Abstract
The midbrain median raphe (MR) and dorsal raphe (DR) nuclei were tested for their capacity to regulate recovery from traumatic brain injury (TBI). An implanted, wireless self-powered stimulator delivered intermittent 8-Hz pulse trains for 7 days to the rat's MR or DR, beginning 4–6 h after a moderate parasagittal (right) fluid-percussion injury. MR stimulation was also examined with a higher frequency (24 Hz) or a delayed start (7 days after injury). Controls had sham injuries, inactive stimulators, or both. The stimulation caused no apparent acute responses or adverse long-term changes. In water-maze trials conducted 5 weeks post-injury, early 8-Hz MR and DR stimulation restored the rate of acquisition of reference memory for a hidden platform of fixed location. Short-term spatial working memory, for a variably located hidden platform, was restored only by early 8-Hz MR stimulation. All stimulation protocols reversed injury-induced asymmetry of spontaneous forelimb reaching movements tested 6 weeks post-injury. Post-mortem histological measurement at 8 weeks post-injury revealed volume losses in parietal-occipital cortex and decussating white matter (corpus callosum plus external capsule), but not hippocampus. The cortical losses were significantly reversed by early 8-Hz MR and DR stimulation, the white matter losses by all forms of MR stimulation. The generally most effective protocol, 8-Hz MR stimulation, was tested 3 days post-injury for its acute effect on forebrain cyclic adenosine monophosphate (cAMP), a key trophic signaling molecule. This procedure reversed injury-induced declines of cAMP levels in both cortex and hippocampus. In conclusion, midbrain raphe nuclei can enduringly enhance recovery from early disseminated TBI, possibly in part through increased signaling by cAMP in efferent targets. A neurosurgical treatment for TBI using interim electrical stimulation in raphe repair centers is suggested.
Key words: cAMP, deep brain stimulation, forebrain trauma, forelimb reaching, neuroanatomical volume, water maze
Introduction
The partial behavioral recovery that is often observed in the days and weeks after a moderate traumatic brain injury (TBI) is presumably a result of adaptive cellular and molecular mechanisms initiated in injured or surrounding regions.1,2 A large class of experimental treatments for TBI aims to intervene in these endogenous processes. For example, some treatments attempt to augment axonal sprouting, remyelination, or neurogenesis in zones that contain progenitor cells.3–6 Alternatively, the suppression of secondary detrimental events can be attempted; these include toxicity from excess glutamate, production of reactive oxygen species, inhibitory effects on fiber growth from the breakdown products of myelin, inflammatory responses, apoptosis, and glial scarring.7
Not all endogenous reactions, however, are unequivocally beneficial or harmful. Regenerative mechanisms risk exuberant growth, axonal misconnection or malformation.8,9 Released substances that are apparently injurious, on the other hand, may be essential for effective neural repair. For example, inflammatory signals can initiate the degradation of damaged tissue, allowing the replacement or removal of dysfunctional elements.10,11
The serotonergic midbrain raphe nuclei, comprising principally the dorsal raphe (DR) and the median raphe (MR), are well suited to the coordination of these potentially conflicting repair processes. Their neurons possess various membrane receptors for injury-associated molecules such as cytokines, eicosanoids, and adenosine triphosphate.12–15 They send partially overlapping axonal projections to the entire gray matter of forebrain and diencephalon, as well as many brainstem regions.16,17 Serotonin, in addition to being a classic neurotransmitter, is known from an extensive literature to have positive developmental, neuroprotective, and regenerative effects.18–20 The majority of serotonergic raphe terminals co-release neuropeptides that have trophic or protective actions, most prominently galanin in the case of the midbrain raphe.21,22
The hindbrain raphe nuclei constitute an analogous descending system, whose neurons release serotonin and neuropeptides such as thyrotropin-releasing hormone and substance P in widespread spinal cord segments and laminae. We reported previously that the hindbrain's nucleus raphe magnus enhances sensorimotor and anatomical recovery from incomplete contusional spinal cord injury when subjected for several days to weeks of intermittent electrical stimulation.23,24 The present article describes a parallel study of the ascending projection.
MR or DR stimulation was delivered for 7 days to rats that had received a controlled fluid percussion injury over the right cortex. The stimulation was usually started 4–6 h after the experimental injury and at a frequency of 8 Hz. Some parametric variations were additionally explored, in which the MR stimulation was either given at the higher frequency of 24 Hz or delayed for 7 days after injury (at 8 Hz). Subsequent recovery of cognitive and sensorimotor behavior was evaluated in the fifth and sixth weeks. Tissue was extracted after euthanasia in the eighth week, so that changes in the volumes of major forebrain regions could be determined. These experiments were intended both to evaluate the MR and DR as repair centers and to explore the possibility of their stimulation as a treatment for TBI.
Methods
Injury model
All experiments were performed with adult male Sprague-Dawley rats (weight 318–326 g), under animal-use protocols approved by the Institutional Animal Care and Use Committee of the University of Miami Miller School of Medicine. The rats received either a fluid percussion injury of moderate intensity25,26 or a sham injury. On the day before the injury or sham procedures, the rats were anesthetized in a closed chamber with 3% isoflurane and then maintained with 1–1.35% isoflurane delivered through a nose-cone.
A 4.8 mm-diameter craniotomy was made over the right parietal-occipital cortex, 3.8 mm posterior to stereotaxic bregma and 2.5 mm lateral to the midline. A sterile plastic tube, 1 cm long, was placed just above the exposed dura, bonded to the skull by cyanoacrylic adhesive, and plugged with a sterile sponge. The scalp was closed by suture thread.
Twenty-four hours later, the rats were anesthetized with 0.5–1% isoflurane in a mixture of 70% nitrous oxide and 30% oxygen, induced in a closed chamber and delivered through a tracheal tube. They were ventilated mechanically and given 1 mg/kg intravenous pancuronium. A catheter inserted in the tail artery monitored arterial blood pressure and provided samples for blood gas analysis. Brain temperature was indirectly measured by a thermistor placed in the left temporalis muscle; body temperature was measured by a rectal thermistor and maintained normothermic (37°C) by a heating lamp.
After stabilization of physiological parameters, the animals received, through the previously inserted intracranial plastic tube, a pressure pulse of an intensity (1.8–2.2 atmospheres) known to produce a moderate injury.27 Surgery for sham injury omitted only the pressure pulse. Post-injury physiological monitoring continued for 20 min, after which the incisions were closed by suturing and the rats were allowed to recover.
Electrical stimulation
The injury or sham procedure was followed 3–4 h later by implantation of an integral stimulator-microelectrode assembly.23,24 The rats were anesthetized with isoflurane, as in the procedure of injury tube placement. An additional opening, 1 mm in diameter, was drilled in the skull above the midbrain. Implants were sterilized with isopropyl alcohol and dried just before use.
The microelectrode, which was oriented at a slight angle (6 or 8 degrees) to the sagittal plane to avoid the dorsal sinus, was aimed at the midline DR or MR. Target coordinates were always 1.2 mm rostral to the interaural line, 3.5 mm vertically above this line for the DR, and 1.5 mm above for the MR. The commercially obtained microelectrodes (Microprobe, Inc., Gaithersberg, MD) were made from platinum-iridium wire of 75 μm diameter, insulated with Epoxylite® except at the tapering tip, whose impedance at 1 kHz was specified to be 0.5 megohm (±20%). A bare, stainless-steel wire served as the anode. The microelectrode and the anode protruded from high-resistivity epoxy resin that encapsulated the custom-built, non-reusable stimulator.
The entire assembly weighed 2±0.1 g. The capsule's dimensions were 18 mm long, 8 mm wide, and 5 mm high. The stainless-steel wire anode was wrapped around one of three small, self-tapping screws placed in the skull. The implant was fixed to these screws and to the exposed dorsal skull surface by dental acrylic. The skin opening was closed by sutures around the implant, which remained partly exposed.
The stimulator circuitry was powered by two silver-silver oxide batteries in series (each 1.6 V), which were capable of providing at least 3 weeks of active stimulation in ex vivo testing. An inductor-based step-up regulator within the circuit delivered constant-current 30-μA rectangular pulses at a compliance of 36 V. The timing of the pulses was set by a microprocessor to be 1 ms in width and 8 or 24 Hz in frequency, with the trains lasting 5 min alternated with a 5-min break over 12 daylight hours; a 12-hour nighttime break occurred between 1800 and 0600 h. The device could be controlled by applying nearby a pulsed magnetic field that was detected by a reed switch. The decoded magnetic pulses could be used to activate or deactivate the stimulation or adjust the various timing parameters (pulse width, frequency, and clock time). Program and parameter status was signaled by a customized pulse-code via an infrared-emitting diode to an external hand-held detector.
