Abstract
Medullary thyroid cancer is an uncommon malignancy for which until recently little effective treatment existed. It is often characterized by mutation and overexpression of the receptor tyrosine kinases RET (rearranged during transfection), VEGFR2 (vascular endothelial growth factor receptor 2) and MET (mesenchymal-epithelial transition factor), which make attractive targets for drug development. Cabozantinib is an orally bioavailable tyrosine kinase inhibitor which blocks MET, VEGRF2 and RET, and has shown considerable activity in medullary thyroid cancer in a Phase III trial, including in heavily pretreated patients. Its novel combination of vascular endothelial growth factor and MET inhibition is believed to address the MET escape pathway, which is thought to be the cause of nonsustained tumor responses resulting from inhibition of vascular endothelial growth factor alone.
Keywords: EXAM trial, VEGF escape pathways, MET, RET
Introduction
Medullary thyroid cancer is an uncommon thyroid malignancy, comprising up to 5% of all thyroid cancers.1 Unlike follicular and papillary thyroid cancers, which are derived from thyroid epithelium, medullary thyroid cancer is derived from calcitonin-secreting parafollicular cells and is of neuroendocrine origin. Therefore, thyroid hormone levels are typically normal in medullary thyroid cancer, while calcitonin is often elevated and can be used as a biomarker, both in diagnosis and in assessment of treatment response. Early disease confined to the thyroid can be cured with surgery. However, more than 50% of cases are locally advanced or have metastasized by the time of diagnosis. There is considerable heterogeneity in the rate of progression, and 10-year survival rates are estimated at 40%.1
Cytotoxic treatment for advanced medullary thyroid cancer has poor response rates and a short duration of effect, and there is no accepted standard regimen.2 The introduction of small molecule tyrosine kinase inhibitors, targeted at known oncogenic pathways, has improved tumor response significantly. Cabozantinib is a potent, orally bioavailable tyrosine kinase inhibitor that targets multiple pathways via the vascular endothelial growth factor receptor 2 (VEGFR2), the rearranged during transfection (RET) receptor, and the mesenchymal-epithelial transition factor (MET) receptor. Cabozantinib has shown considerable antitumor effect in this uncommon disease. This review article will first examine the intracellular signaling pathways that can drive medullary thyroid cancer, and then describe cabozantinib and summarize the results of recent trials using this agent in patients with medullary thyroid cancer.
Key pathways in medullary thyroid cancer
Mutations leading to medullary thyroid cancer can occur sporadically (somatic) or are inherited as an autosomal dominant (germline) mutation. Sporadic medullary thyroid cancer accounts for up to 80% of all disease cases.3 The mutations frequently involve RET, but can also involve MET and VEGFR. The mutations result in constitutive signaling which promotes tumor development and growth.
RET
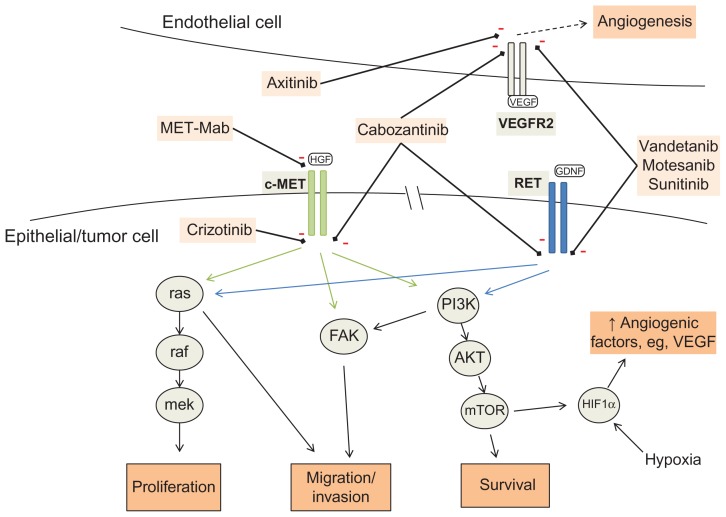
RET is a proto-oncogene located on chromosome 10 that codes for a transmembrane receptor tyrosine kinase usually expressed on epithelial-derived cells. Binding by its ligand, glial cell-line-derived neurotrophic factor, activates multiple signaling pathways that promote cell cycling, motility, and survival (Figure 1).4,5 Mutations in RET are seen in more than 50% of sporadic cases of medullary thyroid cancer and in nearly all inherited cases.6 Somatic RET mutations in medullary thyroid cancer are associated with increased rates of advanced disease at diagnosis, and in one study were correlated with a worse prognosis.3 M918T was the most common somatic mutation, occurring in 80%.
