Abstract
Murine double minute-2 (MDM2) is an intracellular molecule with multiple biologic functions. It serves as a negative regulator of p53 and thereby limits cell cycle arrest and apoptosis. Because MDM2 blockade suppresses tumor cell growth in vitro and in vivo, respective MDM2 inhibition is currently evaluated as anti-cancer therapy in clinical trials. However, the anti-proliferative effects of MDM2 inhibition also impair regenerative cell growth upon tissue injury. This was so far documented for tubular repair upon postischemic acute kidney injury and might apply to wound healing responses in general. Furthermore, MDM2 has numerous p53-independent effects. As a new entry, MDM2 was identified to act as a co-transcription factor for nuclear factor-kappa-light-enhancer of activated B cells (NF-κB) at cytokine promoters. This explains the potent anti-inflammatory effects of MDM2 inhibitors in vitro and in vivo. For example, the NF-κB-antagonistic and p53-agonistic activities of MDM2 inhibitors elicit potent therapeutic effects on experimental lymphoproliferative autoimmune disorders such as systemic lupus erythematosus. In this review, we discuss the classic p53-dependent, the recently discovered p53-independent, and the NF-κB-agonistic biologic functions of MDM2. We describe its complex regulatory role on p53 and NF-κB signaling and name areas of research that may help to foresee previously unexpected effects or potential alternative indications of therapeutic MDM2 blockade.
Introduction
Murine double minute-2 (MDM2) is an intracellular protein with oncoprotein functions, which is considered to be a valuable target for cancer therapy for a variety of reasons: 1) MDM2 is an E3 ubiquitin ligase that negatively regulates p53 mainly by ubiquitin-mediated degradation, as such MDM2 suppresses coordinated cell cycle arrest or apoptosis and promotes cell survival and growth [1]; 2) cell-type-specific deletion of MDM2 recovers p53 and induces cell-type-specific cell death [2]; 3) MDM2 is strongly expressed in many malignancies with wild-type p53 as an alternate mechanism to disrupt the p53 pathway in early cancer development [3,4]; 4) MDM2 overexpression is linked to gain-of-function mutations in many tumors [1]; and 5)MDM2 blockade with suitable antagonists was shown to block tumor growth in a number of models [5]. As such, a clinical trial is currently ongoing to study the effects of the MDM2 antagonist RO5503781, in advanced malignancies other than leukemia (www.clinicaltrials.gov).
There is a huge body of data that describes the p53-MDM2 regulatory feedback loop in various tumor cells, implicating that the scientific rationale of qualifying MDM2 as a valuable therapeutic target is based on tumor cell cycle control [5]. However, MDM2 has a number of p53-independent effects [6] and tumor growth also depends on tumor vasculature, tumor stroma, and an immunosuppressive tumor environment, factors that have not yet been rigorously explored in the context of MDM2. In this context, the recently discovered role of MDM2 in nuclear factor-kappa-light-enhancer of activated B cells (NF-κB) signaling should be of enigmatic importance because it identifies MDM2 as the missing link between inflammation and carcinogenesis [7]. In this review, we focus on the expanding role of MDM2 as a regulator of NF-κB signaling, innate immunity, and tissue inflammation and its potential impact on tumor cell biology, cancer therapy, other abnormal hyperproliferative syndromes, as well as wound healing.
