Abstract
Lung function abnormalities both at rest and during exercise are frequently observed in patients with chronic heart failure, also in the absence of respiratory disease. Alterations of respiratory mechanics and of gas exchange capacity are strictly related to heart failure. Severe heart failure patients often show a restrictive respiratory pattern, secondary to heart enlargement and increased lung fluids, and impairment of alveolar-capillary gas diffusion, mainly due to an increased resistance to molecular diffusion across the alveolar capillary membrane. Reduced gas diffusion contributes to exercise intolerance and to a worse prognosis. Cardiopulmonary exercise test is considered the “gold standard” when studying the cardiovascular, pulmonary, and metabolic adaptations to exercise in cardiac patients. During exercise, hyperventilation and consequent reduction of ventilation efficiency are often observed in heart failure patients, resulting in an increased slope of ventilation/carbon dioxide (VE/VCO2) relationship. Ventilatory efficiency is as strong prognostic and an important stratification marker. This paper describes the pulmonary abnormalities at rest and during exercise in the patients with heart failure, highlighting the principal diagnostic tools for evaluation of lungs function, the possible pharmacological interventions, and the parameters that could be useful in prognostic assessment of heart failure patients.
1. Introduction
Not only heart is involved in chronic heart failure but also lung, kidney, peripheral and respiratory muscles, chemo-ergoreceptors, neurohormonal mechanisms, mitochondria, all play a major role in determining the complex clinical syndrome of chronic heart failure. Indeed, energy deficit is a relevant contributor to the development of cardiac and skeletal myopathy. In heart failure several functions of muscle bioenergetics are altered such as oxygen availability, substrate oxidation, ATP production by the mitochondria, and transfer to contractile apparatus [1]. Notably, the clinical syndrome of heart failure is characterized by symptoms apparently unrelated or partially related to the heart, such as fatigue, dyspnea, anxiety, and exercise intolerance.
Dyspnea, either at rest or during exercise, is one of the main symptoms in heart failure. Indeed, the most often used heart failure grading methodology, the NYHA classification, is based on dyspnea. Dyspnea is the result of a neurological reconstruction of an abnormal physiological condition characterized by hyperventilation and by high ventilation to metabolic demand ratio. This leads to a reduced ventilatory efficiency, which is physiologically defined as the amount of ventilation needed to eliminate a given amount of CO2. The excess of ventilation is due to an increase of dead space/tidal volume (VD/VT) ventilation and of ventilatory drive from peripheral chemo- and ergoreceptors. The reduced ventilatory efficiency during exercise is used more and more as a prognostic marker in heart failure. In addition, an improvement of the efficiency of ventilation is among the goals of heart failure therapy. The following review will examine the role of different mechanisms underlying ventilatory efficiency evaluated with spirometry, lung gas diffusion measurement, and cardiopulmonary exercise test in heart failure patients.
2. Lung Abnormalities at Rest
Pulmonary abnormalities are part of the heart failure syndrome, as both lung mechanics and alveolar-capillary gas exchange are impaired [2–5]. In heart failure, pulmonary abnormalities may be due to respiratory comorbidities but also to heart failure itself. Therefore, standard spirometry and resting lung diffusion for carbon monoxide (DLCO) [6] provide an integrated evaluation of the respiratory function and should be performed in all heart failure patients. Moreover, it may be useful to split DLCO into its two subcomponents either using the classic Roughton and Forster method or nitric oxide lung diffusion. Accordingly, Dm, the resistance to molecular diffusion of carbon monoxide across the alveolar-capillary membrane, and Vcap, the resistance to carbon monoxide binding to hemoglobin, the so-called pulmonary capillary blood volume, can be calculated [7].
2.1. Spirometry
Spirometry is the preliminary pulmonary function test to assess respiratory mechanics.
Wasserman et al. [5] showed in a large multicenter study that, at rest, forced expiratory volume in the first second and vital capacity are either normal or proportionately reduced in heart failure. More recently, Agostoni et al. [8] demonstrated that pulmonary function at rest is usually normal in patients with moderate heart failure, while a restrictive lung disease is observed in 50% of patients with severe heart failure (Figure 1). Cardiac enlargement in heart failure appears to be involved in causing restrictive lung pattern [4, 9]. Indeed, a negative correlation between cardiac size, as analyzed by the cardiothoracic index at chest X-ray, and lung function parameters, including alveolar volume, has been described [4]. Notably, Palermo et al. [10] showed that, in heart failure, pulmonary function varies in relationship with the position of the body, being worst in the lateral decubitus. The larger the heart, the greater the difference in lung function between the sitting position and the lateral decubitus. Moreover, McCormack [11] showed that the restrictive lung disease secondary to severe heart failure seems to be completely reversible after cardiac transplantation, with an increase in forced vital capacity after transplantation, directly correlated with the decrease in cardiac volume.
Figure 1.
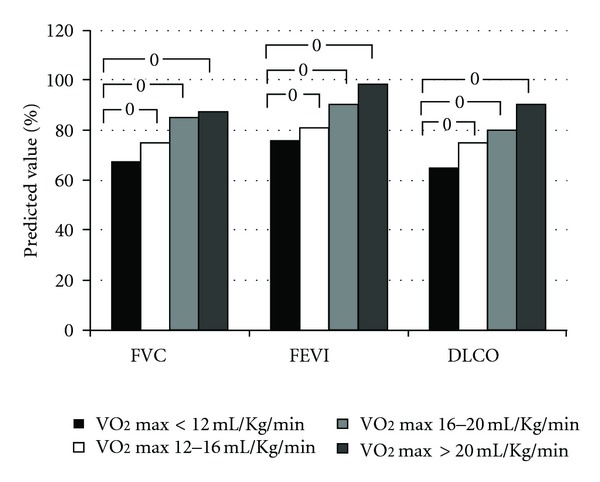
Lung function at rest in 190 heart failure patients in stable clinical condition. Patients were grouped according to exercise capacity. From left to right: peak VO2 < 12 mL/min/kg (black bars), peak VO2 = 12–16 mL/min/kg (white bars), peak VO2 = 16–20 mL/min/kg (grey bars), and peak VO2 > 20 mL/min/kg (dashed bars). FVC: forced vital capacity; FEV1: forced expiratory volume 1 second; DLCO: lung diffusion for carbon monoxide. Data from [8, Table 1].
2.2. Lung Diffusion
Alterations of respiratory mechanics and of gas exchange capacity are strictly related in heart failure patients. Reduction in the pulmonary DLCO is well documented in heart failure [3, 13–15]. The acute pathogenetic mechanisms of lung diffusion abnormalities in heart failure are related to interstitial edema, alveolar-capillary membrane hydrostatic injury, and altered alveolar fluid clearance. These mechanisms result in a remodeling process that causes a persistent reduction in alveolar-capillary membrane conductance and lung diffusion capacity. Indeed, Mettauer et al. [16] demonstrated that DLCO is only partially restored after cardiac transplantation. DLCO improves less in patients with long standing heart failure. This implies that in heart failure the alveolar-capillary membrane undergoes changes that are only partially reversible with heart failure treatment [17].