Figure 1.
Molecular signal transduction pathways for c-MET and RET, with targets of therapeutic agents. VEGFR2 on adjacent endothelial cell showing mechanism of angiogenic signaling.
Note: Dashed arrow represents molecular signal tranduction pathways.
Abbreviations: PI3K, phosphoinositol-3-kinases; FAK, focal adhesion kinase; HIF1α, hypoxia-induced factor 1 alpha; mTOR, mammalian target of rapamycin; GDNF, glial-cell-line-derived neurotrophic factor; HGF, hepatocyte growth factor; VEGFR2, vascular endothelial growth factor receptor 2.
There are a number of inherited RET mutations, most of which result in medullary thyroid cancer, and usually develop during childhood. These form the syndrome MEN2 (multiple endocrine neoplasia 2), which includes MEN2A, MEN2B, and familial medullary thyroid cancer.
RET is a potential target for therapeutic small molecule inhibitors. A number of tyrosine kinase inhibitors have been developed which target the RET receptor, in addition to targeting other receptors, such as VEGFR. These include cabozantinib, vandetanib, sunitinib, and axitinib. However, for each agent, the degree of RET inhibition relative to inhibition of other receptors varies greatly, and similarly the potency against RET differs between agents (Table 1). Furthermore, potency may vary according to the particular type of RET mutation.7 An in vitro study comparing these four agents found cabozantinib to be the most potent inhibitor in MEN2A medullary thyroid cancer and vandetanib to be the most potent in MEN2B medullary thyroid cancer, suggesting that mutation-specific therapy may be of benefit in medullary thyroid cancer.8
Table 1.
Inhibitory activity of cabozantinib in comparison with other tyrosine kinase inhibitors
| Target | Cabozantinib IC50 (nM)21 | Vandetanib IC50 (nM)32 | Motesanib IC50 (nM)33 | Sunitinib IC50 (nM)34 | Axitinib IC50 (nM)35 |
|---|---|---|---|---|---|
| VEGFR-2 | 0.035 | 40 | 3 | 9 | 0.2 |
| c-Met | 1.3–14.6 | – | – | 4000 | – |
| RET | 5.2 | 100 | 59–2500 | 50 | – |
| c-Kit | 4.6 | >20000 | 8 | 1 | 1.7 |
| Flt3 | 11.3 | – | – | 30–250 | <1000 |
| AXL | 7 | – | – | – | – |
| Tie2 | 14.3 | 2500 | – | – | – |
Notes: Where a range is cited, this indicates that IC50 varies according to receptor mutation status. Where a hyphen is shown, no IC50 was reported for that receptor tyrosine kinase.
Abbreviations: VEGFR-2, Vascular endothelial growth factor receptor 2; IC50, concentration at which 50% of maximal inhibition occurs; nM, nanomolar.