The Classic p53-Dependent Role of MDM2 on Cell Cycle Control
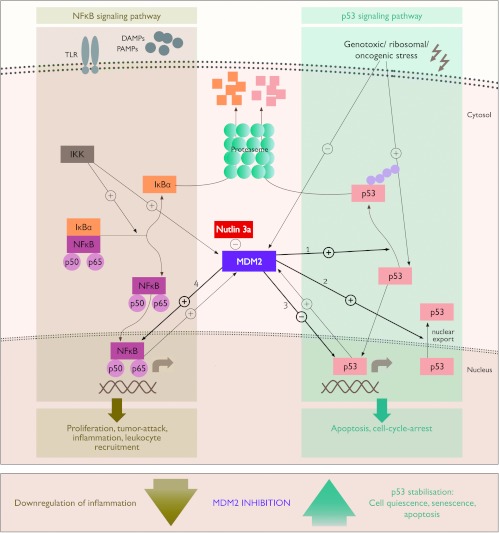
MDM2 is an oncoprotein based on its function as negative regulator of tumor suppressor protein p53, which is one of the central regulators of cell cycle. p53 has a pivotal role in stress response signaling and its activation results in quiescence, senescence, or death of cells with DNA damage, therefore avoiding aberrant mitosis and carcinogenesis. MDM2 inhibits p53 transcriptional activity, it acts as E3 ubiquitin ligase ubiquitinating and targeting p53 for proteasomal degradation, and it promotes the nuclear export of p53 (Figure 1). Vice versa, MDM2 is a p53 target gene illustrating how MDM2 and p53 form a tightly regulated negative feedback loop in which activated p53 upregulates MDM2 expression, which in turn will target p53 for degradation [3,8,9]. MDM2 deficiency leads to p53-driven, uncontrolled cell death already during embryonic development. This phenotype can be entirely rescued by concomitant deletion of p53 [10]. This shows the absolute necessity of tight regulation of p53 by MDM2 in normal tissues, as p53 overexpression results in dramatic apoptosis in multiple organs [11]. However, MDM2 gene duplication or hyper-activation eliminates p53-mediated growth control of cells with significant DNA damage that supports tumorigenesis. This MDM2 effect is most prominent together with concomitant dysfunctional p53, which applies to more than 50% of human cancers [4,9].
Figure 1.
Regulatory effects of MDM2 on NF-κB and p53 signaling pathways. MDM2 negatively regulates p53 in at least three different ways, i.e., 1) MDM2 functions as the E3 ubiquitin ligase promoting ubiquitin-dependent proteasomal degradation of p53; 2) MDM2 facilitates nuclear export of p53 into the cell cytoplasm, moving p53 away from its site of action; 3) MDM2 directly interacts with the p53 transcription activation domain, thus inhibiting p53 transcriptional activity; and 4) MDM2 activates the NF-κB signaling pathway by enhancing the transcription of NF-κB/p65 but also acts as a non-redundant co-transcription factor for NF-κB target genes.
With genotoxic or cytotoxic stress, frequently preceding cell transformation, p53 and MDM2 are activated by phosphorylation and acetylation on specific residues, which results in their dissociation, p53 stabilization, and consequent transcriptional up-regulation of p53 target genes leading to cell cycle arrest and DNA repair or to programmed cell death [12]. There are other crucial players regulating the MDM2-p53 dynamic equilibrium, which form a very complex network. For example, upon oncogenic stress, tumor suppressor ARF is activated and binds to central domain of MDM2 to inhibit MDM2-mediated ubiquitination and degradation of p53 [13]. Similarly, ribosomal stress induces several ribosomal proteins that interact with the same region on MDM2 and, therefore, stabilize p53 [14]. The multi-faceted MDM2-p53 relationship can be further exemplified by studies suggesting opposite function of MDM2, not as a p53 inhibitor but as a p53 enhancer. MDM2 stimulates p53 mRNA translation by binding the p53 mRNA, and moreover, this interaction also suppresses MDM2's capacity to promote p53 ubiquitination and degradation [15].
p53-Independent Roles of MDM2 in Carcinogenesis
There is growing evidence that MDM2 has a number of p53-independent functions in cell cycle regulation, differentiation, transcription, or DNA synthesis [6,8]. Many of these p53-independent MDM2 roles foster cell transformation and tumorigenesis. A number of tumors overexpress MDM2, which overcomes the pro-apoptotic effect of wild-type p53 and fuels tumor growth [4,9]. Alternatively, amplified MDM2 is coupled with non-functional and mutated p53 [4,9]. This suggests that upregulated MDM2 on its own has additional growth advantages independent of p53. For example, estrogen-induced breast cancer cell proliferation requires a p53-independent MDM2-mediated pathway to activate cell proliferation and p53 is not the key target of MDM2 [16].MDM2 also interacts with Rb, MTBP, Smads, and other molecules which play a key role in cell cycle regulation and seem to contribute to MDM2's oncogenic effects independently of p53 [6,8]. p53 is also not required for MDM2 to promote the translation of the anti-apoptotic protein XIAP, which accumulates in cancer cells and supports their resistance to radiation therapy [17]. Furthermore, blocking MDM2 by small-molecule antagonists is known to inhibit MDM2 interactions with p73 [18] and E2F1 [19], resulting in enhanced p53-deficient tumor cell apoptosis, or with HIF1a [20], inhibiting thus tumor promotion and angiogenesis in p53-independent manner.