The stimulator was usually turned on in designated groups of rats 4–6 h after the TBI or sham injury; it was turned on 7 days after TBI in one group. Two groups, one TBI and one sham, were given inactive stimulators as controls. The stimulation was allowed to continue for 7 days and was verified daily with the infrared detector. Untoward effects of the stimulation such as motor twitches or aversive behavior were never seen. The infrared detector was the only means of determining whether the stimulation program was in an active or an inactive phase.
Testing of behavior
A total of 79 rats were subjected to behavioral testing, which was always performed by observers who were unaware of the rat's treatment. A timeline for the behavioral testing in relation to other interventions is shown in Figure 1A. The rats performed three kinds of tasks in a Morris water maze on the fifth week after TBI or sham injury: reference memory trials, a probe trial, and working memory trials.25,28 The water maze, which consisted of a circular pool (122 cm diameter, 60 cm deep) filled with 25°C water made opaque by white, non-toxic paint, was located in a quiet, windowless room. A round platform, 10 cm in diameter, was positioned 5 cm beneath the surface of the water. The animal's movements were videotaped, and analyzed with Ethovision software (Noldus Inc., Leesburg, VA).
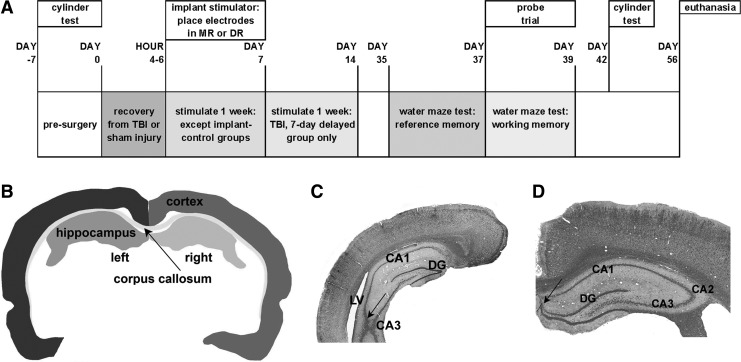
FIG. 1.
Timeline and basis of anatomical analysis. (A) Experimental timeline. (B) Profile of a section halfway through a series from a rat with TBI, delineating the forebrain regions used in the volume measurements. (C, D) Sections taken at caudal (C) and rostral (D) boundaries for the volume measurements, stained with hematoxylin-eosin and Luxol Fast Blue, obtained from two different rats and different sides of the brain. Arrows in C and D point to landmarks used to determine boundaries as described in the Methods section.
The pool was divided into four equal-sized quadrants, whose boundaries were arbitrarily called north, west, south, and east. Fixed, prominent visual cues included three large black cardboard shapes (a plus sign and a circle on the south wall and an equilateral triangle on the east wall), together with a large black equipment cart on the east side, two red heat lamps on a table in the northeast corner, and the experimenter standing always on the west side. Parameters recorded included the time from release to arrival on the hidden platform (referred to here as latency), the path length, and the average speed of swimming. The total and maximum times spent in the target quadrant (for working memory, where the platform was most recently located) were also measured.
To test reference memory, rats were released from the edge of the pool at random quadrant boundaries. The platform's position was fixed in the northeast quadrant. Any rat that successfully arrived at the platform within 60 s was allowed to remain there for 30 s. Rats that were unsuccessful after 60 s were placed on the platform for 30 s. Between trials, the rats were carefully dried and remained under a heat lamp until their next trial.
Four trials per day were performed at 4-min intervals for 3 consecutive days. In the probe trial, performed on the fourth day, the platform was removed from the pool. Animals were then released from the west position and their swim patterns were videotaped for 30 s. Working memory was tested after the probe trial on the fourth day and again on the fifth day. The rat was placed on a submerged platform in a specific quadrant for 60 s and then retrieved from the platform and immediately released into the pool, always from the west release point. This protocol differs from commonly used tests for working memory that do not initially reveal the location.29 The rats were given 60 s to find the submerged platform and were placed on it if they failed. After 10 s on the platform, they were released for the second of each pair of trials with the platform and release point unchanged. Two such pairs of trials were done on each day, each pair using a different platform location, which was consecutively the southeast quadrant, the center of the pool, the south-west quadrant, and the east boundary line.
Sensorimotor performance was evaluated by measuring the symmetry of forelimb reaching movements made during spontaneous rearing,30,31 7 days before TBI or sham injury, and 6 weeks after. The rats were placed for 5 min within a transparent cylinder (20 cm diameter, 30 cm high). From videotape recordings, counts were made of the number of times that one or both forepaws made contact with the cylinder wall during a rearing movement when the rat was standing on the hind legs. Rats producing fewer than six such rearing movements were not included in the analysis.
Neuroanatomical analysis
Eight weeks after injury or sham injury, all rats underwent euthanasia with a ketamine-xylazine mixture. This was followed immediately by intracardiac perfusion with phosphate-buffered saline and then by 4% paraformaldehyde in phosphate buffer. After perfusion, the electrodes were carefully withdrawn by hand along their original track. Brains were then extracted, post-fixed overnight, embedded in paraffin, and sectioned coronally at 10 μm thickness on a microtome. One series of sections, taken at 150 μm intervals, was stained with Luxol Fast Blue and hematoxylin-eosin. These sections were used to estimate the volumes of the left and right parietal-occipital cortex, of the combined corpus callosum and external capsule (CC+EC), and of the left and right dorsal hippocampus (Fig. 1B). Sham-injured, stimulated groups were not analyzed anatomically.
The volume estimation used contouring with Neurolucida software (version 7.50.1, MicroBrightField Inc., Williston, ME). The caudal limit for contouring was set where the dorsal and ventral parts of the hippocampal CA3 unite, typically at about 4.5 mm posterior to bregma (Fig. 1C). The rostral limit was the place where the granule cell layer of the dentate appears to separate from the pyramidal cells of CA1, near 3.0 posterior to bregma (Fig. 1D). The section thickness after histological processing was determined by z-axis measurement, and the borders of the various regions were mapped under a 5×objective. Volumes were estimated from the product of the summed area measurements and the distance between section planes.
Midbrain sections were immunostained for glial fibrillary acidic protein (GFAP) and neuronal nuclei (NeuN) to assess gliosis at the microelectrode tip and to mark neuronal clusters. Sections including the MR or DR target, spaced at 150 μm, were incubated overnight at 4°C in anti-GFAP (1:1000, AB5804, Chemicon) and anti-NeuN (1:250, MAB377, Millipore) diluted in 0.1M phosphate buffered solution at pH 7.4, containing 3% normal donkey serum and 0.04% Tween-20. The tissue was incubated at room temperature in AlexaFluor 488 donkey anti-rabbit IgG and 594 donkey anti-mouse (1:250, InVitrogen) for 2 h. Digital epi-fluorescent images were captured on a Zeiss 200M Axiovert microscope at a 5× magnification. Z-stack images were taken at 25× using a Zeiss LSM 510 confocal microscope.
To confirm the site and efficacy of stimulation, neuronal activity was mapped indirectly with the marker of metabolism, radiolabeled 2-deoxyglucose (2-DG), in an additional set of rats (n=8) 7 days after TBI or sham surgery. Rats with or without TBI either received 8 Hz MR stimulation before and during the 2-DG injection or had an inactive implant. In accordance with previously described methods,32,33 the rats were fasted overnight. During the procedure, which was performed in a quiet, darkened room, they were restrained in a loose plaster cast formed under brief isoflurane anesthesia. After an intravenous injection of 20 μCi 14C- 2-DG (specific activity 45–55 mCi/mmole), arterial blood was sampled at intervals for radioactivity and glucose. The rats were euthanatized by decapitation 45 min after 2-DG injection, and the brains were then quickly removed and frozen in liquid nitrogen. They were embedded and sectioned at 20-μm thickness. Every fifth section (120-μm intervals) was exposed to film for 10 days, along with a calibrated 14C methylmethacrylate standard. Images were digitized and their optical density quantified with reference to the methylmethacrylate standard, to yield the local cerebral glucose utilization.