VEGFR
It is established that angiogenesis is critical for tumor growth and invasion, and that VEGF/VEGFR is a key signaling pathway in many cancers.9 Tumor growth depends on angiogenesis to ensure a supply of nutrients, and this is most typically driven by VEGF, via VEGF receptors 1, 2, or 3. In malignancy, these pathways are often activated constantly, either by mutation of the receptor leading to constitutive activation, or by tumor-driven upregulation of VEGF expression. Increased expression of VEGF-A, VEGFR-1, and VEGFR-2 has been demonstrated in medullary thyroid cancer, and is seen in 50% of primary tumors and 75% of distant metastases.10,11
However, antiangiogenic therapy alone fails to induce durable remissions in many malignancies, and there is evidence that it can ultimately lead to increased tumor invasion and metastasis.12 This is thought to occur in response to VEGF inhibition via increased activity of alternative pathways, such as MET (see below), in response to tissue hypoxia. In a preclinical mouse model of pancreatic carcinoma, anti-VEGF therapy alone reduced initial tumor size, but led to increased local invasiveness and liver metastases. In contrast, dual inhibition of VEGFR and MET with cabozantinib was associated with reduction in both tumor size and invasiveness, and inhibited the development of metastases.13
MET
MET is a proto-oncogene that codes for the hepatocyte growth factor receptor, c-MET, a cell surface receptor tyrosine kinase. In normal tissue, the activated receptor stimulates a downstream signaling cascade to promote cell division and motility, particularly in endothelial cells, and aids in angiogenesis and wound healing.14 Hepatocyte growth factor, also known as scatter factor, is produced in response to tissue hypoxia and promotes neovascularization to restore oxygenation. MET is frequently mutated, overexpressed, or amplified in tumors.15 Activated c-MET has multiple downstream signaling pathways, including FAK, PI3K-Akt, and Ras-Raf-Mek-MAPK, which promote cell replication and reduce apoptosis (thus prolonging tumor cell survival and conferring malignant potential), and also result in cell detachment and migration, and thus invasiveness and formation of metastasis (Figure 1).16 When upregulated in response to VEGF inhibition, these act as “escape pathways”, allowing tumor progression. Increased coexpression of MET and hepatocyte growth factor has been demonstrated in a subset of medullary thyroid cancer, where the increased hepatocyte growth factor may act in a paracrine fashion.17 Increased c-MET expression can also occur as a direct result of RET signaling activity.18
Cabozantinib
Initially identified as XL184, cabozantinib (Exelixis, South San Francisco, CA) was developed as a novel inhibitor of the dual angiogenic pathways VEGFR and MET, and also has activity against RET, KIT (mast/stem cell growth factor), Flt-3 (FMS-like tyrosine kinase 3), AXL, and Tie-2 (tunica interna endothelial cell kinase 2, TEK), which are associated with tumorigenesis and malignancy (Table 1).19 In vitro data showed that in malignant peripheral nerve sheath tumors, cabozantinib at low concentrations was able to inhibit constitutive and inducible MET phosphorylation. This, in turn, inhibited downstream cell signaling.20 Preclinical studies involving tumor xenografts in mice demonstrated reduced tumor cell proliferation and blood vessel density, and increased hypoxia and apoptosis.21 MET inhibition was demonstrated by a reduction in phosphorylation of MET in non-small cell lung cancer xenografts after administration of oral cabozantinib, and tumors were seen to decrease in size in a dose-dependent manner.22 Central nervous system penetration has been demonstrated in whole brain lysates of non-tumor-bearing mice, where XL184 concentrations were detected at 20% of maximum plasma concentration.23
The pharmacokinetics of cabozantinib were established in a Phase I trial.24 Cabozantinib is readily orally bioavailable, reaching peak plasma concentration at 5 hours after administration of a 175 mg capsule on day 1, and displays linear pharmacokinetics. The half-life was reported to be 91.3 ± 33.3 hours, with steady-state occurring at day 15. The maximum tolerated dose was established as 175 mg once daily (equivalent to 140 mg free base, which was used in the Phase III study).
