Abstract
Smoking is a significant risk factor for pancreatic cancer, but the molecular mechanisms by which tobacco smoke components promote the growth and progression of these cancers are not fully understood. While nicotine, the addictive component of tobacco smoke, is not a carcinogen, it has been shown to promote the growth of non-small cell lung and pancreatic cancers in a receptor-dependent fashion. Here, we show that stimulation of pancreatic cancer cells with nicotine concentrations that are within the range of human exposure results in activation of Src kinase, which facilitated the induction of the inhibitor of differentiation-1 (Id1) transcription factor. Depletion of Id1 prevented nicotine-mediated induction of proliferation and invasion of pancreatic cancer cells, indicating that it is a major mediator of nicotine function. Nicotine could promote the growth and metastasis of pancreatic cancers orthotopically implanted into SCID mice; in addition, cells stably expressing a short hairpin RNA for Id1 did not grow or metastasize in response to nicotine. Nicotine could also confer resistance to apoptosis induced by gemcitabine in pancreatic cancer cells in vitro and depletion of Src or Id1 rendered the cells sensitive to gemcitabine. Further, nicotine could effectively inhibit the chemotherapeutic effects of gemcitabine on pancreatic tumors xenografted into mice. Clinical analyses of resected pancreatic cancer specimens demonstrated a statistically significant correlation between Id1 expression and phospho-Src, tumor grade/differentiation, and worsening overall patient survival. These results demonstrate that exposure to tobacco smoke components might promote pancreatic cancer progression, metastasis, and chemoresistance and highlight the role of Id1 in these processes.
Introduction
Pancreatic adenocarcinoma is the fourth leading cause of cancer-related deaths in the United States [1]. In 2011, it is estimated that 44,030 people will be diagnosed with pancreatic adenocarcinoma and 37,660 people will die of this disease [2]. Less than 20% of patients are allowed a surgical option that is presently the only potential for cure. Regardless, the overall 5-year survival remains less than 5%, with gemcitabine-based chemotherapeutic strategies partly due to the development of chemoresistance [1]. The molecular mechanisms that underlie gemcitabine resistance have not been fully defined [3,4]. Recent evidence suggests that deregulation of certain signaling molecules not only play a critical role in pancreatic tumor progression but can also facilitate chemoresistance [5].
Presently, the only established risk factor for pancreatic cancer is tobacco use [6,7]. Although exposure to cigarette smoke components such as polycyclic aromatic hydrocarbons and tobacco-specific N-nitrosamines have resulted in epigenetic events in human pancreatic ductal epithelial cells [8], the mechanism of how tobacco smoke components affects tumor growth and other malignant properties is not clearly understood. Evidence from our laboratory suggests that nicotine, the addictive component of tobacco, acts a tumor promoter in non-small cell lung cancer (NSCLC) cell lines potentially through Src-dependent phosphoinositide 3-kinase (PI3K)/Akt pathways [9]. Nicotine could inhibit apoptosis induced by chemotherapeutic drugs [9,10] in NSCLC cells, through the up-regulation of X-linked inhibitor of apoptosis protein (XIAP) and survivin. Recent evidence supports the role of nicotine on nicotinic acetylcholine receptors (nAChRs), and β-adrenergic receptors stimulate the growth and migration of pancreatic cancer cells [11,12]. It has been suggested that activation of these receptors through an autocrine catecholomine loop contribute to activation of Src, Erk mitogen-activated protein kinase (MAPK), and Akt signaling cascades [13]. Additionally, nicotine and cigarette smoke have been recently implicated in promotion of a metastatic phenotype through the activation of the α7 subunit of nAChR through the JAK2/STAT3 downstream signaling cascade [14]. Additionally, c-Src has been implicated in pancreatic tumor angiogenesis and metastasis [15,16] and its activation and overexpression contributes to a chemoresistant phenotype in pancreatic cancer preclinical models [17–19]. Although Src seems to play a pivotal role in these processes, the mechanisms accelerating Src signaling and the key Src-dependent downstream signaling pathways contributing to these aggressive and chemoresistant phenotype in pancreatic cancer are not clearly understood.
Studies on NSCLC cell lines have identified nicotine as a mitogen that induces proliferation by activating Src, with a concomitant increase in inhibitor of differentiation-1 (Id1) transcription factor [20]. Id1 is a helix-loop-helix (HLH) protein that plays a role in cell growth, senescence, and differentiation [21]. Its ability to form heterodimers with members of the basic HLH family of transcription factors leads to the inhibition of DNA binding and transcriptional repression of certain tumor suppressor proteins, leading to an oncogenic phenotype [22]. In preclinical models of pancreatic cancer, double knockdown of Id1/Id3 resulted in decreased metastatic potential [23]. Additionally, overexpression of Id1 correlates with tumor angiogenesis in resected human pancreatic cancer specimens [23,24].
While Id1 overexpression has been implicated in chemoresistance in certain specific systems [25,26], the role of Id1 in pancreatic tumor chemoresistance and the mechanisms that augment its expression are currently unknown. Studies presented here show that nicotine induces Id1 expression in pancreatic adenocarcinoma cells. Inhibition of Id1 prevented the nicotine-mediated proliferation of pancreatic cancer cells and enhanced their sensitivity to gemcitabine. Further, elimination of Id1 from cells greatly impaired their ability to form tumors in immunocompromised mice and inhibited metastasis. Analysis of human pancreatic cancer tissue microarrays (TMAs) showed a direct correlation between tumor grade, Src phosphorylation, and Id1 levels, suggesting that enhanced Id1 expression might have contributed to the genesis as well as growth of these tumors.
Materials and Methods
Cell Lines and Reagents
Human pancreatic cancer cells of ductal origin, PANC-1 and Mia-PaCa-2, were obtained from ATCC (Rockville, MD) and cultured as described [27]. The cell line CD18/HPAF was originally derived as previously described [28]. The L3.6pl metastatic variant pancreatic cancer cell line was derived from repeated cycle of injecting COLO 357 cells into the pancreas of nude mice, selecting for liver metastases [15,29]. Gemcitabine (Eli Lilly, Indianapolis, IN) was suspended in Dulbecco's phosphate-buffered saline (PBS). Nicotine stimulation was performed on cells that were rendered quiescent by serum starvation for 48 hours with 1 µM nicotine (Sigma Chemical Company, St Louis, MO). Src family kinase inhibitor dasatinib (BMS-354825) was obtained from Bristol-Myers Squibb (Princeton, NJ).
Selection of L3.6plGemRes Gemcitabine-resistant Pancreatic Cancer Cells
Selection of L3.6plGemRes gemcitabine-resistant pancreatic cancer cells was performed as previously described [17,18]. Briefly, L3.6pl gemcitabine-sensitive cells were exposed to 5 µM gemcitabine and steadily increased by 5-µM increments every 2 days to maximal concentration of 30 µM, approximately 12-fold greater than the half-maximal inhibitory concentration (IC50). Single colonies of gemcitabine-resistant clones were expanded for further analysis.
Antibodies, Immunoprecipitation, and Western Blot Analysis
Lysates were prepared from cells and tumor tissues, and immunoprecipitation and Western blots were conducted as previously described [30]. Monoclonal Id1 antibodies from BioCheck (Foster City, CA), monoclonal antibodies to c-Src (Upstate Biotechnology, Lake Placid, NY), p44/42 Erk MAPK and Akt (5G3) (Cell Signaling Technology, Beverly, MA), polyclonal α7 nAChR (Abcam, Cambridge, MA), phospho-Src (pSrc), phospho-AktS473, phospho-p44/42 ErkT202/Y204, β-actin monoclonal antibody (Sigma, St Louis, MO), and PARP (Cell Signaling Technology) antibodies were used in Western blot analysis.