Interestingly, MDM2 can also display tumor suppression properties. MDM2/p53 double-deficient mice had shorter tumor latency compared to p53-null mice with MDM2 expression retained [21]. Furthermore, p53 is also not involved when MDM2 negatively regulates insulin-like growth factor-1 receptor (IGF-1R) that protects cells from DNA damage-induced apoptosis, thus allowing transformed cells to undergo apoptosis [22,23]. MDM2 overexpression is also linked to increased motility, invasive growth, and metastasis of cancer cells through mechanism independent of its function in protein ubiquitination. MDM2 promotes cancer cell mobility through protein-protein interaction with non-metastatic cells 2 protein (NME2). MDM2 overexpression suppresses NME2-mediated inhibition of cell motility [24]. Together, p53-dependent and p53-independent effects of MDM2 mostly promote the proliferation of cells with DNA damage, tumor growth, and metastasis.
The p53-Independent Role of MDM2 in NF-κB Signaling
A novel and unexpected biologic function of MDM2 is its NF-κB-agonistic effect during sterile tissue inflammation. Sterile inflammation is a major element of non-infectious tissue injury, e.g., upon exposure to toxins or ischemia-reperfusion. For example, postischemic acute kidney injury involves a sterile inflammatory response that was dramatically suppressed by MDM2 blockade with nutlin-3a [25]. This effect was independent of p53, as it also occurred in p53-deficient mice, whereas the anti-proliferative effect of MDM2 blockade on epithelial healing was abrogated in p53-deficient mice. MDM2 blockade suppressed the postischemic induction of pro-inflammatory cytokines and chemokines, as well as the subsequent recruitment of leukocytes to the site of injury. The mechanism underlying MDM2-mediated inflammation was identified in vitro by showing that MDM2 acts as a co-factor for NF-κB binding to its target gene promotor binding sites, while upstream the outside-in signaling of NF-κB activation was independent of MDM2 [25]. This was evidenced by electromobility shift assay using lipopolysaccharide (LPS)-stimulated p53-deficient and MDM2/p53 double-deficient mouse embryonic fibroblasts [25]. This observation is consistent with a previous report showing that MDM2 blockade with nutlin suppresses LPS-induced lung inflammation and that nutlin-3a impairs NF-κB DNA binding in neutrophils; however, this effect of nutlin was dependent on the presence of p53 [26]. One may speculate that this p53-independent biologic effect of MDM2 contributes to prolonged recovery of critically ill patients with MDM2 up-regulation [27]. Furthermore, MDM2 is overexpressed in human atherosclerosis and MDM2 inhibition with nutlin-3-suppressed NF-κB-dependent inflammation in vascular smooth muscle cells [28,29].
Complex Regulation among MDM2, NF-κB, and p53
It is an evolving paradigm that MDM2 links NF-κB and p53 signaling by promoting NF-κB and blocking p53. NF-κB and p53 signaling are both important genotoxic and cytotoxic stress response pathways that are both deregulated in cancer [30]. Tissue injury activates NF-κB not only to induce host defense but also to block apoptosis and to stimulate regenerative cell growth. However, these effects become problematic in the context of cancer. The majority of malignancies are associated with long-term activation of NF-κB [31,32]. In contrast, the tumor suppressor p53 is commonly inactivated in the tumor environment, which further impairs cancer cell growth arrest and apoptosis. The opposite functional effects of these two pathways on cell cycle control imply that they need to be tightly co-regulated and kept in balance (Figure 1). In fact, cross talk and reciprocal negative regulation of NF-κB and p53 signaling occurs at multiple levels [30].