Cabozantinib has been trialed in a number of tumor subtypes, including those that have been identified to harbor RET mutations and/or MET mutation, overexpression, or amplification (eg, medullary thyroid cancer, gliomas, non-small cell lung cancer). Responses have been seen in prostate, glioma, non-small cell lung cancer, breast, differentiated thyroid, and hepatocellular carcinoma tumors, among others, but the greatest responses were seen in medullary thyroid cancer, leading to an expansion cohort in the Phase I study followed by the Phase III trial as described below. Ongoing Phase III trials of cabozantinib in other tumor subtypes at the time of writing are in castrate-resistant prostate cancer, where it is being compared with the combination of mitoxantrone plus prednisone (COMET-2 trial).25
Clinical trials
Phase I
An initial Phase I trial of cabozantinib in patients with a variety of solid tumors was designed to identify the maximum tolerated dose. An early analysis reported increased activity in medullary thyroid cancer, noting a reduction in serum calcitonin in all treated patients with this disease. This prompted the addition of an expansion cohort of patients with medullary thyroid cancer within the trial, and the final results of the trial were published in May 2011.24 Of a total of 85 patients receiving treatment, 37 patients had medullary thyroid cancer, including 22 who had a somatic RET mutation, and three who had a confirmed germline RET mutation. Thirty-five of the 37 patients with medullary thyroid cancer had measurable disease as per RECIST (Response Evaluation Criteria In Solid Tumors) criteria. The majority of these patients received the maximum tolerated dose of 175 mg orally daily. Of these, 10 (29%) had confirmed objective response, and 25 (68%) demonstrated a confirmed partial response or stable disease for at least 6 months. Of note was that a response was seen in three patients with medullary thyroid cancer who had been pretreated with vandetanib or sorafenib (which also target VEGFR and RET), supporting the concept of MET being a VEGF inhibition escape pathway and thus a valid target.
Mutational analysis was carried out as part of the trial, and identified 15 patients harboring M918T, a RET mutation associated with poor prognosis. Of these, 12 had a response or stable disease with cabozantinib, noted by the authors to be in contrast with motesanib, which showed no activity in this subset of patients.26 Clear progression was seen in only one patient with medullary thyroid cancer, in whom a functioning b-raf mutation was detected, but no RET mutation. B-raf signaling occurs downstream of VEGFR, RET, and MET, and may account for the lack of response to cabozantinib seen in this patient.
Toxicity was similar to that seen with other tyrosine kinase inhibitors targeting VEGF and RET. The commonest toxicities of any grade were diarrhea, fatigue, anorexia, and nausea, which occurred in >50% of patients receiving 175 mg once daily continuously. Other adverse events included rash, palmar-plantar erythrodysesthesia (hand-foot syndrome), elevated liver transaminases and lipase, hair color change, mucositis, and hypertension. Grade 3 toxicities occurring with 175 mg daily dose in >10% of patients included fatigue (13%), palmar-plantar erythrodysesthesia (20%), and increased lipase level (18%). The only grade 4 event was a single occurrence of pulmonary embolism.
Phase III EXAM trial
The response seen to cabozantinib in medullary thyroid cancer in the Phase I study led directly to a large Phase III trial in medullary thyroid cancer, known as the EXAM (Efficacy of XL184 in Advanced Medullary Thyroid Cancer) trial. Interim results were presented at the 2012 American Society of Clinical Oncology Annual Meeting.27 This was an international, double-blind, randomized, placebo-controlled trial comparing 140 mg of cabozantinib (free base) daily with placebo in patients with locally advanced or metastatic medullary thyroid cancer. Patients were required to have confirmed progression as per RECIST criteria within the previous 14 months, to exclude patients with indolent disease. Again, there was no limit on prior treatment, including other tyrosine kinase inhibitors. A total of 330 patients were randomized to treatment or placebo in a 2:1 ratio. Treatment continued until progression or unacceptable toxicity, and crossover and unblinding were not permitted. The primary endpoint was progression-free survival, with the study having 90% power to detect a 75% increase in progression-free survival. Secondary endpoints included overall response rate and overall survival, as well as toxicity. Tumor response was assessed by computed tomography or magnetic resonance imaging at baseline and 12-week intervals.
