(13):1736–1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Albertsen PC, Moore DF, Shih W, Lin Y, Li H, Lu-Yao GL. Impact of comorbidity on survival among men with localized prostate cancer. J Clin Oncol. 2011; 29(10):1335–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wilt TJ. The VA/NCI/AHRQ CSP#407: Prostate Cancer Intervention Versus Observation Trial (PIVOT): main results from a randomized trial comparing radical prostatectomy to watchful waiting in men with clinically localized prostate cancer. American Urological Association 2011 Annual Meeting; May 2011; Washington, DC [Google Scholar]
- 31. Klotz L. Active surveillance for prostate cancer: patient selection and management. Curr Oncol. 2010; 17(suppl 2):S11–S17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chou R, Croswell JM, Dana T, et al. Screening for prostate cancer: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2011; 155(11):762–771 [DOI] [PubMed] [Google Scholar]
- 33. Fall K, Fang F, Mucci LA, et al. Immediate risk for cardiovascular events and suicide following a prostate cancer diagnosis: prospective cohort study. PLoS Med. 2009; 6(12):e1000197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010; 28(7):1117–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Welch HG, Albertsen PC. Prostate cancer diagnosis and treatment after the introduction of prostate-specific antigen screening: 1986-2005 J Natl Cancer Inst. 2009; 101(19):1325–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008; 358(12):1250–1261 [DOI] [PubMed] [Google Scholar]
- 37. Klotz L, Zhang L, Lam A, Nam R, Mamedov A, Loblaw A. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010; 28(1):126–131 [DOI] [PubMed] [Google Scholar]
- 38. Tosoian JJ, Trock BJ, Landis P, et al. Active surveillance program for prostate cancer: an update of the Johns Hopkins experience. J Clin Oncol. 2011; 29(16):2185–2190 [DOI] [PubMed] [Google Scholar]
- 39. Barocas DA, Cowan JE, Smith JA, Jr, Carroll PR. What percentage of patients with newly diagnosed carcinoma of the prostate are candidates for surveillance? An analysis of the CaPSURE database J Urol. 2008; 180(4):1330–1334; discussion 1334–1335 [DOI] [PubMed] [Google Scholar]
- 40. Gelmann EP. Complexities of prostate-cancer risk. N Engl J Med. 2008; 358(9):961–963 [DOI] [PubMed] [Google Scholar]
- 41. Onik G, Miessau M, Bostwick DG. Three-dimensional prostate mapping biopsy has a potentially significant impact on prostate cancer management. J Clin Oncol. 2009; 27(26):4321–4326 [DOI] [PubMed] [Google Scholar]
- 42. Megwalu II, Ferguson GG, Wei JT, et al. Evaluation of a novel precision template-guided biopsy system for detecting prostate cancer. BJU Int. 2008; 102(5):546–550 [DOI] [PubMed] [Google Scholar]
- 43. Cuzick J, Swanson GP, Fisher G, et al. ; Transatlantic Prostate Group Prognostic value of an RNA expression signature derived from cell cycle proliferation genes in patients with prostate cancer: a retrospective study. Lancet Oncol. 2011; 12(3):245–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lee R, Localio AR, Armstrong K, Malkowicz SB, Schwartz JS. Free PSA Study Group A meta-analysis of the performance characteristics of the free prostate-specific antigen test. Urology. 2006; 67(4):762–768 [DOI] [PubMed] [Google Scholar]
- 45. Vickers A, Cronin A, Roobol M, et al. Reducing unnecessary biopsy during prostate cancer screening using a four-kallikrein panel: an independent replication. J Clin Oncol. 2010; 28(15):2493–2498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vickers AJ, Cronin AM, Aus G, et al. A panel of kallikrein markers can reduce unnecessary biopsy for prostate cancer: data from the European Randomized Study of Prostate Cancer Screening in Göteborg, Sweden. BMC Med. 2008; 6 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shariat SF, Abdel-Aziz KF, Roehrborn CG, Lotan Y. Pre-operative percent free PSA predicts clinical outcomes in patients treated with radical prostatectomy with total PSA levels below 10ng/ml. Eur Urol. 2006; 49(2):293–302 [DOI] [PubMed] [Google Scholar]
- 48. Steuber T, Vickers A, Haese A, et al. Free PSA isoforms and intact and cleaved forms of urokinase plasminogen activator receptor in serum improve selection of patients for prostate cancer biopsy. Int J Cancer. 2007; 120(7):1499–1504 [DOI] [PubMed] [Google Scholar]
- 49. Wenske S, Korets R, Cronin AM, et al. Evaluation of molecular forms of prostate-specific antigen and human kallikrein 2 in predicting biochemical failure after radical prostatectomy. Int J Cancer. 2009; 124(3):659–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Erdem E, Atsü N, Akbal C, Bilen CY, Ergen A, Ozen H. The free-to-total serum prostatic specific antigen ratio as a predictor of the pathological features of prostate cancer. Int Urol Nephrol. 2002; 34(4):519–523 [DOI] [PubMed] [Google Scholar]