Reduced Dm is the main component of impaired pulmonary gas transfer in heart failure [18]. Puri et al. [18] demonstrated that Dm decreases and Vcap increases in relationship with the severity of the disease. The Vcap increase was interpreted as a compensatory mechanism aimed, by pulmonary vessel recruitment, at preserving alveolar-capillary diffusion. Vcap tends to increase in patients with stable heart failure, but could decrease in the advanced stages [19]. Agostoni et al. [8] showed that in severe heart failure in stable clinical condition there are few alveolar-capillary units at work (low alveolar volume), characterized by greater efficiency (high Dm/Vcap ratio) compared to alveolar-capillary units in intermediate chronic heart failure severity. However, the physiological mechanism behind this phenomenon is still undefined. Indeed, Vcap is related to the amount of hemoglobin participating in gas exchange, which, on its turn, depends from hemoconcentration, cardiac output, and the amount of capillary vessels in the ventilated airways. The latter is related to pulmonary venous pressure.
In heart failure, the alveolar-capillary membrane surface area available for gas exchange is reduced and partially responsible for low Dm. Furthermore, Dm reduction remains even after correction for alveolar volume (Dm/VA) [18]. In fact, Dm is affected by several factors including interstitial edema that increases the distance between alveolar gas and red blood cells, fibrosis, inability to further activate the pump mechanism at the alveolar surface which enhances chlorine and sodium transport [20], and peribronchial edema that may reduce ventilation to some lung units. Moreover, there is a strict correlation between hemodynamics and Dm, as proven by the observation that Dm decreases after infusion of a small amount of saline (150 mL) [21] or after exercise [22, 23].
Nevertheless, we must underline that, albeit several heart failure models have been prepared to study the correlation between increased congestion of lung interstitial space and pulmonary function, a reliable model of lung function abnormalities mimicking those present in heart failure does not exist. Studies in healthy humans [24–26] demonstrated a reduced vital capacity, forced expiratory volume, and total lung capacity with a preserved DLCO after a rapid increase fluid content. This observation is consistent with some clinical findings. For instance, ultrafiltration, which acutely reduces the congestion of lung interstitium, improves lung mechanics but not DLCO in heart failure [17, 27].
DLCO is a limiting factor for exercise performance [28, 29], which improves (below sea level, as in the Dead Sea) or worsens at different altitudes more in heart failure patients with reduced DLCO compared to subjects with a normal DLCO [30, 31]. DLCO and Dm abnormalities have a relevant prognostic capacity in heart failure [32], although we do not know whether an improvement of DLCO with treatment is associated with an improvement of prognosis.
More recently, a new biomarker of alveolar-capillary membrane damage has been described [33, 34]. Increased circulating plasma levels of surfactant protein B have been reported in heart failure patients with a good correlation with heart failure severity. The mature form of surfactant protein B plays a crucial role in the formation and stabilization of pulmonary surfactant film. The level of surfactant protein B correlates with lung diffusion as well as peak VO2 and ventilation versus CO2 production (VE/VCO2) slope [34]. The clinical applicability of this biomarker is unclear at present.
3. Lung Abnormalities during Exercise
Cardiopulmonary exercise test (CPET) with incremental increases in workload is considered as the “gold standard” when studying the cardiovascular, pulmonary, and metabolic adaptations to exercise in cardiac patients.
Traditionally, ventilatory limitation to exercise is assessed by measuring the breathing reserve calculated as the difference between minute ventilation at peak exercise and maximal voluntary ventilation or some estimate of the maximal voluntary ventilation (typically the forced expiratory volume in the first second multiplied by 35 or 40). Any difference >15 L/min or breathing reserve >20–40% is interpreted as consistent with exercise not being limited by ventilation [35]. In normal individuals, there is a progressive increase of ventilation (VE) during exercise, due to both VT and respiratory rate increase. The increase in VT mainly occurs at the beginning of exercise, whereas respiratory rate typically increases more toward peak exercise.
CPET reveals an increased VE, at comparable levels of effort, in heart failure patients with respect to age-matched normal individuals [36]. At a given work rate, heart failure patients show a higher VE than normal subjects, the result of an exaggerated respiratory rate response, and a truncated VT response [5] (Figure 2).
Figure 2.
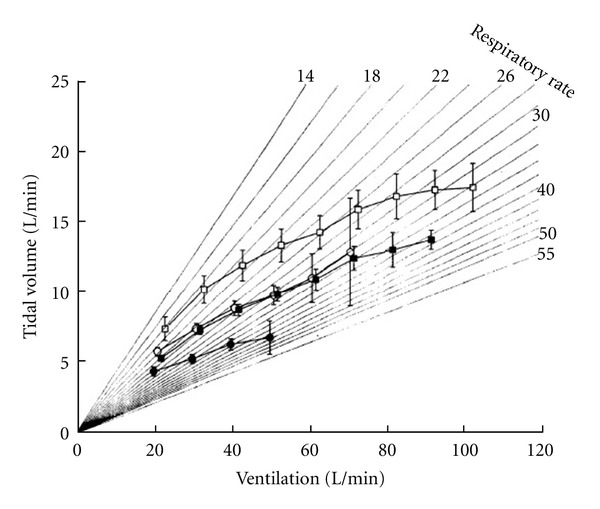
Tidal volume versus ventilation in patients with severe heart failure (peak VO2 < 12 mL/min/kg, black circles), moderate to severe heart failure (peak VO2 12–16 mL/min/kg, black squares), moderate (peak VO2 > 16 mL/min/kg, empty circles), and normal subjects (empty squares). From [5]. Reproduced with permission.
Ventilatory response may be abnormal during exercise despite normal breathing reserve as showed in different settings and in heart failure patients [12, 37, 38]. Lung hyperinflation and expiratory flow limitation can cause fatigue of the inspiratory muscles; therefore, it becomes clear that breathing reserve is insufficient for a comprehensive and precise assessment of the contribution of the respiratory system to physical exercise in physiologic and disease conditions. Indeed, diminished respiratory muscle strength has been demonstrated in heart failure patients. Mouth inspiratory and expiratory pressures are reduced in heart failure compared to normal subjects, and they seem to be correlated with exercise capacity [39]. Moreover, inspiratory muscle strength has independent prognostic value in heart failure. The results of trials with inspiratory muscle training [40] indicate that this intervention improves exercise capacity and quality of life in heart failure. Some benefit from muscle training may be accounted for by the attenuation of the inspiratory muscle metaboreflex. Furthermore, inspiratory muscle training results in improved cardiovascular responses to exercise. These findings suggest that routine screening for intercostal muscle weakness is advisable in patients with heart failure and specific inspiratory muscle training and/or aerobic training are of practical value in the management of these patients.