At the time of the interim analysis, the primary endpoint had been met. Median progression-free survival, as assessed by an independent review committee, was 11.2 months in the treatment arm versus 4.0 months in the placebo arm, ie, a statistically significant difference of 7.2 months with a hazard ratio of 0.28 (95% confidence interval 0.19–0.40). One-year progression-free survival was reported as 47.3% in the treatment arm versus 7.2% in the placebo arm. Progression free survival was increased in the treatment arm in all prespecified subgroups, including number of prior treatments, prior TKI use, and RET mutational status. The secondary endpoint of overall response rate was 28% in the cabozantinib group versus 0% in the placebo group (P ≤ 0.0001), with a duration of response of 14.6 months which was similar in both RET mutation-positive and RET mutation-negative patients. Overall survival data had not reached the required number of events for meaningful analysis, but there was no difference between the two arms at this early stage. Only 44% of the required overall survival events (deaths) had occurred at the time of analysis, and the data are predicted to mature in 2013.
At data cutoff, 45% of patients in the cabozantinib arm remained on treatment compared with 13% in the placebo group. The primary reason for discontinuation was disease progression (20% in the treatment arm versus 60% in the placebo arm). Adverse events accounted for discontinuation in 16% and 8% of cases, respectively.
Prespecified subgroup analysis showed a statistically significant improvement in progression-free survival in the cabozantinib arm regardless of prior chemotherapy, tyrosine kinase inhibitor therapy, radiotherapy, or the presence or absence of bone metastases. Progression-free survival in MET mutation-positive or unknown status patients was significantly improved, while the MET mutation-negative subgroup showed a nonsignificant trend towards improved progression-free survival with treatment, with a wide confidence interval.
The commonest adverse events in the treatment arm were diarrhea and palmar-plantar erythrodysesthesia of all grades in 63% and 50% of patients, and of grade 3/4 in 16% and 27% of patients, respectively. Constitutional symptoms were common in both arms, but more so in the treatment arm. Taste change, hair color change, hypertension, and stomatitis of any grade were seen in 29%–34% of patients on active treatment and only 1%–6% of patients on placebo. Grade 3/4 hypocalcaemia was seen in the treatment arm in 9.3% of patients versus 0% in the placebo arm. Comment was made on the difference in median duration of exposure (cabozantinib 6.7 months versus placebo 3.4 months) and on the fact that symptoms such as diarrhea and fatigue are also common symptoms of active medullary thyroid cancer itself. No comment on the statistical significance of the differences in adverse events between the two arms was made, although this is likely to be reported in the formal publication of this analysis. Four deaths in the treatment arm were from events attributed to VEGF inhibition, namely fistula formation3 and hemorrhage.1
Assessment of the biomarkers calcitonin and carcinoembryonic antigen at 12 weeks after commencement of treatment showed a significant correlation with treatment and tumor response. Calcitonin fell on average by 45% in the cabozantinib arm, and increased by 57% in the placebo arm (P < 0.0001). The increase in calcitonin seen in the placebo arm indicates that, as planned, the study population had active disease.
Discussion
The results of EXAM indicate that cabozantinib has significant activity in patients with medullary thyroid cancer, including those pretreated with cytotoxic and targeted therapies. The full extent of benefit will depend on the final overall survival data. Nevertheless, the initial results from the Phase III trial raise the real possibility of an effective treatment for a disease that previously had no effective systemic therapies. Increased toxicity remains a cost of extended multikinase inhibition, because each pathway has a role in the normal function and homeostasis of cells. Adverse events for cabozantinib were found to be similar to those with other tyrosine kinase inhibitors, suggesting that the agent will be tolerated in a similar fashion to the tyrosine kinase inhibitors already being used in clinical practice.