NF-κB Suppresses p53 Signaling by Inducing MDM2
MDM2 is a target gene of NF-κB signaling; hence, NF-κB negatively regulates p53 through up-regulation of MDM2 [30]. This effect may involve the NF-κB target protein Bcl3 [33] as well as inhibitor of nuclear factor kappa-B kinase subunit beta (IKK2) [34]. In addition, NF-κB induces MDM2 to stimulate T cell activation and proliferation, which in turn inhibits the p53 family tumor suppressor protein p73, independent of p53 [35].
p53 Regulates NF-κB Signaling
p53 negatively regulates NF-κB signaling [30]. For example, p53 competes with NF-κB for limited transcription co-factors such as p300/CBP [36] or suppresses NF-κB transcriptional activity through inhibition of IKKs and histone H3 kinase [37,38]. Obviously, p53-mediated repression of NF-κB occurs rather at the level of protein-protein interactions or protein modifications.
MDM2 Regulates NF-κB Signaling
As described above in detail, MDM2 acts as a co-factor for NF-κB at target gene promoters, a process that is independent of p53 [25]. Furthermore, MDM2 directly induces the transcription of p65 by interacting with Sp-1 binding sites in the p65 gene promoter of leukemia cells, independent of their p53 status [39]. Moreover, MDM2 can upregulate expression of p100/NF-κB2 in lung cells. MDM2 sustains this function also when its p53-interaction domain is blocked by nultin-3 or in p53-deficient lung cancer cells [40]. It is of note that MDM2 can display different regulatory activities dependent on the activation status of NF-κB in transformed cells with inactive p53. In cells with normal levels of NF-κB activity, MDM2 induced NF-κB overactivation and cell proliferation. In contrast, in cells that constitutively overexpress NF-κB, MDM2 suppressed NF-κB signaling and enhanced apoptosis [41].
Together, MDM2 is a regulator of p53 as well as of NF-κB signaling and can tilt the balance of both pathways in both directions. Depending on the context, MDM2 can act either pro-inflammatory and pro-mitogenic or anti-inflammatory and pro-apoptotic.
Clinical Implications of Therapeutic MDM2 Inhibition
The recently discovered additional functions of MDM2 may have certain implications on the clinical use of MDM2 antagonists. These can be divided into effects on tumor cells, on tumor stroma, on potential cancer therapy complications, and on alternative indications of therapeutic MDM2 inhibition.
MDM2 Inhibition in Tumor Cells
The rationale to develop MDM2 inhibitors is based on the well known p53-dependent mitogenic effects of MDM2 on tumor cells. NF-κB signaling also promotes the survival and proliferation of non-immune cells; hence, MDM2 inhibition that blocks NF-κB signaling rather enhances tumor cell death, but this may depend on the tumor cell NF-κB activation status [41]. The broad literature that documents the beneficial effects of MDM2 blockade on tumor cell growth raised the enthusiasm about MDM2 inhibition as a novel cancer therapy.
MDM2 Inhibition and Tumor Stroma
A significant part of the mass of solid tumors is stroma, consisting of tumor vasculature, interstitial mesenchymal tissue, and immune cell infiltrates that create an environment with its own dynamics. Therapies that are designed to target tumor cells may have their own effects on tumor stroma. While anti-proliferative therapies usually act on tumor as well as on stroma cells, anti-inflammatory drugs might also interact with the tumor stroma in a more specific way. The NF-κB-antagonistic effects of MDM2 inhibition should have anti-inflammatory effects on those immune elements that are directed against tumor cells. This way, MDM2 blockade might impair anti-tumor T and B cell responses that help to control tumor mass and enhance the immunosuppressive environment generated by tumor-associated macrophages and regulatory T cells. Even though, MDM2 blockade has proven effective in many in vivo cancer animal models, it is reasonable to believe that the immunosuppressive action of MDM2 inhibitors may limit the efficacy of tumor control in certain malignancies.