Generation of Stable Cell Lines
L3.6pl cells were transfected with pCDNA3.1-luciferase expression construct using HD Fugene transfection reagent (Roche, Indianapolis, IN). Stable cells were selected using G418 (200 mg/ml). Individual clones were maintained in G418 selection media and the expression of luciferase gene was determined by treatment with luciferin (Caliper Life Sciences, Alameda, CA) and imaging on IVIS200.
For generation of Id1 null cells, luciferase expressing L3.6pl pancreatic cancer cells were stably transfected with short hairpin RNA (shRNA) constructs that specifically target Id1 (Open Biosystems, Huntsville, AL). A control cell line was generated by stably transfecting an shRNA that does not target any recognized genes. Luciferase expressing L3.6pl-Id1-shRNA and L3.6pl-control-shRNA cell lines, referred to as shId1 and shcontrol, respectively, were maintained in 1 µg/ml puromycin, 200 mg/ml G418, and 10% FBS.
Cell Viability/Cytotoxicity and Invasion Assays
Cell viability was quantified by 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide assay (Trevigen, Gaithersburg, MD). Cells (1 x 103) were seeded per well in 96-well plates and allowed to adhere overnight. Cells were treated with nicotine (1 µM) and/or 5 µM of gemcitabine (Gemzar) and cellular proliferation and viability assayed after 48 hours using published protocols [16]. Boyden chamber assays were conducted to measure invasion, as described before [10,26].
siRNA Transfections
Id1, Src, and α7 nAChR subunit siRNA oligonucleotides were purchased from Santa Cruz Biotechnology (Sta Cruz, CA). Non-target siRNA was used as the control. Pancreatic cancer cells were transfected either with control (100 pmol), Id1 (100 pmol), Src (100 pmol), or α7 nAChR subunit (50 pmol) siRNA using Oligofetamine (Invitrogen, Grand Island, NY). Western blot analysis was used to monitor the expression level of Id1, Src, and α7 nAChR subunit.
Reverse Transcription-Polymerase Chain Reaction and Quantitative Real-time Polymerase Chain Reaction
RNA was isolated using the RNeasy MiniKit (Qiagen, Valencia, CA) and cDNA was prepared with AMV reverse transcriptase (Promega, Madison, WI). Polymerase Chain Reactions (PCRs) were performed using 1 µg of cDNA. Primers (Integrated DNA Technologies, Coralville, IA) used for amplifying α7 nAChR subunit are previously described [10,31]. PCR products were analyzed on a 1% agarose gel in TAE 1x buffer using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression as a loading control [10].
Id1 mRNA levels were analyzed by quantitative real-time PCR on a Biorad iCycler. The primers used were Id1-forward primer, 5′-GAG CTG AAC TCG GAA TCC GAA G-3′ and Id1-reverse primer, 5′ GAT CGT CCG CAG GAA CGC ATG C3′. Data were normalized using 18S RNA as internal control and the fold change in the expression relative to nontargeting siRNA was determined.
Mouse Xenograft Experiments
Single-cell suspensions in Dulbecco's PBS were used for implantation in 8-week-old female athymic nude mice (Charles River Laboratories, Wilmington, MA); viability of the cells used was greater than 90%, as determined by trypan blue exclusion. Orthotopic implantation of pancreatic tumor cells was performed as previously described [16]. One week after implantation, mice were administered nicotine (1 mg/kg, every Monday/Wednesday/Friday), gemcitabine (250 mg/kg, Tuesday/Thursday), or PBS control through intraperitoneal injections. Tumor growth was monitored by IVIS200 imaging. Mice were killed 4 weeks after implantation, and primary tumors, regional peripancreatic (celiac and para-aortic) lymph nodes, and livers were examined for tumor cells by ex vivo imaging.
Patients
The study cohort included patients who underwent pancreatic resections between 1986 and 2006 at the H. Lee Moffitt Cancer Center and Research Institute. Patients were identified by searching the surgical databases, the pathology information system, and the Moffitt Cancer Registry. Data from the Moffitt Cancer Registry, the patient's medical record, and the Social Security Death Index were reviewed to determine overall survival. The slides of the pancreatic resections were reviewed by a pathologist (B.A.C.), and the histopathologic features including grade and tumor type were established. All data were collected under Institutional Review Board (IRB) approval.
Pancreatic Cancer TMAs
Pancreatic ductal adenocarcinoma resected at the Moffitt Cancer Center from 1987 to 2006 was used to generate TMAs. Tissue specimens were fixed and processed into paraffin blocks, and two separate cores, 0.6 cm in diameter, were obtained for each area of carcinoma and from the nonmalignant areas of the same patient. TMAs were stained with hematoxylin and eosin (H&E) [16] and grade (I–IV) was assigned to the each core using a standard grading system based on tubular differentiation [CAP (College of American Pathologists) protocols]. The first two TMAs consisted of 50 nonmalignant cores and 50 paired carcinoma cores, and the third comprised 44 non-malignant cores and 44 carcinoma cores.
Immunohistochemistry
Immunohistochemical (IHC) staining was performed using a Ventana Discovery XT Automated System (Ventana, Tucson, AZ) as per the manufacturer's protocol [32]. Primary antibodies against Id1 (BioCheck), phospho-Src416 (Millipore, Billerica, MA), and Src (Epitomics, Burlingame, CA) were used for IHC.
Scoring of IHC. Both the intensity and percentage of cells expressing Id1, pSrc, and Src were assessed using a previously established and recognized semiquantitative scoring system for IHC staining [33,34]. The intensity of staining was assessed as 0 (absent), 1+ (weak), 2+ (moderate), and 3+ (strong) for Id1 and as 0 (absent), 1+ (weak), 2+ (moderate), 3+ (strong), and 4+ (very strong) for pSrc and Src. A visual histoscore was assigned to each core, which consisted the sum of the products of the intensities times the percentage of positive cells per intensity.
Densitometric analysis of IHC. TMA slides were scanned using the ScanScope XT (Aperio, Vista, CA) with a x200/0.75 NA objective lens at a rate of 3 minutes per slide via Basler trilinear array.
Regions for analysis were manually selected by the study pathologist (B.A.C.).
Immunopositivity of each biomarker was quantitatively scored using the TissueStudio v3.0 software platform from Definiens (Munich, Germany). The staining intensity for very strong (4+), strong (3+), moderate (2+), weak (1+), and negative (0) compartments were threshold by the pathologist (B.A.C.) and locked to ensure consistency throughout the study. For each case, the number and percentage of each classified group of cells was exported for statistical analysis.