Stimulation-produced changes in cyclic adenosine monophosphate (cAMP)
cAMP was assayed in forebrain regions 3 days after TBI or sham injury in an additional set of rats. Some animals in each condition received inactive implants, and the rest received stimulation at the location and frequency that produced the best overall behavioral outcomes. Stimulation was given for 2 h immediately before extraction. The left and right cortex, the left and right hippocampus, and the thalamus were quickly removed and flash frozen in liquid nitrogen. Analysis of cAMP levels used a commercial enzyme-linked immunosorbent assay kit (ADI-900-066, Enzo Life Sciences, Plymouth Meeting, PA). Tissue was sonicated in 10 volumes of 0.1 N HCl in the presence of the nonselective phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (500 μM) and then centrifuged at 1000 g for 15 min at 4°C to remove particulate debris. Samples were assayed in duplicate according to the manufacturer's protocol for non-acetylated material.
Statistical methods
Statistical testing was performed with SPSS software (version 17), significance being set always at p<0.05. For the analyses of the probe trial, of cAMP concentration and of anatomical volumes, the treatment groups were subjected to one-way analysis of variance (ANOVA) followed by Dunnett post-hoc comparison with the injured, non-stimulated (TBI-NS) group. Reference and working memory were analyzed by repeated measures ANOVA, testing marginal means against the TBI-NS group with Bonferroni protection. Cylinder reaching and, in an alternative analysis of reference memory performance, the trial of peak learning were assumed to be non-Gaussian in distribution and were analyzed by generalized linear models as detailed later with each result. In a summary analysis, to determine the degree of resemblance among treatments in their effects on behavior, 10 variables from the four behavioral tests (reference memory, probe, working memory, cylinder limb asymmetry) were subjected to principal component analysis. The first seven canonical discriminant functions were calculated. To assess findings, categorical independent variables (treatments) were mapped in the plane given by the first two canonical functions.
Results
Observations on injury physiology and stimulation site
The intra-arterial measurements of mean pressure, pH, glucose concentration, and oxygen and carbon dioxide partial pressures, as well as the temperatures of the body and head, were taken 15 min before the injury or sham procedure and 15 min afterward. Two variables, the mean pressure and partial oxygen pressure, showed a significantly greater drop in rats with TBI compared with sham injury (p<0.05, interaction term in two-way ANOVA); oxygen pressure fell by 16.8 mm Hg in TBI versus 9.1 mm Hg in sham injury; arterial pressure fell 6 mm Hg after TBI versus a slight rise of 2 in sham injury (Table 1). Despite the differences, all variables remained within their normal physiological range after TBI.
Table 1.
Systemic Physiological Variables Measured 15 min before and after Surgery, Sampled from 25 Rats with Sham Injury and 66 with Traumatic Brain Injury
| Condition | Body temperature °C | Head tempeerature °C | Blood pH | Blood CO2 mm Hg | Blood O2 mm Hg | Arterial BP mm Hg | Blood glucose mg/dL |
|---|---|---|---|---|---|---|---|
| Pre-sham | 36.7 | 36.7 | 7.44 | 39.7 | 142.0 | 114 | |
| Pre-TBI | 36.7 | 36.7 | 7.46 | 39.7 | 144.3 | 120 | |
| Post-sham | 36.9 | 36.8 | 7.44 | 39.6 | 132.9 | 116 | 106.3 |
| Post-TBI | 36.9 | 36.7 | 7.45 | 39.3 | *127.5 | *114 | 104.2 |
Indicates significance of p<0.05 versus pre-injury in the two-way analysis of variance interaction term. Glucose values were compared post-surgery only, and differences between the two treatments were not significant (unpaired t test).
BP, blood pressure; TBI, traumatic brain injury.
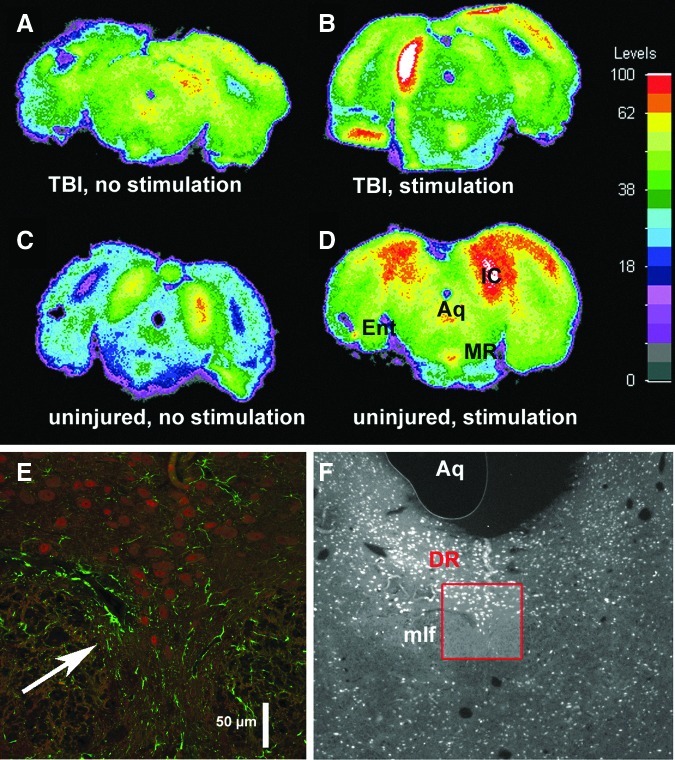
Coronal sections, sampled from 18 rats at the level of the DR or MR, were stained for GFAP. Definite traces of the microelectrode trajectory in the midbrain were never seen, although in a few cases a small degree of astrocytic activation may possibly have remained (Fig. 2E). The lack of anatomical confirmation and the need to verify stimulation efficacy prompted an autoradiographic study of 2-DG uptake in a separate set of rats. These had either 7-day old TBI (n=4) or no injury (n=4); 8 Hz stimulation was applied to two rats from each condition. In both injured and sham-injured rats, metabolic activity was greater at the stimulation site (Figs. 2B, 2D) compared with surrounding tissue and was accurately located in the MR.
FIG. 2.
Localization of microelectrodes. (A–D) Maps of 2-deoxyglucose utilization in the midbrain of individual rats in the coronal plane of the median raphe, showing 7-day traumatic brain injury (TBI) without (A) or with (B) MR stimulation, and 7-day sham-injury without (C) or with (D) stimulation. Structures indicated (on section D only): Aq, aqueduct; MR, median raphe; IC, inferior colliculus; Ent entorhinal cortex. The IC shows strong activation from residual auditory activity, although measures were taken to reduce ambient sound. Levels indicated in the color calibration bar are in units of 10−2 μmoles/g/min; white on the maps indicates saturated values. The uneven steps of the non-linear scale were chosen to highlight regional differences. (E, F) The dorsal raphe (DR) target site of a stimulating electrode after 8 weeks of implantation, viewed in coronal section at higher (×25) and lower power (×5, red box outlining high power region), depicting GFAP (green) and neuroneal nuclei (red) immunoreactivity. The low power micrograph has been color-filtered and converted to grey scale to emphasize red (i.e., neurons). A possible slight cavity with surrounding astrogliosis indicates the former presence of a microelectrode (arrow in E), but typically no residue from the microelectrode's presence was seen, which can be attributed to their construction with very thin and relatively inert platinum-iridium. mlf, median longitudinal fasciculus.