- 51. Graefen M, Karakiewicz PI, Cagiannos I, et al. Percent free prostate specific antigen is not an independent predictor of organ confinement or prostate specific antigen recurrence in unscreened patients with localized prostate cancer treated with radical prostatectomy. J Urol. 2002; 167(3):1306–1309 [PubMed] [Google Scholar]
- 52. Vickers AJ, Cronin AM, Björk T, et al. Prostate specific antigen concentration at age 60 and death or metastasis from prostate cancer: case-control study. BMJ. 2010; 341 c4521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhu X, Albertsen PC, Andriole GL, Roobol MJ, Schroder FH, Vickers AJ. Risk-based prostate cancer screening [published online ahead of print November 24, 2011] Eur Urol. 2011; 61(4):652–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Brandt A, Bermejo JL, Sundquist J, Hemminki K. Age-specific risk of incident prostate cancer and risk of death from prostate cancer defined by the number of affected family members. Eur Urol. 2010; 58(2):275–280 [DOI] [PubMed] [Google Scholar]
- 55. Gann PH. Risk factors for prostate cancer. Rev Urol. 2002; 4(suppl 5):S3–S10 [PMC free article] [PubMed] [Google Scholar]
- 56. Bigler SA, Pound CR, Zhou X. A retrospective study on pathologic features and racial disparities in prostate cancer. Prostate Cancer. 2011; 2011: 239460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hsieh K, Albertsen PC. Populations at high risk for prostate cancer. Urol Clin North Am. 2003; 30(4):669–676 [DOI] [PubMed] [Google Scholar]
- 58. Lilja H, Cronin AM, Dahlin A, et al. Prediction of significant prostate cancer diagnosed 20 to 30 years later with a single measure of prostate-specific antigen at or before age 50. Cancer. 2011; 117(6):1210–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bul M, van Leeuwen PJ, Zhu X, Schröder FH, Roobol MJ. Prostate cancer incidence and disease-specific survival of men with initial prostate-specific antigen less than 3.0ng/ml who are participating in ERSPC Rotterdam. Eur Urol. 2011; 59(4):498–505 [DOI] [PubMed] [Google Scholar]
- 60. Williams SB, Salami S, Regan MM, et al. Selective detection of histologically aggressive prostate cancer: an Early Detection Research Network Prediction model to reduce unnecessary prostate biopsies with validation in the Prostate Cancer Prevention Trial [published online ahead of print October 17, 2011]. Cancer. 2012; 118(10):2651–2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Oesterling JE, Chan DW, Epstein JI, et al. Prostate specific antigen in the preoperative and postoperative evaluation of localized prostatic cancer treated with radical prostatectomy. J Urol. 1988; 139(4):766–772 [DOI] [PubMed] [Google Scholar]
- 62. Partin AW, Carter HB, Chan DW, et al. Prostate specific antigen in the staging of localized prostate cancer: influence of tumor differentiation, tumor volume and benign hyperplasia. J Urol. 1990; 143(4):747–752 [DOI] [PubMed] [Google Scholar]
- 63. Thompson IM, Ankerst DP, Chi C, et al. Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst. 2006; 98(8):529–534 [DOI] [PubMed] [Google Scholar]
- 64. Andriole GL, Bostwick D, Brawley OW, et al. ; REDUCE Study Group The effect of dutasteride on the usefulness of prostate specific antigen for the diagnosis of high grade and clinically relevant prostate cancer in men with a previous negative biopsy: results from the REDUCE study. J Urol. 2011; 185(1):126–131 [DOI] [PubMed] [Google Scholar]
- 65. Marberger M, Freedland SJ, Andriole GL, et al. Usefulness of prostate-specific antigen (PSA) rise as a marker of prostate cancer in men treated with dutasteride: lessons from the REDUCE study. BJU Int. 2012; 109(8):1162–1169 [DOI] [PubMed] [Google Scholar]
- 66. Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003; 349(3):215–224 [DOI] [PubMed] [Google Scholar]
- 67. Andriole GL, Bostwick DG, Brawley OW, et al. ; REDUCE Study Group Effect of dutasteride on the risk of prostate cancer. N Engl J Med. 2010; 362(13):1192–1202 [DOI] [PubMed] [Google Scholar]
- 68. Fleshner N, Lucia MS, Melich K, Nandy IM, Black L, Rittmaster RS. Effect of dutasteride on prostate cancer progression and cancer diagnosis on rebiopsy in the REDEEM active surveillance study. American Society of Clinical Oncology, Genitourinary Cancers Symposium; February 2011; Orlando, FL [Google Scholar]
- 69. Theoret MR, Ning YM, Zhang JJ, Justice R, Keegan P, Pazdur R. The risks and benefits of 5a-reductase inhibitors for prostate-cancer prevention. N Engl J Med. 2011; 365(2):97–99 [DOI] [PubMed] [Google Scholar]
- 70. Crawford ED, Grubb R, III, Black A, et al. Comorbidity and mortality results from a randomized prostate cancer screening trial. J Clin Oncol. 2011; 29(4):355–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Daskivich TJ, Chamie K, Kwan L, et al. Comorbidity and competing risks for mortality in men with prostate cancer [published online ahead of print April 8, 2011]. Cancer. 2011;117(20):4642–4650 [DOI] [PubMed] [Google Scholar]