Statistical Analyses
Statistical analysis for the experiments was performed using two-tailed Student's t test. Experiments were performed in triplicate and values were considered significant when the P value was <.05. Correlation of IHC expression with grade in human samples was performed by comparing the grade within each core to the histoscore for the antibody. To compare IHC expression to patient level variables, such as overall grade and survival, we calculated the mean value for protein expression per patient. To compare grade within each core, or overall grade, to IHC expression, Spearman rho statistic was used, along with associated P values. To compare IHC expression to survival, we fit a Cox proportional hazards regression model. We also split IHC expression into high and low expression using the 75% cut point to compare the high 25% to low 75% IHC expression of Id1. To compare the high and low expression groups, we fit Kaplan-Meier survival curves and tested the difference using a log-rank test. Overall grade was grouped into low (1/2) and high (3/4) grades to fit Kaplan-Meier survival curves and tested the difference using a log-rank test. To compare pathologist to algorithm expression, we considered the Spearman rho statistic, along with associated P value. All tests were considered two-sided unless otherwise specified, and the patient-related analysis was performed using SAS 9.3 and R 2.14.2.
Results
Nicotine Promotes Id1 Expression through a Src-dependent Signaling Axis
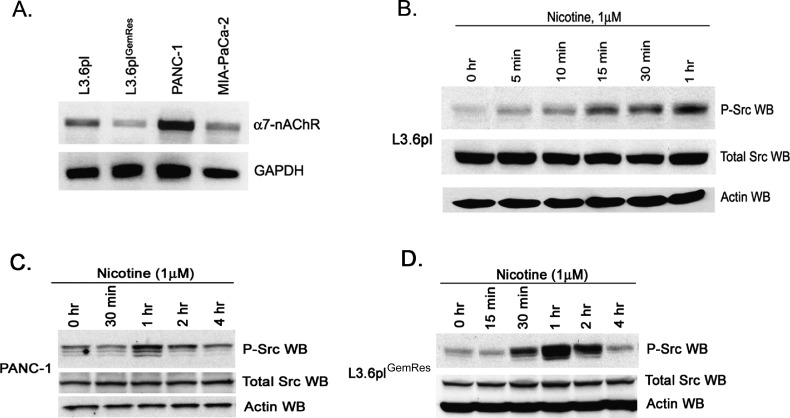
Nicotine has been shown to promote the proliferation of cells, invasion, and angiogenesis through nAChRs [20,35]. As described in previous reports, the tumor promoting effects of nicotine have been mediated primarily by ligand binding to the α7 nAChR subunit [36]. To determine if nicotine could affect pancreatic adenocarcinoma cells through this receptor, we examined the presence of α7 nAChR in pancreatic cancer cell lines L3.6pl, L3.6plGemRes, PANC-1, and Mia-Pa-Ca-2. Semiquantitative reverse transcription. PCR showed the presence of α7 nAChR subunit mRNA in all the four cell lines tested (Figure 1A). This raises the possibility that these pancreatic cancer cell lines would be able to respond to the tumor-promoting effects of nicotine.
Figure 1.
Nicotine induces Src phosphorylation in pancreatic adenocarcinoma cells. (A) Expression of α7 subunit of nAChR as seen by reverse transcription-PCR in a variety of pancreatic cancer cell lines. (B) Quiescent L3.6pl metastatic pancreatic cancer cells stimulated with nicotine induce Src phosphorylation within 1 hour. (C and D) Nicotine induces Src phosphorylation in PANC-1 and acquired gemcitabine-resistant L3.6plGemRes pancreatic cancer cells with a maximal phosphorylation event at 1 hour with return to baseline within 4 hours.
We next determined the effect of nicotine on Src tyrosine activity, which is known to promote proliferation as well as chemoresistance. Stimulation of pancreatic cancer cells L3.6pl with nicotine demonstrated an increase in Src phosphorylation (Figure 1B), as has been reported before [13]. The phosphorylation levels were markedly higher 15 minutes after stimulation and persisted for at least 1 hour and no appreciable change in the levels of total Src protein. Similar results were noted in PANC-1 and L3.6plGemRes pancreatic cancer cells (Figure 1, C and D), suggesting that exposure to nicotine can induce Src kinase activation in multiple pancreatic cancer cell lines.
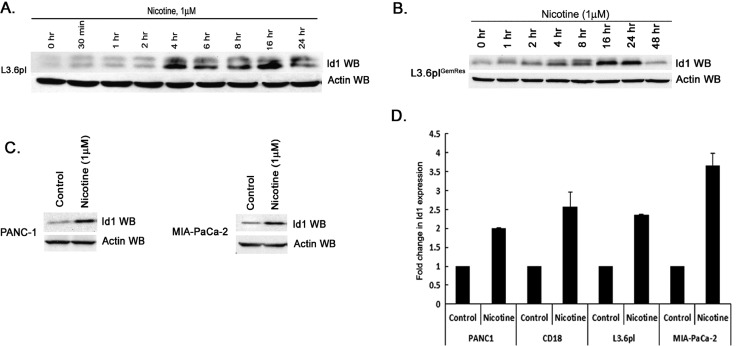
Because Id1 transcription factor is a downstream target of Src signaling in NSCLC cells, experiments were conducted to assess whether nicotine stimulation led to an induction of Id1 expression in multiple pancreatic cancer cell lines. Western blot analysis of lysates from nicotine-stimulated L3.6pl and L3.6plGemRes pancreatic cancer cells showed an induction of Id1 protein 4 hours after nicotine stimulation, and the levels remained elevated at least for 24 hours (Figure 2, A and B, respectively). These results were confirmed in PANC-1 and MIA-PaCa-2 cells at 4 hours (Figure 2C). To examine whether the induction occurred at the transcriptional level, we conducted real-time PCR experiments on quiescent or nicotine-stimulated cells. It was found that stimulation with 1 µM nicotine leads to a two-fold to four-fold induction of Id1 expression in a panel of cell lines, suggesting that nicotine induced Id1 levels at the level of transcription (Figure 2D).
Figure 2.
Nicotine induces Id1 protein expression in pancreatic adenocarcinoma cells. (A and B) L3.6pl and acquired gemcitabine-resistant L3.6plGemRes demonstrate significant increases in Id1 expression when quiescent cells are exposed to 1 µM nicotine. (C) Similarly, nicotine induces Id1 expression in PANC-1 and Mia-PaCa-2 pancreatic cancer cells. (D) Nicotine stimulation leads to increased Id1 mRNA expression levels in a diverse sample of pancreatic cancer cells lines, suggesting that the induction is at the transcriptional level.
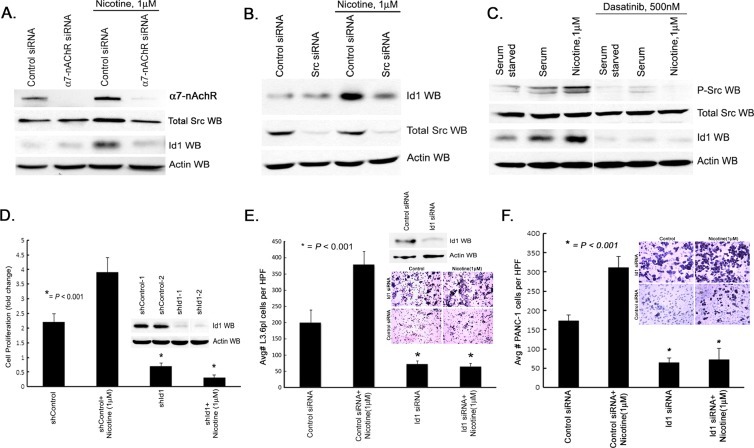
Attempts were then made to determine if the signaling events leading to an increase in Id1 expression are dependent on the α7 nAChR and Src in L3.6pl pancreatic cancer cells. Toward this purpose, L3.6pl cells were transfected with an siRNA to α7 nAChR subunit or a nontargeting control siRNA. Cells were rendered quiescent by serum starvation and stimulated with 1 µM nicotine for 6 hours. Western blot analysis showed that transfection with the α7 nAChR siRNA led to a marked reduction in the levels of this receptor; there was no reduction in the levels of Src (Figure 3A). Nicotine stimulation of control siRNA-transfected cells led to a modest increase in the levels of Src and a significant increase in the levels of Id1 protein as seen by Western blot analysis. There was no increase in cells transfected with an siRNA to α7 nAChR (Figure 3A), suggesting that this subunit is necessary for the nicotine-mediated induction of Id1. Similarly, depletion of Src by siRNA prevented nicotine-mediated induction of Id1 (Figure 3B). The requirement for Src for the nicotine-mediated induction of Id1 was further verified using dasatinib, a Src kinase inhibitor. L3.6pl cells were rendered quiescent by serum starvation and stimulated with serum or 1 µM nicotine in the presence or absence of 500 nM dasatinib. It was found that inhibiting Src kinase completely eliminated Src phosphorylation as well as induction of Id1 by both nicotine as well as serum (Figure 3C). These results show that α7 nAChR and Src are involved in the induction of Id1 by nicotine in pancreatic cancer cells and that Id1 is a mediator of Src function in these cells.