Potential Side Effects of Therapeutic MDM2 Inhibition
The p53-agonistic activity of MDM2 inhibitors impairs regenerative cell growth upon acute kidney injury [25]. Therefore, it is reasonable to believe that this observation applies to other forms of wound healing, e.g., in the surgery setting. One should be reminded that the therapeutic profile of MDM2 inhibitors as anti-proliferative and anti-inflammatory drugs is comparable to that of mammalian target of rapamycin (mTOR) inhibitors. mTOR inhibitors have a proven negative effect on wound healing, which has affected its clinical use, e.g., during the early phase after solid organ transplantation [42]. It will be important to explore during clinical trials whether the use of MDM2 inhibitors is associated with a similar effect on wound healing. Impaired wound healing may affect the outcome of episodes of acute renal failure that commonly occur in patients with cancer after surgery or during treatment with several chemotherapeutics. The same could apply to ischemic limb injury, myocardial infarction, stroke, and chronic ulcers including ulcers of the gastrointestinal tract. Furthermore, the anti-inflammatory effect of MDM2 inhibition may suppress host defense and promote infectious complications.
Potential Other Indications of Therapeutic MDM2 Inhibition
The anti-inflammatory and anti-proliferative potential of MDM2 inhibition can elicit additive effects in other disease settings, especially in autoimmune diseases. For example, systemic lupus erythematosus is a polyclonal lymphoproliferative disorder characterized by expansion of autoreactive T and B cell clones that cause systemic autoimmunity and tissue inflammation. MDM2 inhibition with nutlin-3a dramatically reduces both aspects of the disease by abrogating the abnormal proliferation of lymphocytes and autoantibody production as well as the immunopathology in multiple solid organs [43]. It is noteworthy that MDM2 blockade with nutlin-3a had no effect on hematopoiesis and did not cause neutropenia like it occurs with immunosuppressants such as cyclophosphamide or mycophenolate mofetil, which are currently used for lupus therapy [43]. It remains to be studied whether MDM2 inhibition can have similar advantageous effects on other autoimmune disorders.
Summary and Perspective
MDM2 is a regulator of p53 as well as NF-κB signaling. MDM2 degrades p53 through its E3 ubiquitin ligase activity, but MDM2 also acts as a non-redundant co-transcription factor for NF-κB target genes. This way, MDM2 has additive p53-dependent and NF-κB-dependent (p53-independent) effects on the survival and growth of malignant cells. For the future, it will be important to define the role of MDM2-mediated NF-κB signaling in tumor stroma, tumor vasculature, and metastasis. Furthermore, establishing MDM2 inhibition as a novel cancer therapy will require a careful assessment of its potential suppressive effects on wound healing, tissue repair upon toxic or ischemic injury, as well as host defense, three important and promising areas to work on—experimentally and clinically—in the future. Finally, the NF-κB-antagonistic and p53-agonistic effects of MDM2 inhibitors represent a very promising drug activity profile to suppress lymphoproliferative autoimmune disorders such as systemic lupus erythematosus. Its potential to control lupus or other autoimmune disorders needs to be further evaluated, in both preclinical as well as clinical settings.
Abbreviations
- MDM2
murine double minute-2
Footnotes
The work was supported by a grant from the Deutsche Forschungsgemeinschaft (AN372/12-1). H. Bruns is a fellow of the FöFoLe program of the Medical Faculty, University of Munich. The authors declare no conflict of interest.