Cognitive performance in the Morris water maze
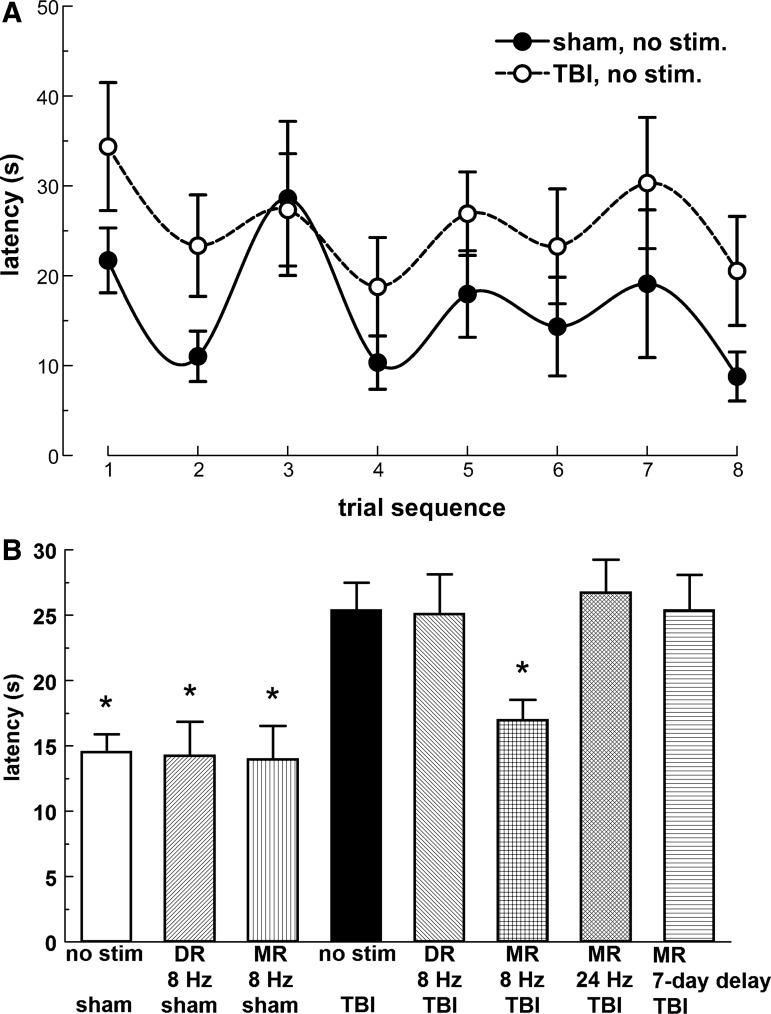
Reference memory testing showed no differences between groups in mean escape latency on the first day. On the second day, a clear difference was seen between the TBI-NS group and the shorter latencies of other groups. By the third day, these differences were smaller or had disappeared (Fig. 3A). In the repeated measures ANOVA, the three trial days and the four trials nested within each day were used as within-subjects factors. The interaction of day and group in a quadratic within-subjects contrast was significant (p=0.02). A significant difference was found between the TBI-NS group and most other groups, except the 24 Hz or 7-day delayed MR (8 Hz) stimulation, comparing marginal means with Bonferroni protection. (Probability test values (p) for significance in post hoc tests are given in Table 2, for all behavioral, anatomical, and neurochemical analyses.) Treatment with 8 Hz MR or DR thus significantly reversed the impairment of latency produced by the TBI. In sham-injured rats, the two stimulated groups resembled the non-stimulated one.
FIG. 3.
Reference memory as determined by escape latency in the Morris water maze. (A) Average escape latency; vertical bars express the group averages from four trials on each day. The asterisks next to the legend indicate the groups that were significantly different from the injured, non-stimulated group (p<0.05). (B) Cumulative fraction of rats in each treatment group that had reached or passed the trial in which the fastest learning occurred, defined as the greatest reduction in swim-time compared with the previous trial. Asterisks next to the legend indicate groups that were significant against the injured, non-stimulated group (Wald chi-square, p<0.05). For this figure and others, calculated p values for significance are listed in Table 2. Stimulation is abbreviated “stim” or omitted when a stimulation frequency or delay is specified, here and in subsequent graphs. Sample sizes of groups: sham, no stimulation (n=7); sham, dorsal raphe (DR) stimulation (n=7); sham, median raphe (MR) stimulation (n=9); traumatic brain injury (TBI), no stimulation (n=10); TBI, DR stimulation (n=8); TBI, 8 Hz MR stimulation (n=12); TBI, 24 Hz MR stimulation (n=11); TBI, 7-day delayed (8 Hz) MR stimulation (n=10). Error bars are standard errors of the mean in this and subsequent figures.
Table 2.
Synopsis of Main Findings with Various Stimulation Protocols in Injured Rats Compared with the Injured, Non-Stimulated Group
|
Stimulation site, condition |
None, sham |
MR, TBI |
DR, TBI |
||
|---|---|---|---|---|---|
| Frequency start time post-TBI | None none | 8 Hz 4–6 hr | 24 Hz 4–6 hr | 8 Hz 7 days | 8 Hz 4–6 hr |
| Reference memory (week 5) | |||||
| Escape latency | <0.001 | 0.04 | NS | NS | 0.03 |
| Peak learning trial | <0.001 | 0.008 | 0.01 | NS | 0.006 |
| Probe (target quadrant) | NS | NS | NS | NS | NS |
| Working memory (week 5) | |||||
| Escape latency | 0.02 | 0.03 | NS | NS | NS |
| Cylinder test (week 6) | |||||
| Reaching asymmetry | 0.02 | 0.04 | 0.04 | 0.008 | 0.001 |
| Forebrain volumes (week 8) | |||||
| Cortex: L, R | 0.03, 0.006 | 0.04, 0.006 | NS | NS | 0.02, 0.03 |
| CC+EC | 0.01 | 0.05 | 0.02 | 0.04 | NS |
| Hippocampus: L, R | NS, NS | NS, NS | NS, NS | NS, NS | NS |
| cAMP (day 3) | |||||
| Cortex: L, R | 0.02, NS | 0.003, 0.01 | nt | nt | nt |
| Hippocampus: L, R | 0.03, 0.01 | 0.004, <0.001 | nt | nt | nt |
| Thalamus | NS | NS | nt | nt | nt |
Significance levels (p values) are rounded to the nearest first significant digit.
MR, median raphe; TBI, traumatic brain injury; DR, dorsal raphe; NS, not significant; L, left side; R, right side (the side of the applied pressure pulse); CC+EC, corpus callosum and external capsule; cAMP, cyclic adenosine monophosphate concentration; nt, not tested;.
Quadrant preference in the probe trial (not illustrated) showed no differences in pairwise comparison with the TBI-NS group (one-way ANOVA with Dunnett post-hoc comparison), which was consistent with the lack of large observable differences in reference memory on day 3.
The time course of the latency reduction in reference memory trials was analyzed further by considering the trial in which the latency showed the greatest drop from the previous trial (Fig. 3B). These trial indices were assumed to have a multinomial distribution and were analyzed with a cumulative logit link function in a generalized linear model. The peak learning trial was delayed in the TBI-NS group compared with all sham-injured groups. This lag was significantly reversed by 8 Hz MR and DR stimulation. Stimulation with 24 Hz MR also reversed the change caused by TBI, an effect not seen in the linear analysis (Fig. 3A). The 7-day delayed MR (8 Hz) stimulation remained non-significant as in the linear analysis.
In the working memory task, preliminary graphical analysis showed that escape latencies from the eight trials averaged for each group formed a non-decaying oscillation of period two (Fig. 4A). There was no evidence that TBI caused oscillation of different amplitude (i.e., had a different fall in latency in repeated trials with the same platform location). Statistical repeated measures analysis of latencies was based on successively nested within-subjects factors: 2 testing days, two platform locations, and two trials per location. Only the trial order was significant (p<0.0005) in testing of within-subjects contrasts; that is, the day and location were not significant. In testing of marginal means, sham-injured rats that received either no stimulation or MR or DR stimulation had significantly lower escape latencies than the TBI-NS group (Fig. 4B). Among rats with TBI, only early 8 Hz MR stimulation significantly lowered the average escape latency compared with the TBI-NS group.
FIG. 4.
Performance in the working memory task. (A) The group means of the time series of latencies, shown for the non-stimulated groups (traumatic brain injury [TBI] and sham) to illustrate the oscillation of period two and the lack of obvious trend or difference in the amplitude of the oscillation. The curves are natural spline interpolations. (B) Average latencies across all groups from all of the eight trials performed over the 2 test days. The asterisks show variables that were significantly different from the injured, non-stimulated group. Group sizes were as in Figure 3. DR, dorsal raphe; MR, median raphe.
Forelimb asymmetry during rearing (cylinder test)
In baseline measurements in the cylinder test, 41.1% of unilateral reaching movements of forelimbs averaged across individuals (n=65, all groups) were made by the right limb alone, 40.5% by the left limb alone, and 18.4% were bilateral (Fig. 5). Implantation of an inactive stimulator in sham-injured rats (n=7) did not significantly change these proportions after 6 weeks: left forelimb movements 40.7%, right forelimb movements 37.3%, bilateral movements 22.0%. In non-stimulated rats with injuries (TBI-NS, n=11), left forelimb movements fell to 34.0% of the total, the direction expected from right-sided cortical injury; right forelimb movements rose to 49.5% and bilateral movements remained little changed at 16.5%. Responses were modeled with a binomial distribution, bilateral movement being assigned arbitrarily to the right side, and analyzed in a generalized linear model with a logit link function; pairwise comparisons were made with estimated marginal means against the TBI-NS group, using Wald chi-square to estimate significance levels. The left forelimb deficit was significantly reversed by all treatments (Fig. 5).
FIG. 5.