Figure 3.
Nicotine induces Id1 expression in a Src-dependent manner and regulates proliferation and invasion of pancreatic adenocarcinoma cells. (A) Silencing of α7 nAChR inhibits nicotine-induced Src and Id1 expression L3.6pl pancreatic cancer cells. (B) Silencing of Src expression inhibits nicotine induction of Id1 expression in L3.6pl pancreatic cancer cells. (C) Pharmacologic inhibition of Src phosphorylation with dasatinib inhibits constitutive and nicotine-induced levels of Id1 expression in L3.6pl pancreatic cancer cells. (D) shRNA to Id1 expression demonstrates significantly less Id1 when compared to controls (inset). Nicotine promotes cell viability and silencing of Id1 significantly diminishes this effect. (E and F) Nicotine promotes invasion in L3.6pl and PANC-1 pancreatic cancer cells and silencing of Id1 significantly diminishes this effect as demonstrated by cells per high-power field (HPF) and hematoxylin staining of both pancreatic cancer cells on membrane (inset).
Depletion of Id1 Inhibits Nicotine-induced Proliferation and Invasion of Pancreatic Cancer Cells
Our earlier studies had shown that nicotine could induce the proliferation and invasion of lung cancer cells and Id1 played a role in this process. Experiments were conducted to examine whether nicotine induced similar events in pancreatic cancer cells and whether Id1 was necessary for this. Toward this purpose, L3.6pl pancreatic cancer cells stably transfected with a control, a nontargeting shRNA, or an shRNA to Id1 were rendered quiescent by serum starvation and subsequently stimulated with 1 µM nicotine. As expected, cells transfected with an shRNA to Id1 had significantly less Id1 (Figure 2D, inset); stimulation with nicotine increased the number of control shRNA-transfected cells but not shId1-transfected cells, as seen by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay (P < .001; Figure 3D). Similarly, Boyden chamber assays were conducted to assess the role of Id1 in nicotine-mediated invasion of L3.6pl and PANC-1 pancreatic cancer cells. As shown in Figure 3, E and F, nicotine-stimulated control cells invaded through the matrix effectively, but invasion was greatly impaired in cells depleted of Id1 (P < .001). This suggests a definite role for Id1 in mediating nicotine-induced proliferation and invasion of pancreatic cancer cells.
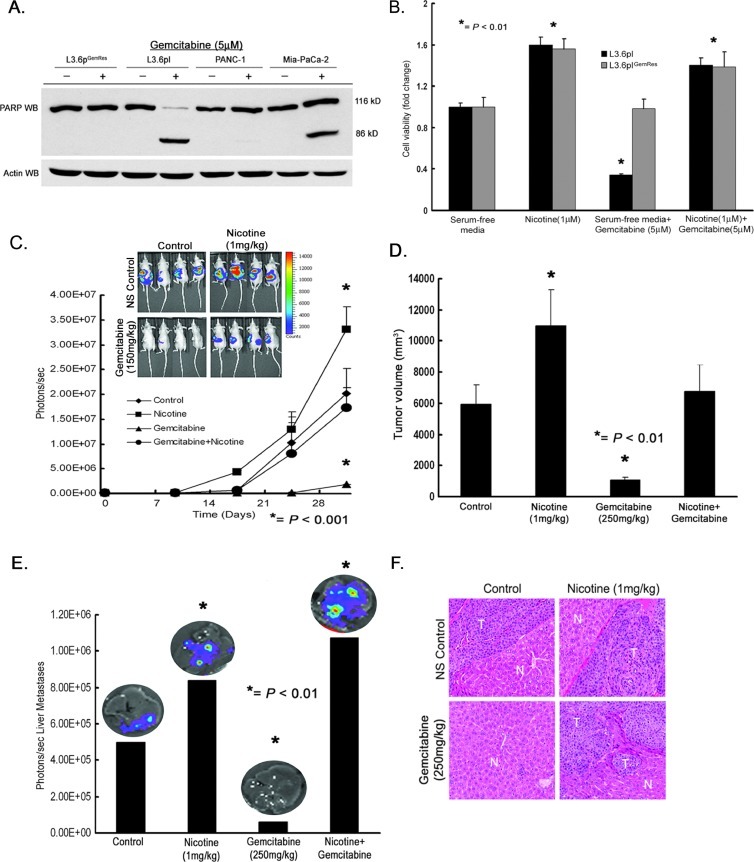
Nicotine Promotes Chemoresistance in Pancreatic Cancer Cells
One clinical challenge in the management of pancreatic cancer is the resistance to chemotherapeutic treatment strategies [5]. Therefore, we identified pancreatic cancer cells with sensitivities to gemcitabine, the first-line chemotherapeutic agent for patients with pancreatic cancer, to determine the role of nicotine and Id1 in chemoresistance. To study acquired chemoresistance, we established gemcitabine resistance in the L3.6pl gemcitabine-sensitive cell line, L3.6plGemRes, as described previously [37]. A panel of pancreatic cancer cells was exposed to 5 µM gemcitabine, and Western blot analyses were conducted for cleavage of PARP, indicative of apoptosis. It was found that gemcitabine induced apoptosis in L3.6pl and Mia-PaCa-2 cells (Figure 4A) but not in PANC-1 cells, which was innately resistant neither to gemcitabine nor in the L3.6plGemRes cells, which had acquired resistance to gemcitabine. To assess whether nicotine affected the sensitivity of the cells to gemcitabine, we treated L3.6pl and L3.6plGemRes cells with gemcitabine in the presence or absence of nicotine. It was found that gemcitabine reduced the viability of L3.6pl cells by about 80% but not L3.6plGemRes cells; nicotine increased the viability of both the cell lines by about 60% (Figure 4B). Interestingly, gemcitabine treatment in the presence of nicotine completely reversed the gemcitabine sensitivity of L3.6pl cells. This suggests that nicotine has protective effects on pancreatic cancer cells as well and might reduce chemosensitivity in patients.
Figure 4.
