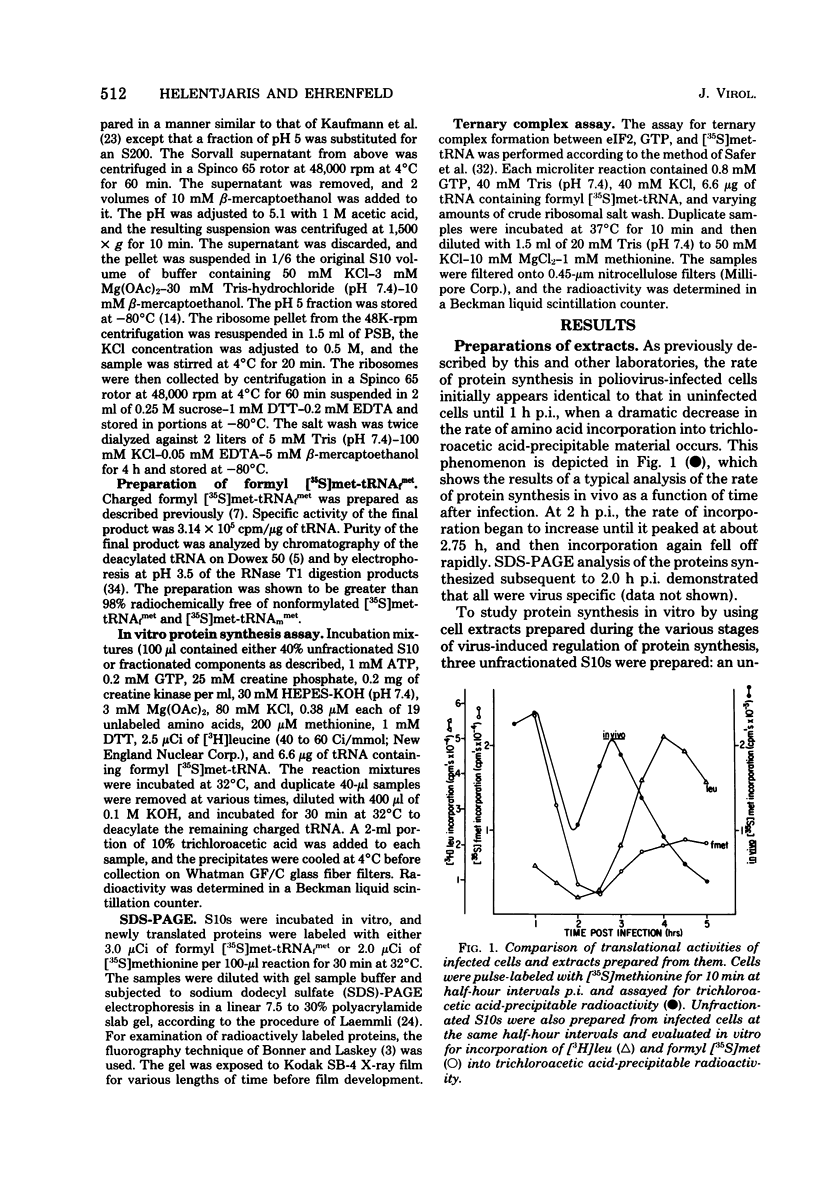
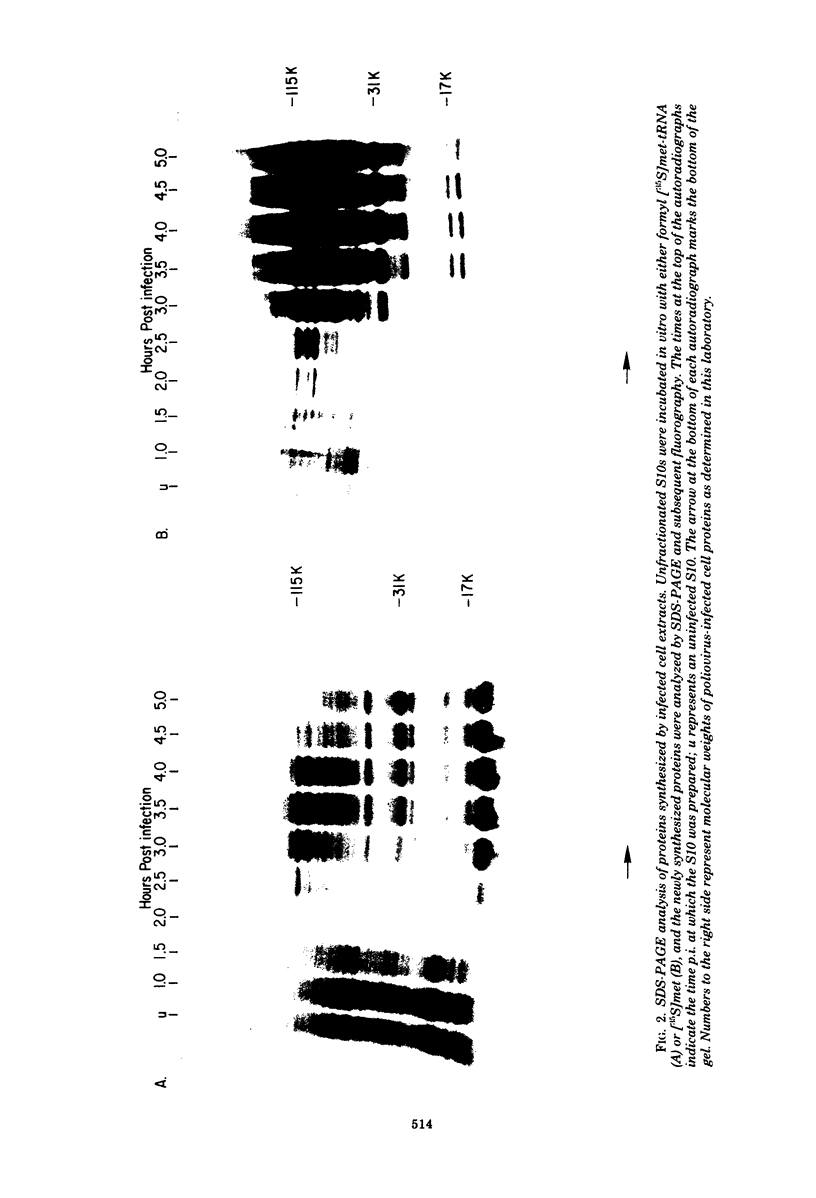
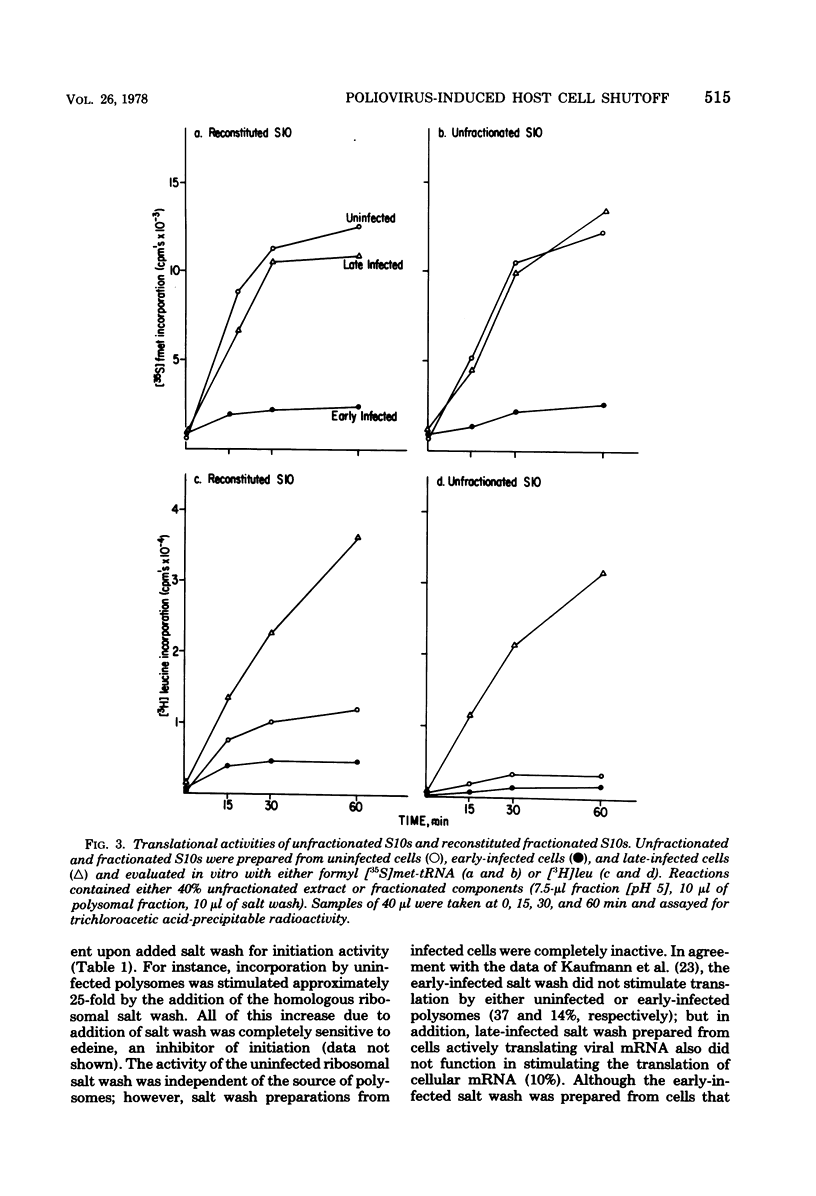
Abstract
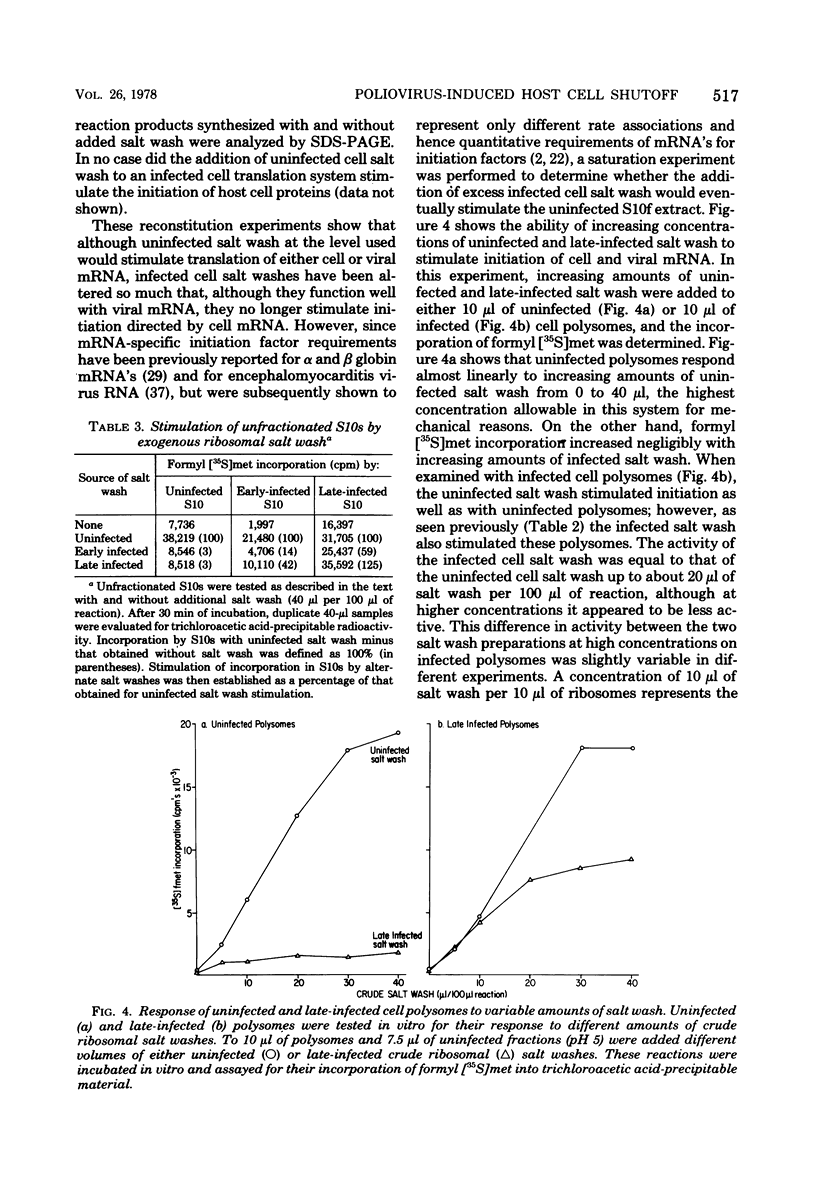
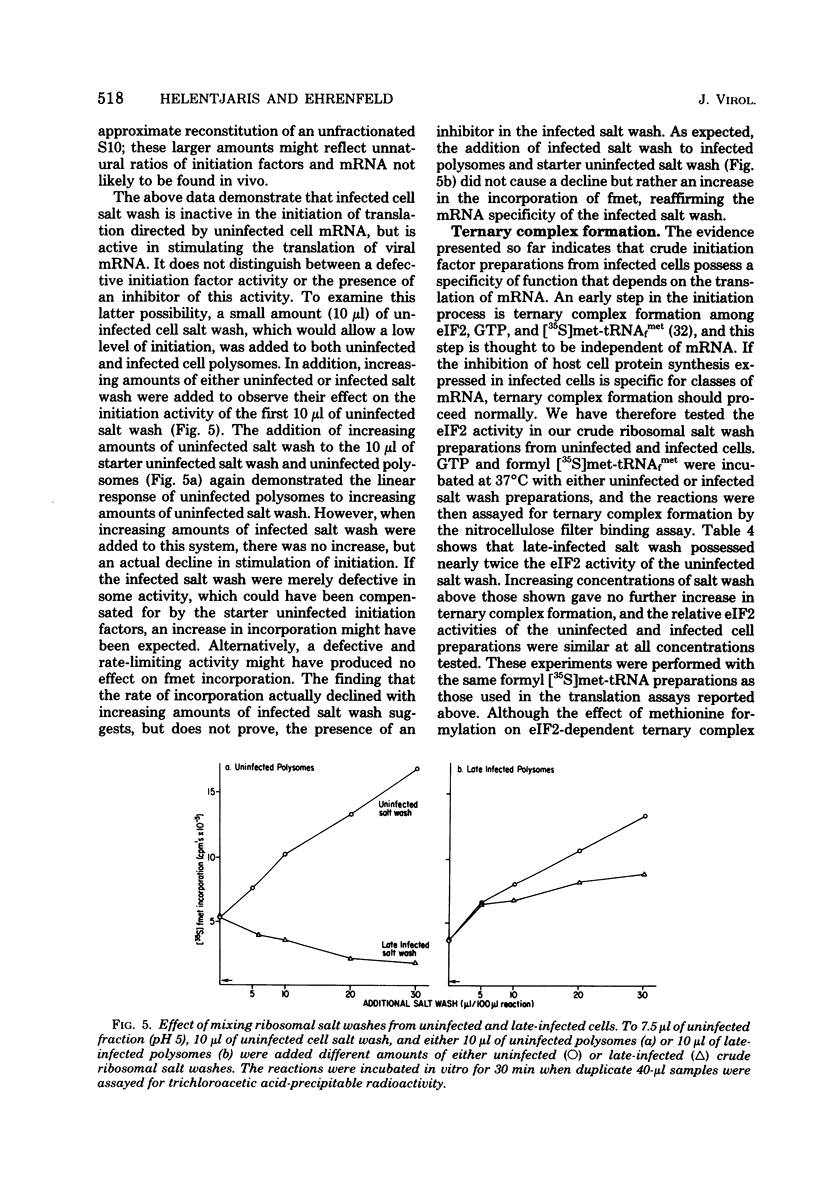
By using cell-free systems prepared from uninfected and poliovirus-infected cells, we have been able to demonstrate that crude preparations of initiation factors from infected cells do not stimulate the initiation of translation by polyribosomes containing endogenous host cell mRNA. When tested with polysomes containing endogenous viral mRNA, however, they were able to stimulate initiation of translation nearly as well as uninfected cell initiation factors. The uninfected cell initiation factor preparations were able to stimulate initiation of translation of both cell and viral mRNA. The results indicate an mRNA-specific activity present in crude initiation factor preparations from infected cells. Furthermore, the ability of eIF2 from infected cells to form a ternary complex with GTP and formyl [35S]methionine-tRNAfmet, an mRNA-independent step in initiation, was found not to be deficient. Implications of these data for proposed mechanisms of poliovirus-induced host