More detailed information about ventilatory abnormalities during exercise in heart failure is provided by analysis of the spontaneous expiratory flow-volume loop relative to the maximal forced curve. Expiratory flow limitation is reached when the right upper corner of the flow-volume loop curve is very close to the maximal flow volume curve registered at rest. When this happens, subjects stop the effort or proceed through the exercise but the flow volume curve shifts to the left, away from the functional residual capacity, in a region where the cost of breathing increases. Johnson et al. [41] and Agostoni et al. [12] showed that in heart failure, because of lung stiffness, the spontaneous flow-volume loop reaches the maximal flow-volume loop and the expiratory flow reserve is dramatically reduced, requiring an increase in end-inspiratory lung volume. An increase in end-inspiratory lung volume raises work of breathing and decreases inspiratory endurance time [41]. In addition, after exercise in healthy subjects, the maximal flow-volume loop is increased due a bronchodilation induced by exercise; this is not the case in heart failure [42]. Figure 3 describes an example of flow-volume curves' behavior during exercise in a normal subject and in a heart failure patient. Moreover, Bussotti et al. [43] showed that maximal flow-volume loops maneuver does not interfere with the main functional parameters used for the interpretation of CPET. Consequently, with a single CPET both flow-volume curve and ventilation efficiency (VE/VCO2 slope) can be evaluated.
Figure 3.
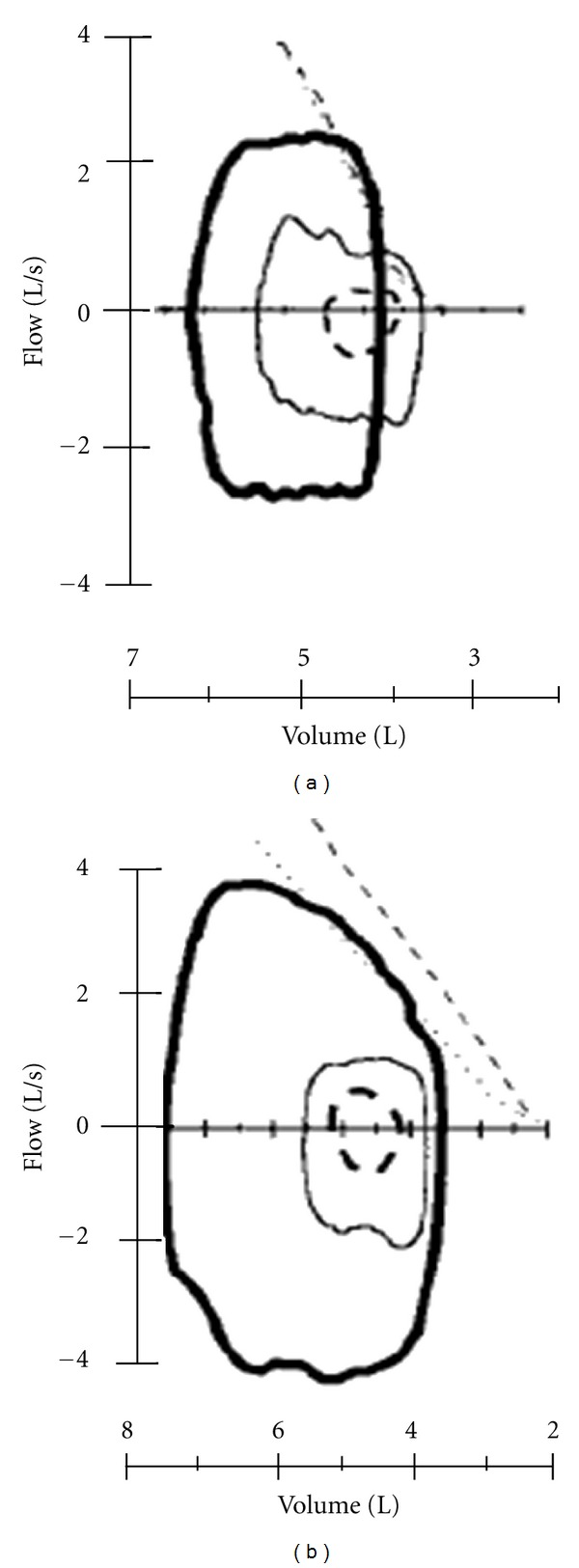
Tidal flow-volume loops at rest (dashed lines) at 40% of maximal ventilation (thin solid lines), and at maximum exercise (thick solid lines) in typical heart failure (a) and normal (b) subjects. The 2 oblique lines on flow-volume loops are partial forced expiratory flows recorded at rest (dotted line) and at maximum exercise (dashed line). From [12].
3.1. Ventilation versus VCO2
In normal subjects the relation of VE versus VCO2 is characterized during CPET progressive work load increase by three linear relationships. The three slopes are progressively steeped. This is due to the change in the functional parameters governing the VE versus VCO2 relationship and specifically VO2, VCO2, and pH [44]. Clinically the VE versus VCO2 slope is measured from the first minute after the beginning of loaded pedaling to the end of isocapnic period (Figure 4), albeit some authors [45] suggest to measure the slope obtained considering the entire exercise as a single linear relationship. Differently the VE/VCO2 ratio declines at the beginning of exercise, reaches a plateau, and increases in the last part of exercise, when metabolic acidosis becomes unbuffered. In heart failure exercise is characterized by hyperventilation, which is likely due to several causes, including alteration of lung mechanics, reduced lung diffusion, increased CO2 production due to early lactic acidosis, increased VD, decreased ventilatory efficiency, and overactive reflexes from metaboreceptors, baroreceptors, and chemoreceptors. In other words, in heart failure patients, besides abnormalities in the lung, either due to mechanics or to gas exchange, also ventilatory control is altered. The latter is likely a part of deranged cardiorespiratory reflex control. Indeed, a direct link between exercise hyperventilation and impaired reflexes which control heart rate and blood pressure has been convincingly proposed [46] providing the physiological base of the link between hyperventilation and poor prognosis in heart failure. Ventilatory efficiency is best defined by the relationship of the amount of ventilation required to eliminate a given amount of CO2. Efficiency of ventilation can be expressed as a slope of the VE/VCO2 relationship or as VE/VCO2 ratio measured either at the anaerobic threshold or as the lowest value recorded during exercise. The modified alveolar equation [44] concisely describes the determinants of the steepness which VE rises with respect to VCO2:
| (1) |
where [K/PaCO2 × (1 − VD/VT)] is the slope, K is constant to adjust for standard temperature pressure dry and body temperature pressure saturated and to convert fractional concentrations to pressures, PaCO2 is partial pressure of CO2 in arterial blood, and VD/VT is the fraction of VT that goes to VD. This equation is linear over a wide range of exercise. Figure 4 describes a theoretical example of VE/VCO2 slope in a normal subject and in a patient with heart failure. If PaCO2 is driven down by a high ventilatory drive from peripheral chemoreceptors or by ergoreceptors in skeletal muscles, the slope of the VE/VCO2 will increase as well as if VD/VT is high. Little is known about chemosensitivity in heart failure. It has been documented that central hypercapnic chemo-sensitivity is enhanced in patients with heart failure with central sleep apnea [47]. Chua et al. [48] demonstrated that there is increased hypoxic and central hypercapnic chemo-sensitivity in patients with heart failure, and that its suppression with dihydrocodeine is associated with a reduction of exercise ventilation, an improvement in exercise tolerance, and a decrease in breathlessness [49]. Piepoli et al. [50] showed that muscle reflex (ergoreflex) has an important effect on the ventilatory responses to exercise in heart failure compared to control subjects, and that training may reduce this exaggerated ergoreflex activity, thereby improving the response to exercise. The increase in reflex sensitivity may serve as a compensatory mechanism producing an increase in ventilatory response during exercise and thereby preserving blood gas homeostasis, also maintaining arterial oxygen concentration. Studies analyzing the effect of drugs interfering with chemo-sensitivity are on-going, and no data are available at present.