Nicotine induces a chemoresistant phenotype in pancreatic cancer cells and tumors. (A) Gemcitabine induces PARP cleavage in L3.6pl and Mia-PaCa-2 cells and not in acquired and innate resistant cells L3.6plGemRes and PANC-1, respectively (left panel). (B) Nicotine induces cell viability/proliferation in both sensitive L3.6pl and acquired chemoresistant L3.6plGemRes cells and nicotine induces a chemoresistant phenotype in the chemosensitive L3.6pl pancreatic cancer cell line. (C) Tumor growth monitored by luciferase activity (photons per second) in L3.6pl gemcitabine-chemosensitive cells demonstrates augmented growth in mice treated with nicotine, containment of tumor growth in mice treated with gemcitabine, and continued tumor growth in mice treated with nicotine and gemcitabine comparable to control with in vivo luciferase imaging of mice at termination of experiment demonstrating these effects (inlet). (D) Graph demonstrating the corresponding tumor volume (mm3) from each animal group. (E) Ex vivo imaging of livers, from each animal group, demonstrates significantly higher liver metastasis in mice treated with nicotine compared to control, no liver metastasis in mice treated with gemcitabine, and overall increased signal from liver in mice treated with nicotine and gemcitabine when compared to all groups as seen by quantification of luciferase activity (photons per second). (F) H&E staining of liver metastases (T) confirming metastatic deposits of pancreatic tumor adjacent to normal liver parenchyma (N).
The effects of nicotine on gemcitabine resistance were also determined in vivo. Luciferase expressing L3.6pl gemcitabine-sensitive metastatic variant cells were orthotopically implanted into the pancreas of SCID mice and randomized to control PBS, nicotine, gemcitabine, or nicotine + gemcitabine. Nicotine was administered 3 days per week at 1 mg/kg and gemcitabine was administered 250 mg/kg 2 days a week. As shown in Figure 4C, administration of nicotine enhanced the growth of tumors in vivo, whereas administration of nicotine significantly reduced the growth of tumors (P < .001), as determined by luciferase expression (photons per second). At the same time, administration of nicotine significantly inhibited the chemotherapeutic effects of gemcitabine, and tumors were comparable in size to control, untreated mice. Measurement of tumor volumes at the termination of the experiment after 4 weeks confirmed these results (Figure 4D). It was also found that nicotine promoted pancreatic tumor liver metastases regardless of gemcitabine exposure (Figure 4E) as quantified by luciferase expression in liver ex vivo. The luciferase expression in the liver, which is suggestive of pancreatic cancer liver metastases, was confirmed by H&E (Figure 4F) for each group. Thus, while gemcitabine had a therapeutic effect on L3.6pl cells in mice, exposure to nicotine abrogated this effect and established a chemoresistant phenotype.
Depletion of Id1 Expression Reverses Acquired and Innate Gemcitabine Chemoresistance and Inhibits Nicotine-induced Primary Tumor Growth and Metastases
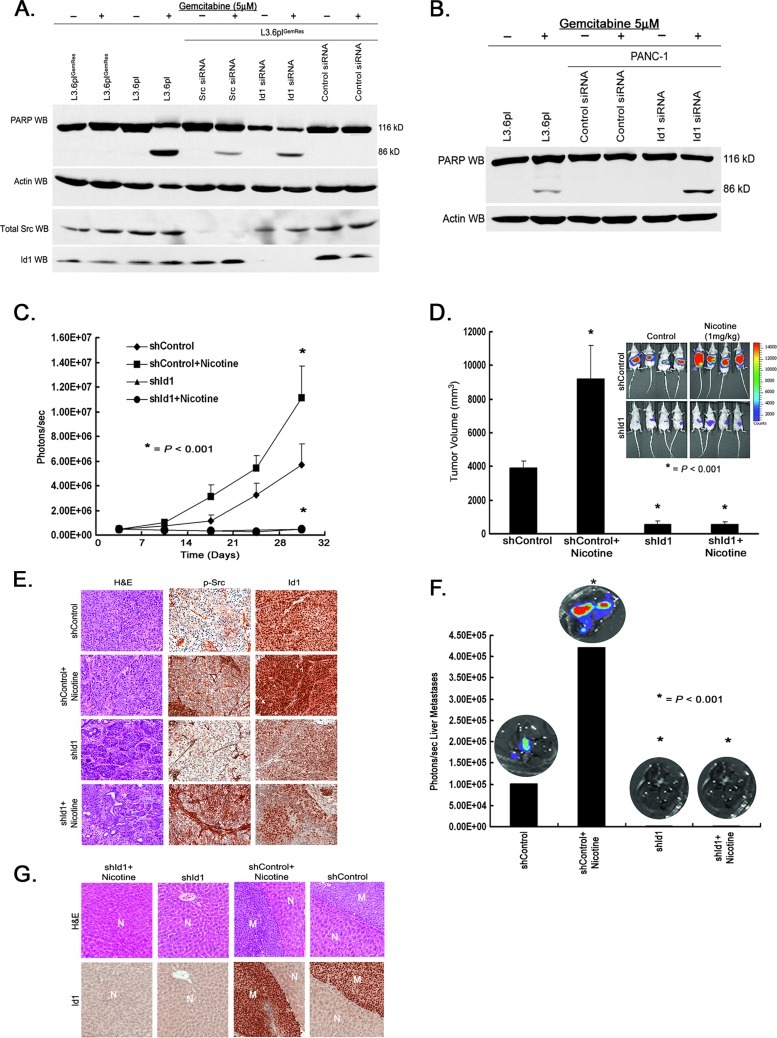
It has been demonstrated that Src activity regulates gemcitabine chemoresistance in preclinical pancreatic cancer models [17,18], but the downstream modulators of Src-mediated chemoresistance are not known. Because we had observed that Id1 is a downstream target of Src, we examined whether Id1 contributes to chemoresistance. Toward this purpose, L3.6plGemRes cells as well as PANC-1 cells were transiently transfected with siRNA to Src as well as Id1 or a nontargeting control siRNA. Cells were treated with 5 µM gemcitabine and apoptosis was assessed by Western blot analysis for PARP cleavage. As shown in Figure 5, A and B, depletion of Id1 expression in acquired and innate gemcitabine-resistant L3.6plGemRes and PANC-1 cells resulted in PARP cleavage and sensitization to gemcitabine. This suggests that Id1 is a potential mediator of chemoresistance induced by Src.
Figure 5.
Depletion of Id1 abrogates chemoresistant phenotype and tumorigenic properties of pancreatic cancer cells. (A) Depletion of Src and Id1 using siRNA induces PARP cleavage in L3.6plGemRes-acquired.resistant pancreatic cancer cells exposed to gemcitabine therapy. (B) Depletion of Id1 expression in innate chemoresistant PANC-1 cells induces PARP cleavage and resensitizes cells to the cytotoxic effects of gemcitabine. (C) Tumor growth monitored by luciferase activity (photons per second) over approximately 4 weeks demonstrates increased tumor burden in mice treated with nicotine, whereas Id1 depletion resulted in inhibition of tumor growth regardless of nicotine exposure. (D) Graph showing the corresponding tumor volume (mm3) from each animal group corresponding to in vivo luciferase imaging of mice at termination of experiment (inset) demonstrating the promoting effects of nicotine and the suppressive effects of Id1 silencing on primary tumor growth. (E) Nicotine-induced pSrc and Id1 expression levels in primary tumors in vivo while having no effect on Id1 expression in tumors originating from cells with stably transfected shRNA silencing of Id1. (F) Ex vivo quantification of luciferase activity (photons per second) from livers demonstrates significantly higher liver metastasis in mice treated with nicotine while demonstrating no liver metastasis in mice with tumors derived from Id1-depleted cells. (G) H&E staining of livers (N) with metastatic pancreatic tumor demonstrates increased expression of Id1 in metastatic deposits (M).