Other tyrosine kinase inhibitors have also shown promise in treating patients with medullary thyroid cancer. These include vandetanib and motesanib. Vandetanib, although not inhibiting MET, does have anti-VEGF, anti-RET, and anti-epidermal growth factor receptor (EGFR) activity, and was found to prolong progression-free survival significantly compared with placebo in a Phase III trial of patients with medullary thyroid cancer, with a hazard ratio of 0.46 (95% confidence interval 0.31–0.69; P < 0.001).28 In April 2011, vandetanib was approved by the US Federal Drug Administration for use in metastatic medullary thyroid cancer.
The activity of vandetanib in medullary thyroid cancer suggests that EGFR inhibition may have a role in its treatment. EGFR pathway activation is a recognized contributor to malignant progression of several tumors, and EGFR and VEGFR2 overexpression and activation have been demonstrated in medullary thyroid cancer metastases.29 However, inhibiting EGFR is associated with upregulation of MET, leading to tumor escape.30 Dual inhibition using erlotinib (an EGFR inhibitor) and cabozantinib has been shown to be active in tumor cell lines that are resistant to either agent alone.22 Whether this combination would yield superior results to cabozantinib as a single agent in humans is yet to be tested.
Targeting MET without knowing the MET mutation or amplification status may potentially reduce overall efficacy. Onartuzumab (MetMAb, Genentech, South San Francisco, CA), a monoclonal antibody that directly inhibits hepatocyte growth factor-MET binding, significantly prolonged overall survival in a cohort of patients who harbored high levels of MET, yet those with low levels performed worse than the control arm when this agent was used in non-small cell lung cancer.31 Whilst these results are not from a Phase 3 trial, the implication for cabozantinib exists: should it be reserved only for medullary thyroid cancer expressing high levels of c-MET or harboring specific MET mutations? Or is the success of cabozantinib related to its ability to prevent the formation of “escape pathways” before they develop (and thus before significant amplification of MET), whilst simultaneously attacking VEGFR and the key proto-oncogene RET? Its broad level of activity in a number of solid tumors regardless of MET status indicates that the latter may be the case.
Ultimately, the role that cabozantinib will play in the management of patients suffering from medullary thyroid cancer will require head-to-head trials with the other agents shown to have activity against this tumor type. Perhaps most importantly, cabozantinib provides a novel targeted combination with proven efficacy, that may have application in a wider variety of tumors and contribute to our understanding of the biology of metastatic disease.
Addendum
Cabozantinib received FDA approval for use in medullary thyroid cancer in November 2012.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A national cancer data base report on 53,856 cases of thyroid carcinoma treated in the US, 1985–1995. Cancer. 1998;83(12):2638–2648. doi: 10.1002/(sici)1097-0142(19981215)83:12<2638::aid-cncr31>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 2.Martins RG, Rajendran JG, Capell P, Byrd DR, Mankoff DA. Medullary thyroid cancer: options for systemic therapy of metastatic disease? J Clin Oncol. 2006;24(11):1653–1655. doi: 10.1200/JCO.2005.05.4106. [DOI] [PubMed] [Google Scholar]
- 3.Elisei R, Cosci B, Romei C, et al. Prognostic significance of somatic RET oncogene mutations in sporadic medullary thyroid cancer: a 10-year follow-up study. J Clin Endocrinol Metab. 2008;93(3):682–687. doi: 10.1210/jc.2007-1714. [DOI] [PubMed] [Google Scholar]
- 4.Lodish MB, Stratakis CA. RET oncogene in MEN2, MEN2B, MTC, and other forms of thyroid cancer: molecular genetics and therapeutic advance. Expert Rev Anticancer Ther. 2008;8(4):625–632. doi: 10.1586/14737140.8.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prazeres H, Torres J, Rodrigues F, et al. How to treat a signal? Current basis for RET-genotype-oriented choice of kinase inhibitors for the treatment of medullary thyroid cancer. J Thyroid Res. 2011;2011:678357. doi: 10.4061/2011/678357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marsh DJ, Learoyd DL, Andrew SD, et al. Somatic mutations in the RET proto-oncogene in sporadic medullary thyroid carcinoma. Clin Endocrinol (Oxf) 1996;44(3):249–257. doi: 10.1046/j.1365-2265.1996.681503.x. [DOI] [PubMed] [Google Scholar]