Figure 4.
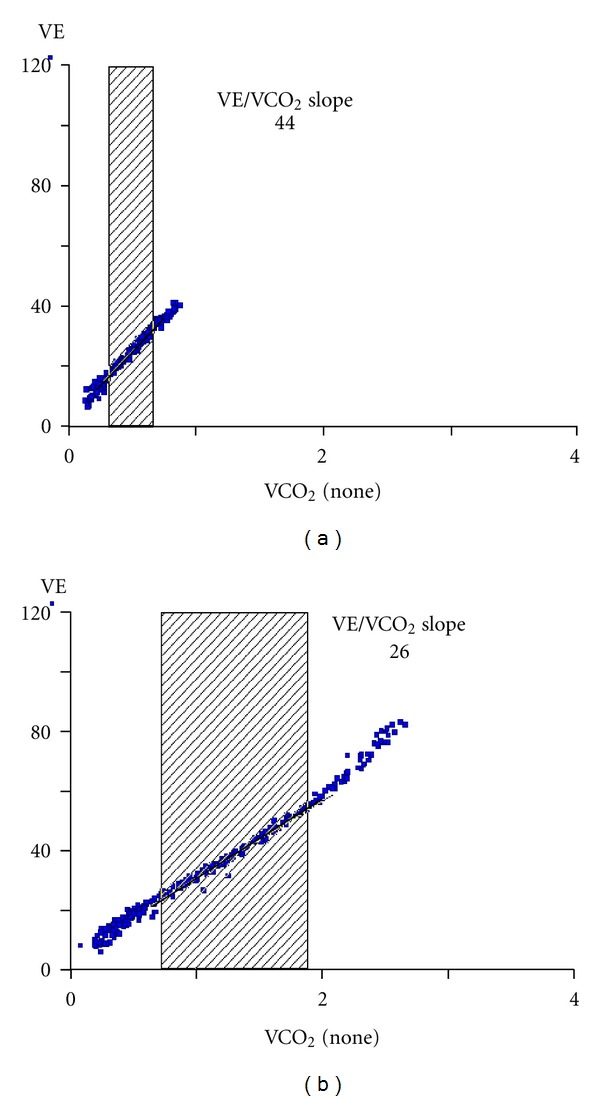
VE/VCO2 slope in an heart failure patient (a) and an healthy control (b).
In normal subjects, VE/VCO2 relationship, for incremental exercise, is normally linear up to the “respiratory compensation point,” when ventilatory compensation begins in response to metabolic (lactic) acidosis. Over the linear phase of the VE/VCO2 relationship, below the respiratory compensation point, the profile of VE/VCO2 closely reflects that of VD/VT, providing information on ventilatory efficiency. In heart failure, hyperventilation is associated with an increased VD/VT and VCO2 and a lower PaCO2 when compared to similar normal subjects at a similar percent of VO2 peak [5]. Patients with heart failure often have a reduced tidal volume at heavy exercise [5], which would increase the VD/VT ratio; however, Buller and Poole-Wilson [51] showed that the increased ventilatory response to exercise in patients with heart failure is largely caused by mechanisms other than increased ventilation of anatomical VD [51]. In heart failure patients, in the absence of coexisting lung disease, this pattern of a high VD/VT ratio with normal arterial blood gases suggests that nonuniformity of ventilation/perfusion (V/Q) ratios in the lung is more likely caused by increased non-uniformity of perfusion than of ventilation [52]. A totally different behavior of the VE/VCO2 relationship is likely when respiratory comorbidities, such as emphysema, are present [53, 54].
The normal VE/VCO2 at the nadir is between 25 and 35. Normal values for these ventilatory equivalents with an end-tidal of CO2 (PetCO2) of approximately 40 mmHg suggest a normal VD/VT and uniform matching of V/Q [44].
In recent years, the VE/VCO2 slope has gained notoriety in the heart failure population as an outstanding prognostic marker. Several studies report the VE/VCO2 slope to be prognostically superior to peak VO2 to predict mortality [55–60]. Arena et al. [55] built a classificatory system based on exercise VE/VCO2 slope to stratify the risk of major cardiovascular events in heart failure. Corrà et al. [56] used VE/VCO2 slope (with a cutoff >34) for efficient prognostic stratification in patients with moderate to severe heart failure (defined as VO2/kg peak 10–18 mL/kg/min). Ponikowski et al. [60] showed that, even in heart failure patients with normal exercise performance and peak VO2 >18 mL/kg/min, abnormal exercise ventilation significantly discriminates survival. Guazzi et al. [61] suggested that the VE/VCO2 slope has a remarkable value for risk stratification even in patients with diastolic heart failure.
Another way to assess ventilation during exercise is to analyze the relationship between end-tidal CO2 pressure (PetCO2) and VE during the isocapnic buffering period [44, 62]. At sea level, during isocapnic buffering period, there is a straight relationship between CO2 set point and ventilation. PetCO2, equivalent to PaCO2, during the isocapnic buffering period, is around 40 mmHg in normal subjects, higher in athletes [63] and lower in heart failure patients. Guazzi et al. [64] suggested that a low peak PaCO2 (and consequently a low peak PetCO2 and an elevated VD/VT) is a strong independent predictor of mortality in stable heart failure, and that a low peak PaCO2 is the most significant determinant of the prognostic value of a steep VE/VCO2 slope. Another ventilation-related index with a prognostic value in heart failure is Oxygen Uptake Efficiency Slope (OUES). OUES is derived from the relation between oxygen uptake (VO2 L/min) and VE (L/min) during incremental exercise. OUES is determined by the linear relation of VO2 (y-axis) versus the logarithm of VE (x-axis) during exercise [65].
The following equation was used to determine the relation between VO2 and VE:
| (2) |
The differential of this equation by VE yields is
| (3) |
where a is the constant that represents the rate of increase in VO2 in response to VE. We define the constant “a” as the OUES. Baba et al. [65] emphasized the value of OUES as a submaximal, effort-independent, and objective parameter to estimate cardiorespiratory functional reserve, and they reported that OUES strongly correlates with VO2max(r = 0.941).