To determine if the effects of nicotine on pancreatic cancer cells observed could be via Id1, in vivo experiments were conducted. Toward this purpose, metastatic L3.6pl cells stably expressing a luciferase gene were transfected with a control shRNA or an shRNA to Id1 and orthotopically implanted into the pancreas of SCID mice. Nicotine (1 mg/kg) was administered by intraperitoneal injection three times a week and tumor growth was assessed by IVIS200 imaging. Correlating with our in vitro data, nicotine promoted primary tumor growth over a period of 4 weeks as determined by luciferase expression as photons per second (Figure 5C). While the growth of control shRNA-transfected cells was promoted by nicotine, there was no stimulation in the growth of cells transfected with Id1 shRNA; indeed, these cells hardly formed any tumors in mice. In agreement with the luciferase expression data (Figure 5D, inset), tumors stimulated with nicotine had a larger volume as measured at the termination of the experiment and the largest tumors were in control cells that were stimulated with nicotine (Figure 5D). IHC analyses of the primary tumors demonstrated an induction of pSrc in tumors exposed to nicotine, compared to tumors from control mice. Although nicotine was able to increase Id1 expression in control tumors, this effect was not observed in tumors derived from pancreatic cancer cells with stable Id1 depletion (Figure 5E), despite the fact that phosphorylation of Src was increased in these tumor cells.
L3.6pl cells are highly metastatic, which resemble human pancreatic cancer; hence, livers of mice were examined for metastatic foci at the termination of the experiment. Liver metastases were detected by luciferase expression ex vivo and confirmed by H&E staining in mice implanted with cells transfected with control shRNA, both vehicle and nicotine-stimulated animals. It was found that nicotine stimulation had promoted the metastasis of L3.6pl cells to the liver; at the same time, stable knockdown of Id1 expression significantly decreased this metastatic potential regardless of nicotine exposure (P < .001; Figure 5F). IHC analyses of liver metastases confirmed disease and also demonstrated an increased Id1 expression level (Figure 5G). These results suggest that nicotine can induce a more aggressive phenotype potentially through by a Src-Id1 signaling axis in vivo and depletion of Id1 expression can inhibit these effects.
Id1 Expression Correlates with Src Activation, Poor Tumor Differentiation, and Reduced Overall Survival in Human Pancreatic Cancer Patients
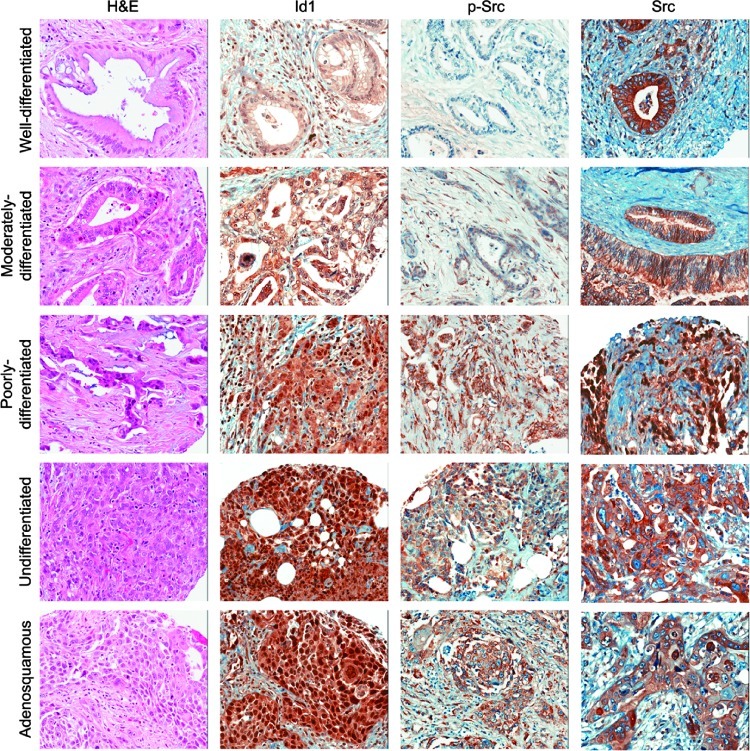
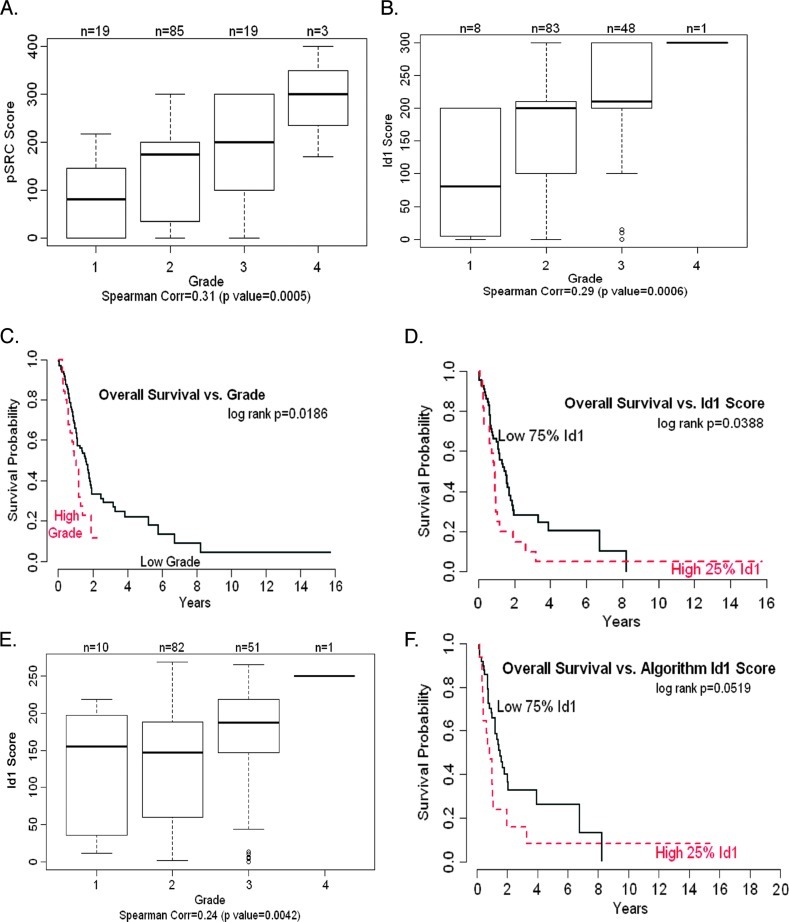
Because Id1 and Src appear to be involved in the proliferation as well as survival of pancreatic adenocarcinoma cells, experiments were conducted to assess whether there is a correlation between the levels of these proteins and tumor grades in surgically resected patients. As a first step, a total of 130 resected specimens were confirmed by H&E for malignancy and subsequently stained for Id1, pSrc, and total Src (Figure 6). Assessment of pathologic grade of the resected specimen showed the following distribution in these patients: 30 grade I carcinomas, 74 grade II, 25 grade III (including 1 squamous cell carcinoma), and 1 grade IV. The grades were combined into two groups, low grade (grade I + grade II) and high grade (grade III + grade IV), for the purpose of statistical analysis. Ninety-two patients had evaluable expression for Id1, of a total of 140 cores analyzed (8 grade I, 83 grade II, 48 grade III, and 1 grade IV). Eighty-seven patients had evaluable pSrc expression, with 136 cores containing cancer analyzed (19 grade I, 85 grade II, 19 grade III, and 3 grade IV). Ninety-seven patients had evaluable Src expression for Src with 166 cores analyzed. IHC staining of pancreatic tumors of worsening differentiation demonstrated increased expression of Id1 and pSrc expression levels (Figure 6). Further quantitative analyses demonstrated a statistically significant (P = .009) correlation between Id1 and phospho-Src levels. Levels of pSrc and Id1 expression significantly correlated independently with tumor grade/differentiation (P = .0005, Figure 7A and P = .0006, Figure 7B, respectively). Analysis of overall survival and tumor grade was also significant (P = .0186, Figure 7C); the correlation between patient overall survival and Id1 expression as a continuous variable was statistically significant (P = .0225). When dichotomizing Id1 expression levels into high and low expression using the 75% cut point to fit Kaplan-Meier survival curves, a significant difference was also observed for overall survival with patients with higher Id1 expression levels having a worse overall survival (P = .0388, Figure 7D). Thus, Id1 and pSrc expression not only correlated with each individual expression level, but they also independently correlated with decreased tumor differentiation. Most importantly, as Id1 expression levels increased in resected pancreatic tumors, patients had a decrease in overall survival.