- 7.Carlomagno F, Guida T, Anaganti S, et al. Disease associated mutations at valine 804 in the RET receptor tyrosine kinase confer resistance to selective kinase inhibitors. Oncogene. 2004;23(36):6056–6063. doi: 10.1038/sj.onc.1207810. [DOI] [PubMed] [Google Scholar]
- 8.Verbeek HH, Alves MM, de Groot JW, et al. The effects of four different tyrosine kinase inhibitors on medullary and papillary thyroid cancer cells. J Clin Endocrinol Metab. 2011;96(6):E991–E995. doi: 10.1210/jc.2010-2381. [DOI] [PubMed] [Google Scholar]
- 9.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358(19):2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Capp C, Wajner SM, Siqueira DR, Brasil BA, Meurer L, Maia AL. Increased expression of vascular endothelial growth factor and its receptors, VEGFR-1 and VEGFR-2, in medullary thyroid carcinoma. Thyroid. 2010;20(8):863–871. doi: 10.1089/thy.2009.0417. [DOI] [PubMed] [Google Scholar]
- 11.Bunone G, Vigneri P, Mariani L, et al. Expression of angiogenesis stimulators and inhibitors in human thyroid tumors and correlation with clinical pathological features. Am J Pathol. 1999;155(6):1967–1976. doi: 10.1016/S0002-9440(10)65515-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pàez-Ribes M, Allen E, Hudock J, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15(3):220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.You W-K, Sennino B, Williamson CW, et al. VEGF and c-Met blockade amplify angiogenesis inhibition in pancreatic islet cancer. Cancer Res. 2011;71(14):4758–4768. doi: 10.1158/0008-5472.CAN-10-2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bussolino F, Di Renzo MF, Ziche M, et al. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol. 1992;119(3):629–641. doi: 10.1083/jcb.119.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Appleman LJ. MET signaling pathway: a rational target for cancer therapy. J Clin Oncol. 2011;29(36):4837–4838. doi: 10.1200/JCO.2011.37.7929. [DOI] [PubMed] [Google Scholar]
- 16.Di Renzo MF, Oliver M, Giacomini A, et al. Overexpression and amplification of the met/HGF receptor gene during the progression of colorectal cancer. Clin Cancer Res. 1995;1(2):147–154. [PubMed] [Google Scholar]
- 17.Papotti M, Olivero M, Volante M, et al. Expression of hepatocyte growth factor (HGF) and its receptor (MET) in medullary carcinoma of the thyroid. Endocr Pathol. 2000;11(1):19–30. doi: 10.1385/ep:11:1:19. [DOI] [PubMed] [Google Scholar]
- 18.Constantini F. Genetic controls and cellular behaviors in branching morphogenesis of the renal collecting system. [Accessed November 12, 2012];WIREs Dev Biol. 2012 1(5):693–713. doi: 10.1002/wdev.52. Available from: http://dx.org/10.1002/wdev.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Exelixis Inc. Press Release. Exelixis files IND application for anticancer compound XL184. [Accessed September 6, 2012]. Available from: http://ir.exelixis.com/phoenix.zhtml?c=120923&p=irol-newsArticle&ID=720188&highlight=
- 20.Torres KE, Zhu QS, Bill K, et al. Activated MET is a molecular prognosticator and potential therapeutic target for malignant peripheral nerve sheath tumors. Clin Cancer Res. 2011;17(12):3943–3955. doi: 10.1158/1078-0432.CCR-11-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yakes FM, Chen J, Tan J, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther. 2011;10(12):2298–2308. doi: 10.1158/1535-7163.MCT-11-0264. [DOI] [PubMed] [Google Scholar]
- 22.Janne PA, Wax M, Leach J, Shangkar G, Engelman J. Targeting MET with XL184 to reverse EGFR tyrosine kinase inhibitor (TKI) resistance in NSCLC: impact of preclinical studies on clinical trial design. Eur J Cancer. 2008;6:552. [Google Scholar]
- 23.De Groot JF, Prados M, Urquhart T, et al. A Phase II study of XL184 in patients (pts) with progressive glioblastoma multiforme (GBM) in first or second relapse. J Clin Oncol. 2009;27(15 Suppl):Abstr 2047. [Google Scholar]
- 24.Kurzrock R, Sherma SI, Ball DW, et al. Activity of XL184 (cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J Clin Oncol. 2011;29(19):2660–2666. doi: 10.1200/JCO.2010.32.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Exelixis. Available from: http://www.exelixis.com.