Symmetry of forelimb reaching movements during rearing in a transparent cylinder, measured 6 weeks after TBI or sham injury and 1 week before. The ordinate is the number of right movements divided by the sum of all movements (left, right, and bilateral). The injured, non-stimulated group was significantly different from all other groups (p<0.05) tested against a generalized linear model using a logit transformation of the binomial distribution. The numbers of rats qualifying for inclusion in each group (see Methods for criterion) were as follows: pre-surgery (n=65); sham, no stimulation (n=7); sham, dorsal raphe (DR) stimulation (n=6); sham, median raphe (MR) stimulation (n=7); traumatic brain injury (TBI), no stimulation (n=10); TBI, DR stimulation (n=6); TBI, 8 Hz MR stimulation (n=11); TBI, 24 Hz MR stimulation (n=11); TBI, 7-day delayed (8 Hz) MR stimulation (n=6).
Combined behavioral analysis
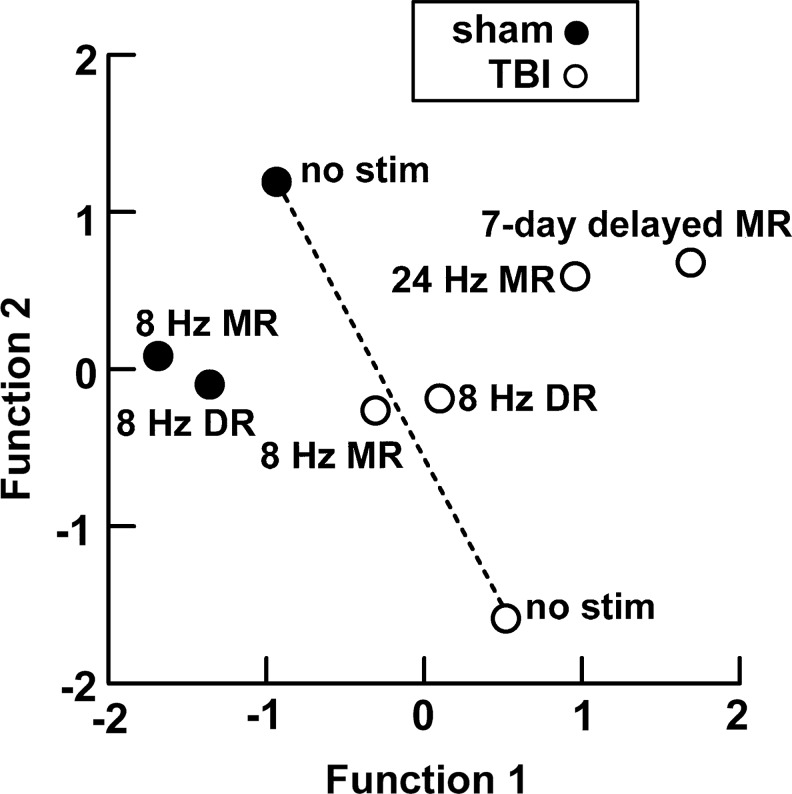
To summarize fundamental similarities and differences among the treatment groups, a discriminant function analysis was applied to 10 diverse variables from the behavioral data. For each categorical independent variable, the original dependent variables (i.e., experimental values) were combined into a defined smaller number of variables, ordered by the amount of the data variance that they incorporate. The reference memory task averaged over trial day 2 provided four variables (swim time, average speed, total distance, and percent time in the target quadrant), the working memory averaged over all trials provided three variables (swim time, average speed, total distance), the probe test provided two variables (distance traveled and percent time in the target quadrant), and the cylinder test provided one (asymmetry, as in Fig. 5). A total of 70 rats gave valid scores for all variables.
The first 7 canonical functions were determined and found to be highly significant by Wilks lambda (p=0.009), with 85.7% of the 70 cases correctly classified. The first two canonical functions accounted for 78.3% of total variance, the first three for 91.3%. To show the main relationships between the eight groups, their centroids are plotted in the plane of the first two canonical functions (Fig. 6). The centroids for 8 Hz DR and 8 Hz MR stimulation in injured rats lie close together and about halfway between non-stimulated sham injury and TBI-NS (the dashed recovery line in Fig. 6), suggesting a valid treatment yielding partial reversal of deficits. Higher frequency (24 Hz) MR stimulation and 7-day delayed MR (8 Hz) stimulation are further from this line, especially the latter, suggesting aberrant or inadequate treatment. The centroids for 8 Hz DR and MR stimulation in sham injury are also near each other, but are not near the recovery line and are further from the non-stimulated sham group. They are nevertheless in the same general region of the plane as the non-stimulated sham group.
FIG. 6.
Discriminant analysis. Group centroids plotted on the plane made by the first two functions derived from discriminant analysis of 10 behavioral parameters. Broken lines join the non-injured and injured non-stimulated groups. Scores had to be valid for all variables in each individual to be included in the analysis. Sample sizes of groups: sham, no stimulation (n=7); sham, dorsal raphe (DR) stimulation (n=4); sham, median raphe (MR) stimulation (n=9); traumatic brain injury (TBI), no stimulation (n=9); TBI, DR stimulation (n=12); TBI, 8 Hz MR stimulation (n=12); TBI, 24 Hz MR stimulation (n=12) TBI, 7-day delayed (8 Hz) MR stimulation (n=12).
Histological analysis of forebrain regions
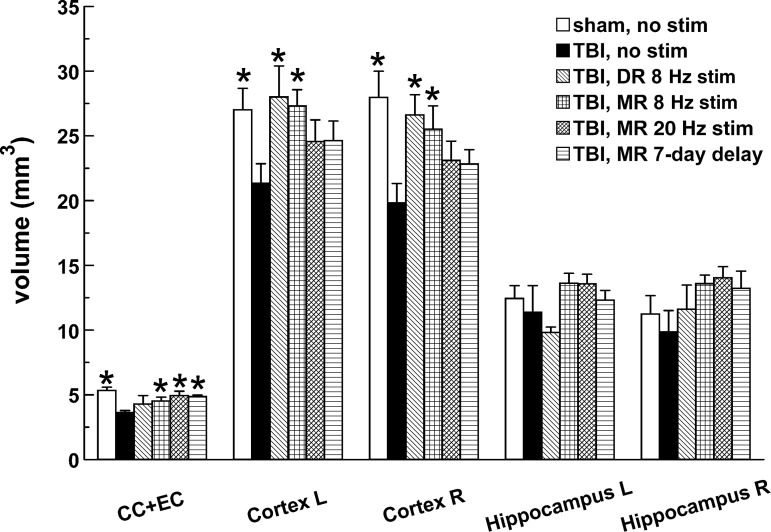
At the eighth week after TBI, non-stimulated rats showed significant reduction in the volumes of the left and right cortex and of the corpus callosum and external capsule combined (CC+EC), but not of the left or right hippocampus (Fig. 7). Losses in the left and right cortex were significantly reversed by early 8 Hz DR and MR stimulation. The CC+EC losses were reversed by all forms of MR stimulation, but not by DR stimulation.
FIG. 7.
Volume measurements in specific forebrain regions. Significant differences from the injured, non-stimulated group are marked by asterisks (p<0.05). Sample sizes of groups: sham, no stimulation (n=5); traumatic brain injury (TBI), no stimulation (n=7); TBI, dorsal raphe (DR) stimulation (n=5); TBI, 8 Hz median raphe (MR) stimulation (n=6); TBI, 24 Hz MR stimulation (n=6); TBI, 7-day delayed (8 Hz) MR stimulation (n=5). Of rats tested behaviorally, between two and six rats per group were omitted from the anatomical analysis because of losses in histological processing (perfusion or embedding). CC+EC, corpus callosum and external capsule.