References
- 1.Vazquez A, Bond EE, Levine AJ, Bond GL. The genetics of the p53 pathway, apoptosis and cancer therapy. Nat Rev Drug Discov. 2008;7:979–987. doi: 10.1038/nrd2656. [DOI] [PubMed] [Google Scholar]
- 2.Grier JD, Xiong S, Elizondo-Fraire AC, Parant JM, Lozano G. Tissue-specific differences of p53 inhibition by Mdm2 and Mdm4. Mol Cell Biol. 2006;26:192–198. doi: 10.1128/MCB.26.1.192-198.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eischen CM, Lozano G. p53 and MDM2: antagonists or partners in crime? Cancer Cell. 2009;15:161–162. doi: 10.1016/j.ccr.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Leach FS, Tokino T, Meltzer P, Burrell M, Oliner JD, Smith S, Hill DE, Sidransky D, Kinzler KW, Vogelstein B. p53 Mutation and MDM2 amplification in human soft tissue sarcomas. Cancer Res. 1993;53:2231–2234. [PubMed] [Google Scholar]
- 5.Shangary S, Wang S. Small-molecule inhibitors of the MDM2-p53 protein-protein interaction to reactivate p53 function: a novel approach for cancer therapy. Annu Rev Pharmacol Toxicol. 2009;49:223–241. doi: 10.1146/annurev.pharmtox.48.113006.094723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouska A, Eischen CM. Mdm2 affects genome stability independent of p53. Cancer Res. 2009;69:1697–1701. doi: 10.1158/0008-5472.CAN-08-3732. [DOI] [PubMed] [Google Scholar]
- 7.Mantovani A. Molecular pathways linking inflammation and cancer. Curr Mol Med. 2010;10:369–373. doi: 10.2174/156652410791316968. [DOI] [PubMed] [Google Scholar]
- 8.Marine JC, Lozano G. Mdm2-mediated ubiquitylation: p53 and beyond. Cell Death Differ. 2010;17:93–102. doi: 10.1038/cdd.2009.68. [DOI] [PubMed] [Google Scholar]
- 9.Clegg HV, Itahana K, Zhang Y. Unlocking the Mdm2-p53 loop: ubiquitin is the key. Cell Cycle. 2008;7:287–292. doi: 10.4161/cc.7.3.5358. [DOI] [PubMed] [Google Scholar]
- 10.Montes de Oca Luna R, Wagner DS, Lozano G. Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature. 1995;378:203–206. doi: 10.1038/378203a0. [DOI] [PubMed] [Google Scholar]
- 11.Ringshausen I, O'Shea CC, Finch AJ, Swigart LB, Evan GI. Mdm2 is critically and continuously required to suppress lethal p53 activity in vivo. Cancer Cell. 2006;10:501–514. doi: 10.1016/j.ccr.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Tang Y, Zhao W, Chen Y, Zhao Y, Gu W. Acetylation is indispensable for p53 activation. Cell. 2008;133:612–626. doi: 10.1016/j.cell.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sherr CJ. Divorcing ARF and p53: an unsettled case. Nat Rev Cancer. 2006;6:663–673. doi: 10.1038/nrc1954. [DOI] [PubMed] [Google Scholar]
- 14.Deisenroth C, Zhang Y. Ribosome biogenesis surveillance: probing the ribosomal protein-Mdm2-p53 pathway. Oncogene. 2010;29:4253–4260. doi: 10.1038/onc.2010.189. [DOI] [PubMed] [Google Scholar]
- 15.Gajjar M, Candeias MM, Malbert-Colas L, Mazars A, Fujita J, Olivares-Illana V, Fahraeus R. The p53 mRNA-Mdm2 interaction controls Mdm2 nuclear trafficking and is required for p53 activation following DNA damage. Cancer Cell. 2012;21:25–35. doi: 10.1016/j.ccr.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 16.Brekman A, Singh KE, Polotskaia A, Kundu N, Bargonetti J. A p53-independent role of Mdm2 in estrogen-mediated activation of breast cancer cell proliferation. Breast Cancer Res. 2011;13:R3. doi: 10.1186/bcr2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu L, Zhu N, Zhang H, Durden DL, Feng Y, Zhou M. Regulation of XIAP translation and induction by MDM2 following irradiation. Cancer Cell. 2009;15:363–375. doi: 10.1016/j.ccr.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lau LM, Nugent JK, Zhao X, Irwin MS. HDM2 antagonist Nutlin-3 disrupts p73-HDM2 binding and enhances p73 function. Oncogene. 2008;27:997–1003. doi: 10.1038/sj.onc.1210707. [DOI] [PubMed] [Google Scholar]