Myers et al. [66], when defining a CPET score for predicting outcomes in heart failure, considered OUES to be a stronger predictor of risk than peak VO2. Davies et al. [67] claimed OUES as a prognostic marker in heart failure patients. Sun et al. [68] have recently described the Oxygen Uptake Efficiency highest Plateau (OUEP, i.e., oxygen uptake/ventilation = VO2/VE) as the best single predictor of early death (six months), in a cohort of 508 patients with low ejection fraction (>35%), with an Odds ratio for mortality of 13. When OUEP is combined with periodic breathing, the Odds ratio increases to 56.
3.2. Periodic Breathing
Periodic breathing is a ventilatory pattern, which is present in some heart failure patients both at rest and during exercise. Exercise-induced periodic breathing has been defined by Kremser et al. [69] as the presence of cyclic fluctuation of VE lasting longer than 66% of the exercise, with an amplitude of more than 15% of the average value at rest. It can be observed during the entire exercise or it disappears after a few minutes. The origin of periodic breathing is still unclear and several mechanisms have been proposed, mainly grouped into ventilatory (instability in the feedback ventilatory system) and hemodynamic (pulmonary blood flow fluctuations). Agostoni et al. [70], showed that, adding 250 and 500 mL of dead space, respectively, periodic breathing disappears earlier during exercise and suggested that low tidal volume and carbon dioxide apnea threshold are important contributors to periodic breathing. Schmid et al. [71] supposed that periodic breathing in heart failure might potentiate the negative effects of low cardiac output and high ventilation on exercise performance, and they concluded that the presence of periodic breathing negatively influences the exercise performance of heart failure patients, likely because of an increased cost of breathing. Indeed, periodic breathing disappearance during exercise showed to be associated with a more efficient oxygen delivery in most cases. Regardless, periodic breathing is associated with a worse prognosis [64, 72] in heart failure and reflects disease severity [73].
4. Therapeutic Interventions
Several reports showed that the respiratory system can be one of the targets for proper heart failure treatment. On this regard, several therapeutic interventions affect the ventilatory abnormalities both at rest and during exercise in heart failure patients. Indeed, ACE-inhibition improves pulmonary diffusion in heart failure [74]. ACE-inhibition could improve pulmonary hemodynamic, remove interstitial fluid and pulmonary vasoconstriction, and improve DLCO. The effect of ACE-inhibitors is counteracted by aspirin, suggesting that bradykinin metabolism has a role, and bradykinin is likely to participate to abnormal alveolar capillary gas diffusion regulation in heart failure. Most importantly the studies on ACE-inhibitors, with and without aspirin, showed a direct effect of these drugs on lung gas diffusion, in the absence of a hemodynamic effect [74]. Agostoni et al. [75] showed that spironolactone improves gas diffusion through the lungs in stable heart failure patients with impaired DLCO, possibly through a reduction of pulmonary fibrosis.
Also Beta-blockers affect DLCO diffusion. Beta-receptors in the lung are located on the alveoli, mainly Beta2-receptors, and on the airways (mainly Beta1-receptor). Carvedilol reduces DLCO, due to a reduction of membrane diffusion [62], while Bisoprolol does not modify DLCO [76]. Carvedilol and Bisoprolol have a different pharmacological action, blocking both Beta1- and Beta2-receptors (Carvedilol) or selectively Beta1-receptor (Bisoprolol). The pharmacological action of Beta-blockers can explain the different actions on DLCO diffusion. Carvedilol improves clinical conditions, without affecting exercise performance. Carvedilol, but not Bisoprolol, reduces hyperventilation through exercise [77], as shown by a lower VE/VCO2 slope, consequent to an increase in the arterial CO2 set point [62, 78]. The improvement of the clinical conditions of heart failure patients treated with Carvedilol could be associated with reduction of the inappropriately elevated ventilation levels observed during exercise and consequently dyspnea.
A direct effect of Carvedilol on chemoreceptors activity has been recently suggested [79]. This reduction of hyperventilation during exercise is present both in normoxia (equivalent to sea level) and in hypoxia (equivalent to 2,000 m altitude). The reduction of hyperventilation by Carvedilol has a negative influence on arterial PO2 during exercise at a simulated altitude of 2,000 m [78].
In conclusion, lung abnormalities have a major role in heart failure syndrome. Indeed, lung abnormalities influence the clinical setting being dyspnea, a frequently reported heart failure symptom, affecting exercise performance, providing a tool to grade heart failure severity and to predict its prognosis, and finally being the target of therapy with several drugs commonly used in heart failure.
Acknowledgments
The authors are grateful to Michela Palmieri and Elisabetta Salvioni, P.h.D, for their contribution in preparation of this paper.
References
- 1.Rosca MG, Hoppel CL. Mitochondrial dysfunction in heart failure. doi: 10.1007/s10741-012-9340-0. Heart Failure Reviews. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chua TP, Coats AJS. The lungs in chronic heart failure. European Heart Journal. 1995;16(7):882–887. doi: 10.1093/oxfordjournals.eurheartj.a061019. [DOI] [PubMed] [Google Scholar]
- 3.Wright RS, Levine MS, Bellamy PE, et al. Ventilatory and diffusion abnormalities in potential heart transplant recipients. Chest. 1990;98(4):816–820. doi: 10.1378/chest.98.4.816. [DOI] [PubMed] [Google Scholar]
- 4.Agostoni P, Cattadori G, Guazzi M, Palermo P, Bussotti M, Marenzi G. Cardiomegaly as a possible cause of lung dysfunction in patients with heart failure. American Heart Journal. 2000;140(5, article e24) doi: 10.1067/mhj.2000.110282. [DOI] [PubMed] [Google Scholar]
- 5.Wasserman K, Zhang YY, Gilt A, et al. Lung function and exercise gas exchange in chronic heart failure. Circulation. 1997;96(7):2221–2227. doi: 10.1161/01.cir.96.7.2221. [DOI] [PubMed] [Google Scholar]