Effect of stimulation on cAMP levels
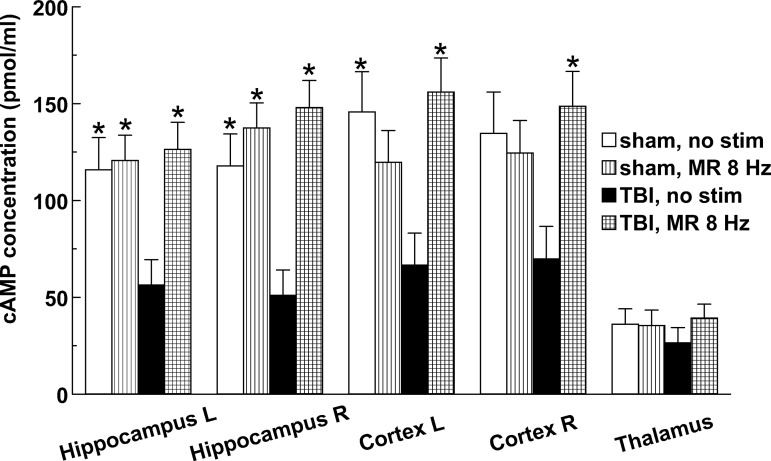
MR stimulation at 8 Hz was tested for its effect on cAMP levels 3 days after injury. This site and frequency were selected for examination because they produced the best overall behavioral restoration. The 3-day time point is nearly midway through the treatment given to rats tested behaviorally, and thus was selected as the optimal point for a single sample. After injury, cAMP levels in non-stimulated rats were <50% of normal (sham-injured) in cortex and hippocampus (reaching significance in three of the four regions), but the measured decline was weak and statistically insignificant in the thalamus (Fig. 8). MR stimulation returned the cAMP level bilaterally in cortex and hippocampus to slightly above normal in rats with TBI, but sham-injured rats showed no significant difference when stimulated (Fig. 8).
FIG. 8.
Effects of 2 h of median raphe (MR) stimulation on the concentration of cyclic adenosine monophosphate (cAMP) measured by enzyme-linked immunosorbent assay in selected forebrain regions 3 days after TBI or sham injury. Asterisks mark significant differences from the injured, non-stimulated group (p<0.05). Sample sizes of groups: sham, no stimulation (n=5); sham, 8 Hz MR stimulation (n=8); traumatic brain injury (TBI), no stimulation (n=8); TBI, 8 Hz MR stimulation (n=7).
Discussion
Restorative outcome of raphe stimulation
One week of midbrain raphe stimulation, starting either a few hours or 1 week after TBI, was effective in restoring one or more behavioral variables measured 5–6 weeks later. The main findings are summarized in Table 2. The most successful protocol, 8 Hz early MR stimulation, fully or partly restored forelimb reaching symmetry, reference memory, working memory, and the volume of the parietal-occipital cortex. These enduring effects occurred against a background of considerable spontaneous recovery, which is a function of the severity of injury.27 Thus, in the reference memory test, mean latencies were similar in all groups on the third trial day, including in sham and TBI non-stimulated animals, although acquisition of reference memory on the previous day had been slowed by the injury. Because the maximal incremental effect achievable with treatment was relatively modest, a more severe injury could perhaps have shown larger benefits and given a stronger verdict on clinical efficacy.
A large range of stimulation parameters were possible. The final choice was based on the following rationale. The current amplitude (-30 μA monopolar at 1 ms) was chosen to limit the spread of above-threshold current for cell body excitation to a radius of roughly 250 μm, as estimated by published threshold-distance relations for neurons in a hindbrain raphe region34 and elsewhere in the CNS.35,36 A fairly low stimulation frequency of 8 Hz was used in most treated groups, which was just at the higher limit of the range of firing of midbrain serotonergic neurons.37,38 Pauses of 5 min were alternated with 5-min bouts of stimulation, allowing recovery of serotonin release mechanisms, as suggested to be needed by real-time measurements of evoked serotonin release.39 Because firing in midbrain raphe neurons is related strongly to general arousal,40 a 12-h daily hiatus was imposed. Larger effects on recovery could perhaps have been produced with different stimulation parameters or electrode configurations, as well as with other start times and numbers of days of the treatment.
In univariate analyses of cognitive and motor performance (water maze trials and forelimb reaching during rearing movements, Figs. 3–5), there was no indication that MR or DR stimulation affected sham-injured animals. Although considerations of statistical power prevented direct comparison among sham groups in post-hoc analysis, they were clearly very similar in behavioral outcome. This finding is consistent with a lack of effect of 8 Hz MR stimulation on cAMP concentration in sham-injury (Fig. 8). However, the multivariate discriminant analysis using multiple parameters from each type of water maze trial indicates that 7 days of MR or DR stimulation did leave some residual effects on behavior in sham-injured rats (Fig. 6).
Physiologic, cellular, and molecular repair mechanisms
The MR and DR both send serotonergic axonal projections to widespread forebrain and brainstem regions. The MR tends to project more medially, prominently targeting the dorsal hippocampus, with weak innervation of the neocortex (except for perirhinal and entorhinal regions).16,17 The DR projects more strongly to the neocortex, sending little direct input to the hippocampus.41,42 Because long-term spatial memory requires the hippocampus43 but short-term cued learning is less strongly affected by hippocampal deficits,44,45 a larger effect was predicted for DR stimulation on working memory and for MR stimulation on reference memory. Yet the influence of DR stimulation was limited to reference memory, while MR stimulation (8 Hz, early post-injury) reversed both working and reference memory deficits.
There are reciprocal connections between the two nuclei,17,40 but some evidence suggests that they are not symmetrical functionally. Activating the DR with glutamate is reported to increase serotonin in the MR, but not vice versa.46 If the released serotonin has a predominantly inhibitory effect in the MR, for example, via 5-HT1a receptors, then this difference can account for the limited efficacy of DR compared with MR stimulation on recovery from TBI.
The differences in effects between low and high frequency MR stimulation (8 and 24 Hz) could stem from the presence of both serotonergic and non-serotonergic neurons in midbrain raphe nuclei. The upper bound of the normal firing range for serotonergic neurons is about 8 Hz, whereas non-serotonergic neurons in raphe nuclei can fire at higher frequencies.37,38 Thus, the serotonergic neurons may not have been able to follow the 24 Hz stimulation during an entire 5-min train. Low-frequency MR stimulation (0.5 Hz) but not high frequency (100 Hz) reportedly causes a state-dependent reset of phase of the theta rhythm,47 which is thought to increase stimulus-processing in hippocampal networks.48 (Williams, J.M. and Givens, B. 2003).
In cats, the firing rates of serotonergic raphe neurons are directly correlated with arousal, highest during waking, less during slow-wave sleep, and least during paradoxical sleep.40,49 Stimulation during 12 daylight hours for 1 week, rats being nocturnal, thus may have boosted neuronal activity in phases of lower activity. A significant fraction of neurons in the rat's DR, however, have been reported more recently to exhibit an inverse or more complex relationship with arousal.50 Furthermore, midbrain raphe stimulation resets these diurnal cycles,51 and injury may also affect them.52
A brain region controlling neural repair can be expected to mobilize the widest array of relevant cellular and molecular processes after injury. These could include protective physiological responses related to oxidative metabolism or brain swelling, in addition to corrective trophic responses. By the same token, no potent neurotrophic signaling pathway is likely to remain quiescent. cAMP is a well established signaling molecule,53 whose levels are increased by several of the major serotonin receptors, 5-HT4, 5-HT6, and 5-HT7.54,55 It was therefore selected for assay in this study. Depression of cAMP levels 24 h after parasagittal fluid percussion injury in the parietal cortex and hippocampus has been shown to be reversed by the phosphodiesterase IV inhibitor rolipram, which by 3 days had reduced local forebrain inflammation, axonal damage, and the cortical lesion volume.56 This is consistent with the present findings of MR-produced restoration of cAMP levels bilaterally in cortex and hippocampus at 3 days, leading to the anatomical improvement of bilateral cortical volume that was subsequently measured at 8 weeks post-injury.
Clinical implications of restoration by midbrain raphe nuclei
Deep brain stimulation (DBS) has been applied to neurotrauma in man rarely, and almost exclusively for concurrently reviving awareness in long-term comatose patients.57,58 Preclinical studies of restorative brain stimulation have been mainly concerned with plasticity in the motor cortex, which is amenable to non-invasive magnetic stimulation as well as to DBS.59,60 Unlike the raphe nuclei, however, this region is not divergent in output topography and so probably produces a much less general boost in functional recovery. Restorative DBS has the well known advantages of any form of DBS in comparison with systemic drug therapy, including confinement of activation to a few regions and the ability to titrate the dose or to stop completely.61 In the present experiments, neither the MR nor the DR caused an overt behavioral change or other adverse effect when stimulated, which is an added clinical advantage.
Restorative DBS furthermore offers the possibility of dispensing with permanent device implantation, because the electrode and stimulator electronics can be removed as soon as the maximum benefit has been produced. Indeed, the entire treatment may conceivably be given during hospitalization immediately after trauma. There are, however, neurosurgical risks associated with the acute condition—for example, from polytrauma or cerebral coagulopathy, that can be avoided if restorative DBS is begun in the sub-acute or chronic injury condition. Treatment after a 1-week delay in the present experiments proved much less effective than treatment started within hours of the injury, unfortunately. An important experimental question for the future is whether a different protocol for raphe stimulation, for example, prolonged application over many weeks, can yield improved function in older injuries. This would imply some lower but still exploitable level of responsiveness in cellular and molecular repair processes that remains beyond the first days or weeks after TBI.