- 19.Ambrosini G, Sambol EB, Carvajal D, Vassilev LT, Singer S, Schwartz GK. Mouse double minute antagonist Nutlin-3a enhances chemotherapy-induced apoptosis in cancer cells with mutant p53 by activating E2F1. Oncogene. 2007;26:3473–3481. doi: 10.1038/sj.onc.1210136. [DOI] [PubMed] [Google Scholar]
- 20.Lee YM, Lim JH, Chun YS, Moon HE, Lee MK, Huang LE, Park JW. Nutlin-3, an Hdm2 antagonist, inhibits tumor adaptation to hypoxia by stimulating the FIH-mediated inactivation of HIF-1α. Carcinogenesis. 2009;30:1768–1775. doi: 10.1093/carcin/bgp196. [DOI] [PubMed] [Google Scholar]
- 21.McDonnell TJ, Montes de Oca Luna R, Cho S, Amelse LL, Chavez-Reyes A, Lozano G. Loss of one but not two mdm2 null alleles alters the tumour spectrum in p53 null mice. J Pathol. 1999;188:322–328. doi: 10.1002/(SICI)1096-9896(199907)188:3<322::AID-PATH372>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 22.Froment P, Dupont J, Christophe-Marine J. Mdm2 exerts pro-apoptotic activities by antagonizing insulin-like growth factor-I-mediated survival. Cell Cycle. 2008;7:3098–3103. doi: 10.4161/cc.7.19.6807. [DOI] [PubMed] [Google Scholar]
- 23.Di Conza G, Buttarelli M, Monti O, Pellegrino M, Mancini F, Pontecorvi A, Scotlandi K, Moretti F. IGF-1R/MDM2 relationship confers enhanced sensitivity to RITA in Ewing sarcoma cells. Mol Cancer Ther. 2012;11:1247–1256. doi: 10.1158/1535-7163.MCT-11-0913. [DOI] [PubMed] [Google Scholar]
- 24.Polanski R, Maguire M, Nield PC, Jenkins RE, Park BK, Krawczynska K, Devling T, Ray-Sinha A, Rubbi CP, Vlatkovic N, et al. MDM2 interacts with NME2 (non-metastatic cells 2, protein) and suppresses the ability of NME2 to negatively regulate cell motility. Carcinogenesis. 2011;32:1133–1142. doi: 10.1093/carcin/bgr070. [DOI] [PubMed] [Google Scholar]
- 25.Mulay SR, Thomasova D, Ryu M, Anders HJ. MDM2 (murine double minute-2) links inflammation and tubular cell healing during acute kidney injury in mice. Kidney Int. 2012;81:1199–1211. doi: 10.1038/ki.2011.482. [DOI] [PubMed] [Google Scholar]
- 26.Liu G, Park YJ, Tsuruta Y, Lorne E, Abraham E. p53 Attenuates lipopolysaccharide-induced NF-κB activation and acute lung injury. J Immunol. 2009;182:5063–5071. doi: 10.4049/jimmunol.0803526. [DOI] [PubMed] [Google Scholar]
- 27.Kleiman DA, Calvano JE, Coyle SM, Macor MA, Calvano SE, Lowry SF. A single nucleotide polymorphism in the Mdm2 promoter and risk of sepsis. Am J Surg. 2009;197:43–48. doi: 10.1016/j.amjsurg.2007.12.049. [DOI] [PubMed] [Google Scholar]
- 28.Ihling C, Haendeler J, Menzel G, Hess RD, Fraedrich G, Schaefer HE, Zeiher AM. Co-expression of p53 and MDM2 in human atherosclerosis: implications for the regulation of cellularity of atherosclerotic lesions. J Pathol. 1998;185:303–312. doi: 10.1002/(SICI)1096-9896(199807)185:3<303::AID-PATH106>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 29.Hashimoto T, Ichiki T, Ikeda J, Narabayashi E, Matsuura H, Miyazaki R, Inanaga K, Takeda K, Sunagawa K. Inhibition of MDM2 attenuates neointimal hyperplasia via suppression of vascular proliferation and inflammation. Cardiovasc Res. 2011;91:711–719. doi: 10.1093/cvr/cvr108. [DOI] [PubMed] [Google Scholar]