- 6.Huang YCT, Helms MJ, MacIntyre NR. Normal values for single exhalation diffusing capacity and pulmonary capillary blood flow in sitting, supine positions, and during mild exercise. Chest. 1994;105(2):501–508. doi: 10.1378/chest.105.2.501. [DOI] [PubMed] [Google Scholar]
- 7.Roughton FJ, Forster RE. Relative importance of diffusion and chemical reaction rates in determining rate of exchange of gases in the human lung, with special reference to true diffusing capacity of pulmonary membrane and volume of blood in the lung capillaries. Journal of Applied Physiology. 1957;11(2):290–302. doi: 10.1152/jappl.1957.11.2.290. [DOI] [PubMed] [Google Scholar]
- 8.Agostoni P, Bussotti M, Cattadori G, et al. Gas diffusion and alveolar-capillary unit in chronic heart failure. European Heart Journal. 2006;27(21):2538–2543. doi: 10.1093/eurheartj/ehl302. [DOI] [PubMed] [Google Scholar]
- 9.Olson TP, Beck KC, Johnson BD. Pulmonary function changes associated with cardiomegaly in chronic heart failure. Journal of Cardiac Failure. 2007;13(2):100–107. doi: 10.1016/j.cardfail.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palermo P, Cattadori G, Bussotti M, Apostolo A, Contini M, Agostoni P. Lateral decubitus position generates discomfort and worsens lung function in chronic heart failure. Chest. 2005;128(3):1511–1516. doi: 10.1378/chest.128.3.1511. [DOI] [PubMed] [Google Scholar]
- 11.McCormack DG. Increase in vital capacity after cardiac transplantation. The American Journal of Medicine. 1991;90(5):660–661. [PubMed] [Google Scholar]
- 12.Agostoni P, Pellegrino R, Conca C, Rodarte JR, Brusasco V. Exercise hyperpnea in chronic heart failure: relationships to lung stiffness and expiratory flow limitation. Journal of Applied Physiology. 2002;92(4):1409–1416. doi: 10.1152/japplphysiol.00724.2001. [DOI] [PubMed] [Google Scholar]
- 13.Guazzi M. Alveolar gas diffusion abnormalities in heart failure. Journal of Cardiac Failure. 2008;14(8):695–702. doi: 10.1016/j.cardfail.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Naum CC, Sciurba FC, Rogers RM. Pulmonary function abnormalities in chronic severe cardiomyopathy preceding cardiac transplantation. American Review of Respiratory Disease. 1992;145(6):1334–1338. doi: 10.1164/ajrccm/145.6.1334. [DOI] [PubMed] [Google Scholar]
- 15.Siegel JL, Miller A, Brown LK, DeLuca A, Teirstein AS. Pulmonary diffusing capacity in left ventricular dysfunction. Chest. 1990;98(3):550–553. doi: 10.1378/chest.98.3.550. [DOI] [PubMed] [Google Scholar]
- 16.Mettauer B, Lampert E, Charloux A, et al. Lung membrane diffusing capacity, heart failure, and heart transplantation. American Journal of Cardiology. 1999;83(1):62–67. doi: 10.1016/s0002-9149(98)00784-x. [DOI] [PubMed] [Google Scholar]
- 17.Agostoni PG, Marenzi GC, Pepi M, et al. Isolated ultrafiltration in moderate congestive heart failure. Journal of the American College of Cardiology. 1993;21(2):424–431. doi: 10.1016/0735-1097(93)90685-t. [DOI] [PubMed] [Google Scholar]
- 18.Puri S, Baker BL, Dutka DP, Oakley CM, Hughes JMB, Cleland JGF. Reduced alveolar-capillary membrane diffusing capacity in chronic heart failure: its pathophysiological relevance and relationship to exercise performance. Circulation. 1995;91(11):2769–2774. doi: 10.1161/01.cir.91.11.2769. [DOI] [PubMed] [Google Scholar]
- 19.Al-Rawas OA, Carter R, Stevenson RD, Naik SK, Wheatley DJ. Exercise intolerance following heart transplantation: the role of pulmonary diffusing capacity impairment. Chest. 2000;118(6):1661–1670. doi: 10.1378/chest.118.6.1661. [DOI] [PubMed] [Google Scholar]
- 20.Mutlu GM, Factor P. Alveolar epithelial beta2-adrenergic receptors. American Journal of Respiratory Cell and Molecular Biology. 2008;38:127–134. doi: 10.1165/rcmb.2007-0198TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guazzi M, Agostoni P, Bussotti M, Guazzi MD. Impeded alveolar-capillary gas transfer with saline infusion in heart failure. Hypertension. 1999;34(6):1202–1207. doi: 10.1161/01.hyp.34.6.1202. [DOI] [PubMed] [Google Scholar]
- 22.Cattadori G, Wasserman K, Meloni C, et al. Alveolar membrane conductance decreases as BNP increases during exercise in heart failure. Rationale for BNP in the evaluation of dyspnea. Journal of Cardiac Failure. 2009;15(2):136–144. doi: 10.1016/j.cardfail.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Agostoni P, Cattadori G, Bianchi M, Wasserman K. Exercise-induced pulmonary edema in heart failure. Circulation. 2003;108(21):2666–2671. doi: 10.1161/01.CIR.0000097115.61309.59. [DOI] [PubMed] [Google Scholar]
- 24.Pellegrino R, Dellacà R, Macklem PT, et al. Effects of rapid saline infusion on lung mechanics and airway responsiveness in humans. Journal of Applied Physiology. 2003;95(2):728–734. doi: 10.1152/japplphysiol.00310.2003. [DOI] [PubMed] [Google Scholar]
- 25.Robertson HT, Pellegrino R, Pini D, et al. Exercise response after rapid intravenous infusion of saline in healthy humans. Journal of Applied Physiology. 2004;97(2):697–703. doi: 10.1152/japplphysiol.00108.2004. [DOI] [PubMed] [Google Scholar]
- 26.Muir AL, Flenley DC, Kirby BJ. Cardiorespiratory effects of rapid saline infusion in normal man. Journal of Applied Physiology. 1975;38(5):786–793. doi: 10.1152/jappl.1975.38.5.786. [DOI] [PubMed] [Google Scholar]
- 27.Agostoni P, Marenzi G, Lauri G, et al. Sustained improvement in functional capacity after removal of body fluid with isolated ultrafiltration in chronic cardiac insufficiency: failure of furosemide to provide the same result. American Journal of Medicine. 1994;96(3):191–199. doi: 10.1016/0002-9343(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 28.Lalande S, Yerly P, Faoro V, Naeije R. Pulmonary vascular distensibility predicts aerobic capacity in healthy individuals. Journal of Physiology. 2012;590(17):4279–4288. doi: 10.1113/jphysiol.2012.234310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Bisschop C, Martinot J-B, Leurquin-Sterk G, Faoro V, Guénard H, Naeije R. Improvement in lung diffusion by endothelin A receptor blockade at high altitude. Journal of Applied Physiology. 2012;112(1):20–25. doi: 10.1152/japplphysiol.00670.2011. [DOI] [PubMed] [Google Scholar]
- 30.Agostoni PG, Bussotti M, Palermo P, Guazzi M. Does lung diffusion impairment affect exercise capacity in patients with heart failure? Heart. 2002;88(5):453–459. doi: 10.1136/heart.88.5.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abinader EG, Sharif DS, Goldhammer E. Effects of low altitude on exercise performance in patients with congestive heart failure after healing of acute myocardial infarction. American Journal of Cardiology. 1999;83(3):383–387. doi: 10.1016/s0002-9149(98)00873-x. [DOI] [PubMed] [Google Scholar]