Acknowledgments
The work described in this article was supported by the Department of Defense (CDMPRP grant W81XWH-08-1-0288) and State of Florida funding. We thank Jessie S. Truettner, William J. Moreno, Scott S. Burns, Allison Irvine, Clayton Jackson, and Lizbeth Manoah for technical assistance.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Jang S.H. Review of motor recovery in patients with traumatic brain injury. NeuroRehabilitation. 2009;24:349–353. doi: 10.3233/NRE-2009-0489. [DOI] [PubMed] [Google Scholar]
- 2.Wagner A.K. Zitelli K.T. A Rehabilomics focused perspective on molecular mechanisms underlying neurological injury, complications, and recovery after severe TBI. Pathophysiology. 2012 Mar 21; doi: 10.1016/j.pathophys.2012.02.007. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 3.Garcia A.N. Shah M.A. Dixon C.E. Wagner A.K. Kline A.E. Biologic and plastic effects of experimental traumatic brain injury treatment paradigms and their relevance to clinical rehabilitation. PM. R. 2011;3(Suppl. 1):S18–S27. doi: 10.1016/j.pmrj.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liepert J. Pharmacotherapy in restorative neurology. Curr. Opin. Neurol. 2008;21:639–643. doi: 10.1097/WCO.0b013e32831897a3. [DOI] [PubMed] [Google Scholar]
- 5.Richardson R.M. Singh A. Sun D. Fillmore H.L. Dietrich D.W., 3rd Bullock M.R. Stem cell biology in traumatic brain injury: effects of injury and strategies for repair. J. Neurosurg. 2010;112:1125–1138. doi: 10.3171/2009.4.JNS081087. [DOI] [PubMed] [Google Scholar]
- 6.Xiong Y. Mahmood A. Chopp M. Neurorestorative treatments for traumatic brain injury. Discov. Med. 2010;10:434–442. [PMC free article] [PubMed] [Google Scholar]
- 7.Bramlett H.M. Dietrich W.D. Progressive damage after brain and spinal cord injury: pathomechanisms and treatment strategies. Prog. Brain Res. 2007;161:125–141. doi: 10.1016/S0079-6123(06)61009-1. [DOI] [PubMed] [Google Scholar]
- 8.Avramescu S. Timofeev I. Synaptic strength modulation after cortical trauma: a role in epileptogenesis. J. Neurosci. 2008;28:6760–6772. doi: 10.1523/JNEUROSCI.0643-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prince D.A. Parada I. Scalise K. Graber K. Jin X. Shen F. Epilepsy following cortical injury: cellular and molecular mechanisms as targets for potential prophylaxis. Epilepsia. 2009;50(Suppl 2):30–40. doi: 10.1111/j.1528-1167.2008.02008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lenzlinger P.M. Morganti-Kossmann M.C. Laurer H.L. McIntosh T.K. The duality of the inflammatory response to traumatic brain injury. Mol Neurobiol. 2001;24:169–181. doi: 10.1385/MN:24:1-3:169. [DOI] [PubMed] [Google Scholar]
- 11.Ziebell J.M. Morganti-Kossmann M.C. Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics. 2010;7:22–30. doi: 10.1016/j.nurt.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cunningham E.T., Jr. Wada E. Carter D.B. Tracey D.E. Battey J.F. De Souza E.B. In situ histochemical localization of type I interleukin-1 receptor messenger RNA in the central nervous system, pituitary, and adrenal gland of the mouse. J. Neurosci. 1992;12:1101–1114. doi: 10.1523/JNEUROSCI.12-03-01101.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanjhan R. Housley G.D. Burton L.D. Christie D.L. Kippenberger A. Thorne P.R. Luo L. Ryan A.F. Distribution of the P2X2 receptor subunit of the ATP-gated ion channels in the rat central nervous system. J. Comp. Neurol. 1999;407:11–32. [PubMed] [Google Scholar]
- 14.Langhans W. Signals generating anorexia during acute illness. Proc Nutr Soc. 2007;66:321–330. doi: 10.1017/S0029665107005587. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura K. Li Y.Q. Kaneko T. Katoh H. Negishi M. Prostaglandin EP3 receptor protein in serotonin and catecholamine cell groups: a double immunofluorescence study in the rat brain. Neuroscience. 2001;103:763–775. doi: 10.1016/s0306-4522(01)00027-6. [DOI] [PubMed] [Google Scholar]
- 16.Molliver M.E. Serotonergic neuronal systems: what their anatomic organization tells us about function. J. Clin. Psychopharmacol. 1987;7(Suppl.):3S–23S. [PubMed] [Google Scholar]
- 17.Vertes R.P. Fortin W.J. Crane A.M. Projections of the median raphe nucleus in the rat. J. Comp. Neurol. 1999;407:555–582. [PubMed] [Google Scholar]
- 18.Azmitia E.C. Serotonin and brain: evolution, neuroplasticity, and homeostasis. Int. Rev. Neurobiol. 2007;77:31–56. doi: 10.1016/S0074-7742(06)77002-7. [DOI] [PubMed] [Google Scholar]
- 19.Berger M. Gray J.A. Roth B.L. The expanded biology of serotonin. Annu. Rev. Med. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turlejski K. Evolutionary ancient roles of serotonin: long-lasting regulation of activity and development. Acta Neurobiol. Exp. (Wars) 1996;56:619–636. doi: 10.55782/ane-1996-1167. [DOI] [PubMed] [Google Scholar]
- 21.Hobson S.A. Bacon A. Elliot-Hunt C.R. Holmes F.E. Kerr N.C. Pope R. Vanderplank P. Wynick D. Galanin acts as a trophic factor to the central and peripheral nervous systems. EXS. 2010;102:25–38. doi: 10.1007/978-3-0346-0228-0_3. [DOI] [PubMed] [Google Scholar]
- 22.Hökfelt T. Xu Z.Q. Shi T.J. Holmberg K. Zhang X. Galanin in ascending systems. Focus on coexistence with 5-hydroxytryptamine and noradrenaline. Ann. N. Y. Acad. Sci. 1998;863:252–263. doi: 10.1111/j.1749-6632.1998.tb10700.x. [DOI] [PubMed] [Google Scholar]
- 23.Hentall I.D. Burns S.B. Restorative effects of stimulating medullary raphe after spinal cord injury. J. Rehabil. Res. Dev. 2009;46:109–122. [PubMed] [Google Scholar]
- 24.Hentall I.D. Gonzalez M.M. Promotion of recovery from thoracic spinal cord contusion in rats by stimulation of medullary raphe or its midbrain input. Neurorehabil. Neural. Repair. 2012;26:374–384. doi: 10.1177/1545968311425178. [DOI] [PubMed] [Google Scholar]
- 25.Bramlett H.M. Green E.J. Dietrich W.D. Hippocampally dependent and independent chronic spatial navigational deficits following parasagittal fluid percussion brain injury in the rat. Brain Res. 1997;762:195–202. doi: 10.1016/s0006-8993(97)00387-9. [DOI] [PubMed] [Google Scholar]
- 26.Thompson H.J. Lifshitz J. Marklund N. Grady M.S. Graham D.I. Hovda D.A. McIntosh T.K. Lateral fluid percussion brain injury: a 15-year review and evaluation. J. Neurotrauma. 2005;22:42–75. doi: 10.1089/neu.2005.22.42. [DOI] [PubMed] [Google Scholar]
- 27.Sanders M.J. Dietrich W.D. Green E.J. Cognitive function following traumatic brain injury: effects of injury severity and recovery period in a parasagittal fluid-percussive injury model. J. Neurotrauma. 1999;16:915–925. doi: 10.1089/neu.1999.16.915. [DOI] [PubMed] [Google Scholar]
- 28.D'Hooge R. De Deyn P.P. Applications of the Morris water maze in the study of learning and memory. Brain Res. Brain Res. Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- 29.Hamm R.J. Temple M.D. Pike B.R. O'Dell D.M. Buck D.L. Lyeth B.G. Working memory deficits following traumatic brain injury in the rat. J. Neurotrauma. 1996;13:317–323. doi: 10.1089/neu.1996.13.317. [DOI] [PubMed] [Google Scholar]