Figure 6.
Id1 expression in resected human pancreatic adenocarcinoma. TMAs constructed from resected pancreatic ductal adenocarcinoma were stained for Id1, pSrc (activated form), and total Src using standard IHC techniques. A statistically significant correlation was observed between Id1 and tumor grade/differentiation and pSrc expression levels. No significant correlation was seen between total Src and Id1 expression.
Figure 7.
Worsening tumor grade correlates with increased pSrc and Id1 expression levels and increased Id1 expression correlates with poor overall survival in pancreatic cancer patients. (A) Increased tumor grade level demonstrates a clear correlation with higher pSrc expression levels (score) with a Spearman correlation estimate of 0.31 (P < .001). (B) Increased tumor grade level is associated with increased Id1 expression (score) with a Spearman correlation estimate of 0.29 (P < .001). (C) Patients with high histologic grade have significantly worse survival than patients with low histologic grade (P = .0235). (D) Dichotomizing Id1 scores into low and high scores using the 75% score demonstrates significant correlation with overall patient survival, with high Id1 expression levels resulting in a worse overall survival (P = .0388). (E) Increased tumor grade level demonstrates a significant correlation with increased Id1 expression (algorithmic densitometric analyses) with Spearman correlation estimate of 0.24 (P = .0042). (F) Dichotomizing Id1 scores (determined by quantitative algorithm) into low and high scores with 75% score demonstrates a significant correlation with overall patient survival with high Id1 expression levels resulting in a worse overall survival (P = .0519).
Supporting our results above, quantitative densitometric analyses were performed on the IHC correlations of Id1, pSrc, and Src. To this effect, TMAs were scanned and analyzed as described in Materials and Methods section. Pathologist semiquantitative histoscore and algorithmic values produced from our densitometric analyses were compared using the Spearman correlation coefficient. There was a strong correlation between pSrc and Id1 pathologist when compared to algorithmic values, with a correlation coefficient of 0.71 (P < .001) and 0.65 (P < .001), respectively, and a moderate correlation between pathologist and algorithmic values of Src, with a correlation coefficient of 0.31 (P < .001). Comparison of algorithmic Id1 to pSrc expression levels also demonstrated a significant correlation coefficient of 0.72 (P < .0001). Supporting our semiquantitative analyses, algorithmic Id1 expression levels significantly correlated independently with tumor grade/differentiation (P = .0186). When comparing overall patient survival with Id1 algorithmic values, the significance level increases only slightly, to P = .0519, but comparable to semiquantitative pathologic values. These results indicate that the pathologist semiquantitative histoscore method is an effective method for assessing protein expression in the case of the antibodies used in this study.
Altogether, these results suggest a Src-Id1 signaling axis that might contribute to pancreatic tumor progression, decreased tumor differentiation, and worse clinical outcomes.
Discussion
Although humans express four types of Id1 protein, increasing evidence supports the function of the HLH protein Id1 as an oncogene in more than 15 types of epithelial cancers [22,38–40] through inactivation of p16INK4a/RB and p53 tumor suppressors, two early hallmark mutations in pancreatic ductal adenocarcinoma [21]. Id1 facilitates the growth and metastases of NSCLC cells in response to nicotine activation of the α7 nAChR [20]. Previous studies demonstrate that nicotine can also inhibit apoptosis by up-regulation of XIAP and survivin through up-regulation of a Src-dependent PI3K/Akt pathway [9] and these effects might be through an autocrine catecholamine loop that is activated by nAChRs [13], or directly through α7 receptors as seen in NSCLC cells. The tumor-promoting effects of nicotine and cigarette smoke have also been recently reported in pancreatic cancer through MUC4-mediated signaling [14]. Although Id1 has been implicated as a tumor promoter in a variety of systems, the effects of tobacco components such as nicotine on Id1 and how it effects pancreatic tumor progression and chemoresistance are poorly understood. Previous data have reported that inactivation of Id1 promotes chemosensitivity in prostate and nasopharyngeal cancer cells, through activation of the c-Jun N-terminal kinase and Raf/MAP kinase kinases (MEK) pathways, respectively [25,41]. In our studies, we demonstrated that nicotine induction of Id1 expression results in enhanced malignant properties and chemoresistance in pancreatic cancer.
Pancreatic cancer remains an insistent disease partly due to innate or the development of acquired resistance to chemotherapy [5]. Patients with intrinsic drug resistance suggest that therapy will be ineffective from the initiation of therapy, whereas those with acquired drug resistance develop this phenotype after exposure to therapy. Interestingly, cancer cells that develop acquired resistance initially demonstrate some efficacy to treatment, but continued treatment can lead to progression of disease. The mechanisms into acquired chemoresistance are the acquisition of ATP-binding cassette transporters, which clear chemotherapeutic drugs from cancer cells, unresponsive drug-induced apoptosis, and initiation drug detoxification mechanisms [42], whereas the tumor microenvironment has been implicated in innate chemoresistance [43]. Signaling proteins such as Akt, nuclear factor-kappaB (NF-κB), and MAP/ERK kinase, as well as secreted proteins such as vascular endothelial growth factor, have been identified as having important roles in drug resistance in pancreatic cancer [44–46], and previous reports suggest that Src kinase is an upstream signaling such proteins mentioned above [47–49]. Therefore, it is not surprising that Src tyrosine kinase overactivity represents a chemoresistant mechanism in pancreatic cancer preclinical models. Specifically, inhibition of Src activity or expression augmented gemcitabine-induced caspase-mediated apoptosis, suppressed RRM2 expression, and decreased activity of the RRM2-regulating transcription factor E2F1 [17,18]. Smoking is a principal risk factor for pancreatic cancer with nicotine as the additive component and nicotine has been demonstrated to promote tumor growth and angiogenesis and protect cancer cells from apoptosis in lung cancer models in a Src-dependent manner [9,20,50]. In our studies, we present a novel Src-dependent mechanism for gemcitabine resistance regulated by the tumor-promoting effects of nicotine.