- 32.Guazzi M, Pontone G, Brambilla R, Agostoni P, Rèina G. Alveolar-capillary membrane gas conductance: a novel prognostic indicator in chronic heart failure. European Heart Journal. 2002;23(6):467–476. doi: 10.1053/euhj.2001.2803. [DOI] [PubMed] [Google Scholar]
- 33.De Pasquale CG, Arnolda LF, Doyle IR, Grant RL, Aylward PE, Bersten AD. Prolonged alveolocapillary barrier damage after acute cardiogenic pulmonary edema. Critical Care Medicine. 2003;31(4):1060–1067. doi: 10.1097/01.CCM.0000059649.31659.22. [DOI] [PubMed] [Google Scholar]
- 34.Magrì D, Brioschi M, Banfi C, et al. Circulating plasma surfactant protein type B as biological marker of alveolar-capillary barrier damage in chronic heart failure. Circulation. 2009;2(3):175–180. doi: 10.1161/CIRCHEARTFAILURE.108.819607. [DOI] [PubMed] [Google Scholar]
- 35.Babb TG, Viggiano R, Hurley B, Staats B, Rodarte JR. Effect of mild-to-moderate airflow limitation on exercise capacity. Journal of Applied Physiology. 1991;70(1):223–230. doi: 10.1152/jappl.1991.70.1.223. [DOI] [PubMed] [Google Scholar]
- 36.Johnson RL., Jr. Gas exchange efficiency in congestive heart failure. Circulation. 2000;101(24):2774–2776. doi: 10.1161/01.cir.101.24.2774. [DOI] [PubMed] [Google Scholar]
- 37.Johnson BD, Beck KC, Olson LJ, et al. Ventilatory constraints during exercise in patients with chronic heart failure. Chest. 2000;117(2):321–332. doi: 10.1378/chest.117.2.321. [DOI] [PubMed] [Google Scholar]
- 38.O’Donnell DE. Hyperinflation, dyspnea, and exercise intolerance in chronic obstructive pulmonary disease. Proceedings of the American Thoracic Society. 2006;3(2):180–184. doi: 10.1513/pats.200508-093DO. [DOI] [PubMed] [Google Scholar]
- 39.Mancini DM, Henson D, LaManca J, Levine S. Respiratory muscle function and dyspnea in patients with chronic congestive heart failure. Circulation. 1992;86(3):909–918. doi: 10.1161/01.cir.86.3.909. [DOI] [PubMed] [Google Scholar]
- 40.Mancini DM, Henson D, La Manca J, Donchez L, Levine S. Benefit of selective respiratory muscle training on exercise capacity in patients with chronic congestive heart failure. Circulation. 1995;91(2):320–329. doi: 10.1161/01.cir.91.2.320. [DOI] [PubMed] [Google Scholar]
- 41.Johnson BD, Weisman IM, Zeballos RJ, Beck KC. Emerging concepts in the evaluation of ventilatory limitation during exercise: the exercise tidal flow-volume loop. Chest. 1999;116(2):488–503. doi: 10.1378/chest.116.2.488. [DOI] [PubMed] [Google Scholar]
- 42.Agostoni PG, Cattadori G. Patterns of response diagnostic for cardiac disease. European Respiratory Monograph. 2007;40:1–16. [Google Scholar]
- 43.Bussotti M, Agostoni P, Durigato A, et al. Do maximum flow-volume loops collected during maximum exercise test alter the main cardiopulmonary parameters? Chest. 2009;135(2):425–433. doi: 10.1378/chest.08-1477. [DOI] [PubMed] [Google Scholar]
- 44.Wasserman K, Hansen JE, Sue DY. Principles of Exercise Testing and Interpretationed. 3rd edition. Baltimore, Md, USA: Lippincott Williams & Wilkins; 1999. [Google Scholar]
- 45.Tabet JY, Beauvais F, Thabut G, Tartière JM, Logeart D, Cohen-Solal A. A critical appraisal of the prognostic value of the VE/VCO2 slope in chronic heart failure. European Journal of Cardiovascular Prevention and Rehabilitation. 2003;10(4):267–272. doi: 10.1097/00149831-200308000-00008. [DOI] [PubMed] [Google Scholar]
- 46.Johnson RL., Jr. Gas exchange efficiency in congestive heart failure II. Circulation. 2001;103(7):916–918. doi: 10.1161/01.cir.103.7.916. [DOI] [PubMed] [Google Scholar]
- 47.Wilcox I, Grunstein RR, Collins FL, Berthon-Jones M, Kelly DT, Sullivan CE. The role of central chemosensitivity in central apnea of heart failure. Sleep. 1993;16(8):S37–S38. doi: 10.1093/sleep/16.suppl_8.s37. [DOI] [PubMed] [Google Scholar]
- 48.Chua TP, Clark AL, Amadi AA, Coats AJS. Relation between chemosensitivity and the ventilatory response to exercise in chronic heart failure. Journal of the American College of Cardiology. 1996;27(3):650–657. doi: 10.1016/0735-1097(95)00523-4. [DOI] [PubMed] [Google Scholar]
- 49.Chua TP, Harrington D, Ponikowski P, Webb-Peploe K, Poole-Wilson PA, Coats AJS. Effects of dihydrocodeine on chemosensitivity and exercise tolerance in patients with chronic heart failure. Journal of the American College of Cardiology. 1997;29(1):147–152. doi: 10.1016/s0735-1097(96)00446-9. [DOI] [PubMed] [Google Scholar]
- 50.Piepoli M, Clark AL, Volterrani M, Adamopoulos S, Sleight P, Coats AJS. Contribution of muscle afferents to the hemodynamic, autonomic, and ventilatory responses to exercise in patients with chronic heart failure: effects of physical training. Circulation. 1996;93(5):940–952. doi: 10.1161/01.cir.93.5.940. [DOI] [PubMed] [Google Scholar]
- 51.Buller NP, Poole-Wilson PA. Mechanism of the increased ventilatory response to exercise in patients with chronic heart failure. British Heart Journal. 1990;63(5):281–283. doi: 10.1136/hrt.63.5.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kleber FX, Vietzke G, Wernecke KD, et al. Impairment of ventilatory efficiency in heart failure: prognostic impact. Circulation. 2000;101(24):2803–2809. doi: 10.1161/01.cir.101.24.2803. [DOI] [PubMed] [Google Scholar]
- 53.Paoletti P, De Filippis F, Fraioli F, et al. Cardiopulmonary exercise testing (CPET) in pulmonary emphysema. Respiratory Physiology and Neurobiology. 2011;179(2-3):167–173. doi: 10.1016/j.resp.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 54.Agostoni P, Apostolo A, Sciomer S. Evolution of the concept of ventilatory limitation during exercise. Combining the pneumologist and cardiologist point of view. Respiratory Physiology and Neurobiology. 2011;179(2-3):127–128. doi: 10.1016/j.resp.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 55.Arena R, Myers J, Abella J, et al. Development of a ventilatory classification system in patients with heart failure. Circulation. 2007;115(18):2410–2417. doi: 10.1161/CIRCULATIONAHA.107.686576. [DOI] [PubMed] [Google Scholar]
- 56.Corrà U, Mezzani A, Bosimini E, Scapellato F, Imparato A, Giannuzzi P. Ventilatory response to exercise improves risk stratification in patients with chronic heart failure and intermediate functional capacity. American Heart Journal. 2002;143(3):418–426. doi: 10.1067/mhj.2002.120772. [DOI] [PubMed] [Google Scholar]