- 30.Schallert T. Fleming S.M. Leasure J.L. Tillerson J.L. Bland S.T. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- 31.Vazey E.M. Chen K. Hughes S.M. Connor B. Transplanted adult neural progenitor cells survive, differentiate and reduce motor function impairment in a rodent model of Huntington's disease. Exp. Neurol. 2006;199:384–396. doi: 10.1016/j.expneurol.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 32.Dietrich W.D. Ginsberg M.D. Busto R. Effect of transient cerebral ischemia on metabolic activation of a somatosensory circuit. J. Cereb. Blood Flow Metab. 1986;6:405–413. doi: 10.1038/jcbfm.1986.73. [DOI] [PubMed] [Google Scholar]
- 33.Passineau M.J. Zhao W. Busto R. Dietrich W.D. Alonso O. Loor J.Y. Bramlett H.M. Ginsberg M.D. Chronic metabolic sequelae of traumatic brain injury: prolonged suppression of somatosensory activation. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H924–931. doi: 10.1152/ajpheart.2000.279.3.H924. [DOI] [PubMed] [Google Scholar]
- 34.Hentall I.D. Zorman G. Kansky S. Fields H.L. Relations among threshold, spike height, electrode distance, and conduction velocity in electrical stimulation of certain medullospinal neurons. J. Neurophysiol. 1984;51:968–977. doi: 10.1152/jn.1984.51.5.968. [DOI] [PubMed] [Google Scholar]
- 35.Ranck J.B., Jr Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res. 1975;98:417–440. doi: 10.1016/0006-8993(75)90364-9. [DOI] [PubMed] [Google Scholar]
- 36.Shannon R.V. Moore J.K. McCreery D.B. Portillo F. Threshold-distance measures from electrical stimulation of human brainstem. IEEE Trans Rehabil Eng. 1997;5:70–74. doi: 10.1109/86.559351. [DOI] [PubMed] [Google Scholar]
- 37.Allers K.A. Sharp T. Neurochemical and anatomical identification of fast- and slow-firing neurones in the rat dorsal raphe nucleus using juxtacellular labelling methods in vivo. Neuroscience. 2003;122:193–204. doi: 10.1016/s0306-4522(03)00518-9. [DOI] [PubMed] [Google Scholar]
- 38.Kocsis B. Varga V. Dahan L. Sik A. Serotonergic neuron diversity: identification of raphe neurons with discharges time-locked to the hippocampal theta rhythm. Proc. Natl. Acad. Sci. U. S. A. 2006;103:1059–1064. doi: 10.1073/pnas.0508360103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hentall I.D. Pinzon A. Noga B.R. Spatial and temporal patterns of serotonin release in the rat's lumbar spinal cord following electrical stimulation of the nucleus raphe magnus. Neuroscience. 2006;142:893–903. doi: 10.1016/j.neuroscience.2006.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacobs B.L. Azmitia E.C. Structure and function of the brain serotonin system. Physiol. Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- 41.Vertes R.P. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J. Comp. Neurol. 1991;313:643–668. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- 42.Vertes R.P. Kocsis B. Projections of the dorsal raphe nucleus to the brainstem: PHA-L analysis in the rat. J. Comp. Neurol. 1994;340:11–26. doi: 10.1002/cne.903400103. [DOI] [PubMed] [Google Scholar]
- 43.Morris R.G. Garrud P. Rawlins J.N. O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 44.Sutherland R.J. McDonald R.J. Hill C.R. Rudy J.W. Damage to the hippocampal formation in rats selectively impairs the ability to learn cue relationships. Behav. Neural Biology. 1989;52:331–356. doi: 10.1016/s0163-1047(89)90457-3. [DOI] [PubMed] [Google Scholar]
- 45.Whishaw I.Q. Kolb B. Decortication abolishes place but not cue learning in rats. Behav. Brain Res. 1984;11:123–134. doi: 10.1016/0166-4328(84)90135-9. [DOI] [PubMed] [Google Scholar]
- 46.Mokler D.J. Dugal J.R. Hoffman J.M. Morgane P.J. Functional interrelations between nucleus raphe dorsalis and nucleus raphe medianus: a dual probe microdialysis study of glutamate-stimulated serotonin release. Brain Res. Bull. 2009;78:132–138. doi: 10.1016/j.brainresbull.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jackson J. Dickson C.T. Bland B.H. Median raphe stimulation disrupts hippocampal theta via rapid inhibition and state-dependent phase reset of theta-related neural circuitry. J. Neurophysiol. 2008;99:3009–3026. doi: 10.1152/jn.00065.2008. [DOI] [PubMed] [Google Scholar]
- 48.Williams J.M. Givens B. Stimulation-induced reset of hippocampal theta in the freely performing rat. Hippocampus. 2003;13:109–116. doi: 10.1002/hipo.10082. [DOI] [PubMed] [Google Scholar]
- 49.Trulson M.E. Jacobs B.L. Raphe unit activity in freely moving cats: correlation with level of behavioral arousal. Brain Res. 1979;163:135–150. doi: 10.1016/0006-8993(79)90157-4. [DOI] [PubMed] [Google Scholar]
- 50.Urbain N. Creamer K. Debonnel G. Electrophysiological diversity of the dorsal raphe cells across the sleep-wake cycle of the rat. J. Physiol. 2006;573:679–695. doi: 10.1113/jphysiol.2006.108514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meyer-Bernstein E.L. Morin L.P. Electrical stimulation of the median or dorsal raphe nuclei reduces light-induced FOS protein in the suprachiasmatic nucleus and causes circadian activity rhythm phase shifts. Neuroscience. 1999;92:267–279. doi: 10.1016/s0306-4522(98)00733-7. [DOI] [PubMed] [Google Scholar]
- 52.Mathias J.L. Alvaro P.K. Prevalence of sleep disturbances, disorders, and problems following traumatic brain injury: A meta-analysis. Sleep Med. 2012;13:898–905. doi: 10.1016/j.sleep.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 53.Spencer T. Filbin M.T. A role for cAMP in regeneration of the adult mammalian CNS. J. Anat. 2004;204:49–55. doi: 10.1111/j.1469-7580.2004.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kitson S.L. 5-hydroxytryptamine (5-HT) receptor ligands. Curr. Pharm. Des. 2007;13:2621–2637. doi: 10.2174/138161207781663000. [DOI] [PubMed] [Google Scholar]
- 55.Raymond J.R. Mukhin Y.V. Gelasco A. Turner J. Collinsworth G. Gettys T.W. Grewal J.S. Garnovskaya M.N. Multiplicity of mechanisms of serotonin receptor signal transduction. Pharmacol. Ther. 2001;92:179–212. doi: 10.1016/s0163-7258(01)00169-3. [DOI] [PubMed] [Google Scholar]
- 56.Atkins C.M. Oliva A.A., Jr. Alonso O.F. Pearse D.D. Bramlett H.M. Dietrich W.D. Modulation of the cAMP signaling pathway after traumatic brain injury. Exp. Neurol. 2007;208:145–158. doi: 10.1016/j.expneurol.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schiff N.D. Giacino J.T. Kalmar K. Victor J.D. Baker K. Gerber M. Fritz B. Eisenberg B. Biondi T. O'Connor J. Kobylarz E.J. Farris S. Machado A. McCagg C. Plum F. Fins J.J. Rezai A.R. Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature. 2007;448:600–603. doi: 10.1038/nature06041. [DOI] [PubMed] [Google Scholar]
- 58.Yamamoto T. Katayama Y. Deep brain stimulation therapy for the vegetative state. Neuropsychol. Rehabil. 2005;15:406–413. doi: 10.1080/09602010443000353. [DOI] [PubMed] [Google Scholar]
- 59.Adkins-Muir D.L. Jones T.A. Cortical electrical stimulation combined with rehabilitative training: enhanced functional recovery and dendritic plasticity following focal cortical ischemia in rats. Neurol. Res. 2003;25:780–788. doi: 10.1179/016164103771953853. [DOI] [PubMed] [Google Scholar]
- 60.Jackson A. Mavoori J. Fetz E.E. Long-term motor cortex plasticity induced by an electronic neural implant. Nature. 2006;444:56–60. doi: 10.1038/nature05226. [DOI] [PubMed] [Google Scholar]
- 61.Kringelbach M.L. Jenkinson N. Owen S.L. Aziz T.Z. Translational principles of deep brain stimulation. Nat. Rev. Neurosci. 2007;8:623–635. doi: 10.1038/nrn2196. [DOI] [PubMed] [Google Scholar]