cell shutoff are discussed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abreu S. L., Lucas-Lenard J. Cellular protein synthesis shutoff by mengovirus: translation of nonviral and viral mRNA's in extracts from uninfected and infected Ehrlich ascites tumor cells. J Virol. 1976 Apr;18(1):182–194. doi: 10.1128/jvi.18.1.182-194.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair G. E., Dahl H. H., Truelsen E., Lelong J. C. Functional identity of a mouse ascites and a rabbit reticulocyte initiation factor required for natural mRNA translation. Nature. 1977 Feb 17;265(5595):651–653. doi: 10.1038/265651a0. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Both G. W., Furuichi Y., Muthukrishnan S., Shatkin A. J. Ribosome binding to reovirus mRNA in protein synthesis requires 5' terminal 7-methylguanosine. Cell. 1975 Oct;6(2):185–195. doi: 10.1016/0092-8674(75)90009-4. [DOI] [PubMed] [Google Scholar]
- Capecchi M. R. Initiation of E. coli proteins. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1517–1524. doi: 10.1073/pnas.55.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celma M. L., Ehrenfeld E. Effect of poliovirus double-stranded RNA on viral and host-cell protein synthesis. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2440–2444. doi: 10.1073/pnas.71.6.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celma M. L., Ehrenfeld E. Translation of poliovirus RNA in vitro: detection of two different initiation sites. J Mol Biol. 1975 Nov 15;98(4):761–780. doi: 10.1016/s0022-2836(75)80009-x. [DOI] [PubMed] [Google Scholar]
- Colby D. S., Finnerty V., Lucas-Lenard J. Fate of mRNA of L-cells infected with mengovirus. J Virol. 1974 Apr;13(4):858–869. doi: 10.1128/jvi.13.4.858-869.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole C. N., Baltimore D. Defective interfering particles of poliovirus. II. Nature of the defect. J Mol Biol. 1973 May 25;76(3):325–343. doi: 10.1016/0022-2836(73)90508-1. [DOI] [PubMed] [Google Scholar]
- Ehrenfeld E., Lund H. Untranslated vesicular stomatitis virus messenger RNA after poliovirus infection. Virology. 1977 Jul 15;80(2):297–308. doi: 10.1016/s0042-6822(77)80006-8. [DOI] [PubMed] [Google Scholar]
- Falvey A. K., Staehelin T. Structure and function of mammalian ribosomes. I. Isolation and characterization of active liver ribosomal subunits. J Mol Biol. 1970 Oct 14;53(1):1–19. doi: 10.1016/0022-2836(70)90042-2. [DOI] [PubMed] [Google Scholar]