- 57.Francis DP, Shamim W, Davies LC, et al. Cardiopulmonary exercise testing for prognosis in chronic heart failure: continuous and independent prognostic value from VE/VCO2 slope and peak VO2 . European Heart Journal. 2000;21(2):154–161. doi: 10.1053/euhj.1999.1863. [DOI] [PubMed] [Google Scholar]
- 58.Gitt AK, Wasserman K, Kilkowski C, et al. Exercise anaerobic threshold and ventilatory efficiency identify heart failure patients for high risk of early death. Circulation. 2002;106(24):3079–3084. doi: 10.1161/01.cir.0000041428.99427.06. [DOI] [PubMed] [Google Scholar]
- 59.Guazzi M, De Vita S, Cardano P, Barlera S, Guazzi MD. Normalization for peak oxygen uptake increases the prognostic power of the ventilatory response to exercise in patients with chronic heart failure. American Heart Journal. 2003;146(3):542–548. doi: 10.1016/S0002-8703(03)00321-1. [DOI] [PubMed] [Google Scholar]
- 60.Ponikowski P, Francis DP, Piepoli MF, et al. Enhanced ventilatory response to exercise in patients with chronic heart failure and preserved exercise tolerance: marker of abnormal cardiorespiratory reflex control and predictor of poor prognosis. Circulation. 2001;103(7):967–972. doi: 10.1161/01.cir.103.7.967. [DOI] [PubMed] [Google Scholar]
- 61.Guazzi M, Myers J, Arena R. Cardiopulmonary exercise testing in the clinical and prognostic assessment of diastolic heart failure. Journal of the American College of Cardiology. 2005;46(10):1883–1890. doi: 10.1016/j.jacc.2005.07.051. [DOI] [PubMed] [Google Scholar]
- 62.Agostoni P, Guazzi M, Bussotti M, De Vita S, Palermo P. Carvedilol reduces the inappropriate increase of ventilation during exercise in heart failure patients. Chest. 2002;122(6):2062–2067. doi: 10.1378/chest.122.6.2062. [DOI] [PubMed] [Google Scholar]
- 63.Bussotti M, Magrí D, Previtali E, et al. End-tidal pressure of CO2 and exercise performance in healthy subjects. European Journal of Applied Physiology. 2008;103(6):727–732. doi: 10.1007/s00421-008-0773-z. [DOI] [PubMed] [Google Scholar]
- 64.Guazzi M, Arena R, Ascione A, Piepoli M, Guazzi MD. Exercise oscillatory breathing and increased ventilation to carbon dioxide production slope in heart failure: an unfavorable combination with high prognostic value. American Heart Journal. 2007;153(5):859–867. doi: 10.1016/j.ahj.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 65.Baba R, Nagashima M, Goto M, et al. Oxygen uptake efficiency slope: a new index of cardiorespiratory functional reserve derived from the relation between oxygen uptake and minute ventilation during incremental exercise. Journal of the American College of Cardiology. 1996;28(6):1567–1572. doi: 10.1016/s0735-1097(96)00412-3. [DOI] [PubMed] [Google Scholar]
- 66.Myers J, Arena R, Dewey F, et al. A cardiopulmonary exercise testing score for predicting outcomes in patients with heart failure. American Heart Journal. 2008;156(6):1177–1183. doi: 10.1016/j.ahj.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 67.Davies LC, Wensel R, Georgiadou P, et al. Enhanced prognostic value from cardiopulmonary exercise testing in chronic heart failure by non-linear analysis: oxygen uptake efficiency slope. European Heart Journal. 2006;27(6):684–690. doi: 10.1093/eurheartj/ehi672. [DOI] [PubMed] [Google Scholar]
- 68.Sun X-G, Hansen JE, Stringer WW. Oxygen uptake efficiency plateau best predicts early death in heart failure. Chest. 2012;141(5):1284–1294. doi: 10.1378/chest.11-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kremser CB, O’Toole MF, Leff AR. Oscillatory hyperventilation in severe congestive heart failure secondary to idiopathic dilated cardiomyopathy or to ischemic cardiomyopathy. American Journal of Cardiology. 1987;59(8):900–905. doi: 10.1016/0002-9149(87)91116-7. [DOI] [PubMed] [Google Scholar]
- 70.Agostoni P, Apostolo A, Albert RK. Mechanisms of periodic breathing during exercise in patients with chronic heart failure. Chest. 2008;133(1):197–203. doi: 10.1378/chest.07-1439. [DOI] [PubMed] [Google Scholar]
- 71.Schmid JP, Apostolo A, Antonioli L, et al. Influence of exertional oscillatory ventilation on exercise performance in heart failure. European Journal of Cardiovascular Prevention and Rehabilitation. 2008;15(6):688–692. doi: 10.1097/HJR.0b013e32830fdfdb. [DOI] [PubMed] [Google Scholar]
- 72.Sun XG, Hansen JE, Beshai JF, Wasserman K. Oscillatory breathing and exercise gas exchange abnormalities prognosticate early mortality and morbidity in heart failure. Journal of the American College of Cardiology. 2010;55(17):1814–1823. doi: 10.1016/j.jacc.2009.10.075. [DOI] [PubMed] [Google Scholar]
- 73.Corrà U, Giordano A, Bosimini E, et al. Oscillatory ventilation during exercise in patients with chronic heart failure: clinical correlates and prognostic implications. Chest. 2002;121(5):1572–1580. doi: 10.1378/chest.121.5.1572. [DOI] [PubMed] [Google Scholar]
- 74.Guazzi M, Marenzi G, Alimento M, Contini M, Agostoni P. Improvement of alveolar-capillary membrane diffusing capacity with enalapril in chronic heart failure and counteracting effect of aspirin. Circulation. 1997;95(7):1930–1936. doi: 10.1161/01.cir.95.7.1930. [DOI] [PubMed] [Google Scholar]
- 75.Agostoni P, Magini A, Andreini D, et al. Spironolactone improves lung diffussion in chronic heart failure. European Heart Journal. 2005;26(2):159–164. doi: 10.1093/eurheartj/ehi023. [DOI] [PubMed] [Google Scholar]
- 76.Agostoni P, Contini M, Cattadori G, et al. Lung function with carvedilol and bisoprolol in chronic heart failure: is β selectivity relevant? European Journal of Heart Failure. 2007;9(8):827–833. doi: 10.1016/j.ejheart.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 77.Agostoni P, Apostolo A, Cattadori G, et al. Effects of β-blockers on ventilation efficiency in heart failure. American Heart Journal. 2010;159(6):1067–1073. doi: 10.1016/j.ahj.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 78.Agostoni P, Contini M, Magini A, et al. Carvedilol reduces exercise-induced hyperventilation: a benefit in normoxia and a problem with hypoxia. European Journal of Heart Failure. 2006;8(7):729–735. doi: 10.1016/j.ejheart.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 79.Contini M, Paolillo S, Iorio A, et al. Comparison of carvedilol, nebivolol and bisoprolol on cardiopulmonary function in moderate heart failure. European Heart Journal. 2012;33:42–43. [Google Scholar]