- 26.Schlumberger MJ, Elisei R, Bastholt L, et al. Phase II study of safety and efficacy of motesanib in patients with progressive or symptomatic, advanced or metastatic medullary thyroid cancer. J Clin Oncol. 2009;27(23):3794–3801. doi: 10.1200/JCO.2008.18.7815. [DOI] [PubMed] [Google Scholar]
- 27.Schoffski P, Elisei R, Muller S, et al. An international, double-blind, randomized, placebo-controlled Phase III trial (EXAM) of cabozantinib (XL184) in medullary thyroid carcinoma (MTC) patients (pts) with documented RECIST progression at baseline. J Clin Oncol. 2012;30(Suppl):Abstr 5508. [Google Scholar]
- 28.Wells SA, Jr, Robinson BG, Gagel RF, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2012;30(2):134–141. doi: 10.1200/JCO.2011.35.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodríguez-Antona C, Pallares J, Montero-Conde C, et al. Overexpression and activation of EGFR and VEGFR2 in medullary thyroid carcinomas is related to metastasis. Endocr Relat Cancer. 2012;17(1):7–16. doi: 10.1677/ERC-08-0304. [DOI] [PubMed] [Google Scholar]
- 30.Engelman JA, Zejnullahu K, Mitsdomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316(5827):1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 31.Spigel DR, Ervin TJ, Ramlau R, et al. Final efficacy results from OAM4558G, a randomized Phase II study evaluating MetMAb or placebo in combination with erlotinib in advanced NSCLC. Oral abstract session. J Clin Oncol. 2011;29(Suppl):Abstr 7505. [Google Scholar]
- 32.Wedge SR, Ogilvie DJ, Dukes M, et al. ZD6474 inhibits vascular endothelial growth factor signaling, angiogenesis, and tumor growth following oral administration. Cancer Res. 2002 Aug 15;62(16):4645–4655. [PubMed] [Google Scholar]
- 33.Polverino A, Coxon A, Starnes C, et al. AMG 706, an oral, mulitkinase inhibitor that selectively targets VEGF, PDGF, and Kit receptors, potently inhibits angiogenesis and induces regression in tumor xenografts. Cancer Res. 2006 Sep 1;66(17):8715–8721. doi: 10.1158/0008-5472.CAN-05-4665. [DOI] [PubMed] [Google Scholar]
- 34.Mendel DB, Laird AD, Xin X, et al. In vivo antitumour activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003 Jan;9(1):327–337. [PubMed] [Google Scholar]
- 35.Hu-Lowe DD, Zou HY, Grazzini ML, et al. Nonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clin Cancer Res. 2008 Nov 15;14(22):7272–7283. doi: 10.1158/1078-0432.CCR-08-0652. [DOI] [PubMed] [Google Scholar]