- Golini F., Thach S. S., Birge C. H., Safer B., Merrick W. C., Thach R. E. Competition between cellular and viral mRNAs in vitro is regulated by a messenger discriminatory initiation factor. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3040–3044. doi: 10.1073/pnas.73.9.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helentjaris T., Ehrenfeld E. Inhibition of host cell protein synthesis by UV-inactivated poliovirus. J Virol. 1977 Jan;21(1):259–267. doi: 10.1128/jvi.21.1.259-267.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett M. J., Rose J. K., Baltimore D. 5'-terminal structure of poliovirus polyribosomal RNA is pUp. Proc Natl Acad Sci U S A. 1976 Feb;73(2):327–330. doi: 10.1073/pnas.73.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat D., Chappell M. R. Competition between globin messenger ribonucleic acids for a discriminating initiation factor. J Biol Chem. 1977 Apr 25;252(8):2684–2690. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nomoto A., Detjen B., Pozzatti R., Wimmer E. The location of the polio genome protein in viral RNAs and its implication for RNA synthesis. Nature. 1977 Jul 21;268(5617):208–213. doi: 10.1038/268208a0. [DOI] [PubMed] [Google Scholar]
- Nuss D. L., Oppermann H., Koch G. Selective blockage of initiation of host protein synthesis in RNA-virus-infected cells. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1258–1262. doi: 10.1073/pnas.72.4.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafritz D. A., Weinstein J. A., Safer B., Merrick W. C., Weber L. A., Hickey E. D., Baglioni C. Evidence for role of m7G5'-phosphate group in recognition of eukaryotic mRNA by initiation factor IF-M3. Nature. 1976 May 27;261(5558):291–294. doi: 10.1038/261291a0. [DOI] [PubMed] [Google Scholar]
- Smith A. E., Marcker K. A. Cytoplasmic methionine transfer RNAs from eukaryotes. Nature. 1970 May 16;226(5246):607–610. doi: 10.1038/226607a0. [DOI] [PubMed] [Google Scholar]
- Willems M., Penman S. The mechanism of host cell protein synthesis inhibition by poliovirus. Virology. 1966 Nov;30(3):355–367. doi: 10.1016/0042-6822(66)90114-0. [DOI] [PubMed] [Google Scholar]
- Wright P. J., Cooper P. D. Poliovirus proteins associated with ribosomal structures in infected cells. Virology. 1974 May;59(1):1–20. doi: 10.1016/0042-6822(74)90201-3. [DOI] [PubMed] [Google Scholar]