- 30.Gudkov AV, Gurova KV, Komarova EA. Inflammation and p53: a tale of two stresses. Genes Cancer. 2011;2:503–516. doi: 10.1177/1947601911409747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-κB as the matchmaker. Nat Immunol. 2011;12:715–723. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- 32.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kashatus D, Cogswell P, Baldwin AS. Expression of the Bcl-3 protooncogene suppresses p53 activation. Genes Dev. 2006;20:225–235. doi: 10.1101/gad.1352206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tergaonkar V, Pando M, Vafa O, Wahl G, Verma I. p53 stabilization is decreased upon NFκB activation: a role for NFκB in acquisition of resistance to chemotherapy. Cancer Cell. 2002;1:493–503. doi: 10.1016/s1535-6108(02)00068-5. [DOI] [PubMed] [Google Scholar]
- 35.Busuttil V, Droin N, McCormick L, Bernassola F, Candi E, Melino G, Green DR. NF-κB inhibits T-cell activation-induced, p73-dependent cell death by induction of MDM2. Proc Natl Acad Sci USA. 2010;107:18061–18066. doi: 10.1073/pnas.1006163107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ikeda A, Sun X, Li Y, Zhang Y, Eckner R, Doi TS, Takahashi T, Obata Y, Yoshioka K, Yamamoto K. p300/CBP-dependent and -independent transcriptional interference between NF-κB RelA and p53. Biochem Biophys Res Commun. 2000;272:375–379. doi: 10.1006/bbrc.2000.2786. [DOI] [PubMed] [Google Scholar]
- 37.Gu L, Zhu N, Findley HW, Woods WG, Zhou M. Identification and characterization of the IKKα promoter: positive and negative regulation by ETS-1 and p53, respectively. J Biol Chem. 2004;279:52141–52149. doi: 10.1074/jbc.M407915200. [DOI] [PubMed] [Google Scholar]
- 38.Kawauchi K, Araki K, Tobiume K, Tanaka N. Activated p53 induces NF-κB DNA binding but suppresses its transcriptional activation. Biochem Biophys Res Commun. 2008;372:137–141. doi: 10.1016/j.bbrc.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 39.Gu L, Findley HW, Zhou M. MDM2 induces NF-κB/p65 expression transcriptionally through Sp1-binding sites: a novel, p53-independent role of MDM2 in doxorubicin resistance in acute lymphoblastic leukemia. Blood. 2002;99:3367–3375. doi: 10.1182/blood.v99.9.3367. [DOI] [PubMed] [Google Scholar]
- 40.Vaughan C, Mohanraj L, Singh S, Dumur CI, Ramamoorthy M, Garrett CT, Windle B, Yeudall WA, Deb S, Deb SP. Human oncoprotein MDM2 up-regulates expression of NF-κB2 precursor p100 conferring a survival advantage to lung cells. Genes Cancer. 2011;2:943–955. doi: 10.1177/1947601911436008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheney MD, McKenzie PP, Volk EL, Fan L, Harris LC. MDM2 displays differential activities dependent upon the activation status of NFκB. Cancer Biol Ther. 2008;7:38–44. doi: 10.4161/cbt.7.1.5125. [DOI] [PubMed] [Google Scholar]
- 42.Veroux M, Tallarita T, Corona D, D'Assoro A, Gurrieri C, Veroux P. Sirolimus in solid organ transplantation: current therapies and new frontiers. Immunotherapy. 2011;3:1487–1497. doi: 10.2217/imt.11.143. [DOI] [PubMed] [Google Scholar]
- 43.Allam R, Sayyed SG, Kulkarni OP, Lichtnekert J, Anders HJ. Mdm2 promotes systemic lupus erythematosus and lupus nephritis. J Am Soc Nephrol. 2011;22:2016–2027. doi: 10.1681/ASN.2011010045. [DOI] [PMC free article] [PubMed] [Google Scholar]