In the present era, pancreatic cancer remains a devastating disease with adjuvant therapies providing modest clinical results. Identification of key molecular targets that enhance chemoresistance to these therapies is critical. We believe that the malignant and chemoresistant properties of pancreatic cancer might be enhanced by tobacco exposure act through a Src-dependent Id1 signaling axis. Our work provides support for population-based studies that show patients who continue to smoke after cancer diagnosis have a poor prognosis compared to nonsmokers. Our studies also suggest that the clinical assessment of Id1 expression might be a good biomarker of prognosis and support further investigations toward targeting this protein for potential pancreatic cancer therapy.
Acknowledgments
Support of the Core Facilities at Moffitt Cancer Center is greatly appreciated.
Footnotes
These studies were supported by grant CA139612 from the National Cancer Institute.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 3.Wang Z, Li Y, Kong D, Banerjee S, Ahmad A, Azmi AS, Ali S, Abbruzzese JL, Gallick GE, Sarkar FH. Acquisition of epithelial-mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathway. Cancer Res. 2009;69:2400–2407. doi: 10.1158/0008-5472.CAN-08-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hagmann W, Jesnowski R, Lohr JM. Interdependence of gemcitabine treatment, transporter expression, and resistance in human pancreatic carcinoma cells. Neoplasia. 2010;12:740–747. doi: 10.1593/neo.10576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Z, Li Y, Ahmad A, Banerjee S, Azmi AS, Kong D, Sarkar FH. Pancreatic cancer: understanding and overcoming chemoresistance. Nat Rev Gastroenterol Hepatol. 2011;8:27–33. doi: 10.1038/nrgastro.2010.188. [DOI] [PubMed] [Google Scholar]
- 6.Alguacil J, Silverman DT. Smokeless and other noncigarette tobacco use and pancreatic cancer: a case-control study based on direct interviews. Cancer Epidemiol Biomarkers Prev. 2004;13:55–58. doi: 10.1158/1055-9965.epi-03-0033. [DOI] [PubMed] [Google Scholar]
- 7.Michaud DS. Epidemiology of pancreatic cancer. Minerva Chir. 2004;59:99–111. [PubMed] [Google Scholar]
- 8.Tai MH, Upham BL, Olson LK, Tsao MS, Reed DN, Jr, Trosko JE. Cigarette smoke components inhibited intercellular communication and differentiation in human pancreatic ductal epithelial cells. Int J Cancer. 2007;120:1855–1862. doi: 10.1002/ijc.22530. [DOI] [PubMed] [Google Scholar]
- 9.Dasgupta P, Kinkade R, Joshi B, Decook C, Haura E, Chellappan S. Nicotine inhibits apoptosis induced by chemotherapeutic drugs by up-regulating XIAP and survivin. Proc Natl Acad Sci USA. 2006;103:6332–6337. doi: 10.1073/pnas.0509313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dasgupta P, Rizwani W, Pillai S, Kinkade R, Kovacs M, Rastogi S, Banerjee S, Carless M, Kim E, Coppola D, et al. Nicotine induces cell proliferation, invasion and epithelial-mesenchymal transition in a variety of human cancer cell lines. Int J Cancer. 2009;124:36–45. doi: 10.1002/ijc.23894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Wadei HA, Plummer HK, III, Schuller HM. Nicotine stimulates pancreatic cancer xenografts by systemic increase in stress neurotransmitters and suppression of the inhibitory neurotransmitter γ-aminobutyric acid. Carcinogenesis. 2009;30:506–511. doi: 10.1093/carcin/bgp010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Wadei MH, Al-Wadei HA, Schuller HM. Effects of chronic nicotine on the autocrine regulation of pancreatic cancer cells and pancreatic duct epithelial cells by stimulatory and inhibitory neurotransmitters. Carcinogenesis. 2012;33:1745–1753. doi: 10.1093/carcin/bgs229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Wadei MH, Al-Wadei HA, Schuller HM. Pancreatic cancer cells and normal pancreatic duct epithelial cells express an autocrine catecholamine loop that is activated by nicotinic acetylcholine receptors α3, α5, and α7. Mol Cancer Res. 2012;10:239–249. doi: 10.1158/1541-7786.MCR-11-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Momi N, Ponnusamy MP, Kaur S, Rachagani S, Kunigal SS, Chellappan S, Ouellette MM, Batra SK. Nicotine/cigarette smoke promotes metastasis of pancreatic cancer through α7nAChR-mediated MUC4 upregulation. Oncogene. doi: 10.1038/onc.2012.163. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trevino JG, Summy JM, Gray MJ, Nilsson MB, Lesslie DP, Baker CH, Gallick GE. Expression and activity of SRC regulate interleukin-8 expression in pancreatic adenocarcinoma cells: implications for angiogenesis. Cancer Res. 2005;65:7214–7222. doi: 10.1158/0008-5472.CAN-04-3858. [DOI] [PubMed] [Google Scholar]
- 16.Trevino JG, Summy JM, Lesslie DP, Parikh NU, Hong DS, Lee FY, Donato NJ, Abbruzzese JL, Baker CH, Gallick GE. Inhibition of SRC expression and activity inhibits tumor progression and metastasis of human pancreatic adenocarcinoma cells in an orthotopic nude mouse model. Am J Pathol. 2006;168:962–972. doi: 10.2353/ajpath.2006.050570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE. siRNA directed against c-Src enhances pancreatic adenocarcinoma cell gemcitabine chemosensitivity. J Am Coll Surg. 2004;198:953–959. doi: 10.1016/j.jamcollsurg.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 18.Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE. Inhibition of SRC tyrosine kinase impairs inherent and acquired gemcitabine resistance in human pancreatic adenocarcinoma cells. Clin Cancer Res. 2004;10:2307–2318. doi: 10.1158/1078-0432.ccr-1183-3. [DOI] [PubMed] [Google Scholar]
- 19.Ischenko I, Camaj P, Seeliger H, Kleespies A, Guba M, De Toni EN, Schwarz B, Graeb C, Eichhorn ME, Jauch KW, et al. Inhibition of Src tyrosine kinase reverts chemoresistance toward 5-fluorouracil in human pancreatic carcinoma cells: an involvement of epidermal growth factor receptor signaling. Oncogene. 2008;27:7212–7222. doi: 10.1038/onc.2008.326. [DOI] [PubMed] [Google Scholar]
- 20.Pillai S, Rizwani W, Li X, Rawal B, Nair S, Schell MJ, Bepler G, Haura E, Coppola D, Chellappan S. ID1 facilitates the growth and metastasis of non-small cell lung cancer in response to nicotinic acetylcholine receptor and epidermal growth factor receptor signaling. Mol Cell Biol. 2011;31:3052–3067. doi: 10.1128/MCB.01311-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong YC, Wang X, Ling MT. Id-1 expression and cell survival. Apoptosis. 2004;9:279–289. doi: 10.1023/b:appt.0000025804.25396.79. [DOI] [PubMed] [Google Scholar]
- 22.Lasorella A, Uo T, Iavarone A. Id proteins at the cross-road of development and cancer. Oncogene. 2001;20:8326–8333. doi: 10.1038/sj.onc.1205093. [DOI] [PubMed] [Google Scholar]
- 23.Shuno Y, Tsuno NH, Okaji Y, Tsuchiya T, Sakurai D, Nishikawa T, Yoshikawa N, Sasaki K, Hongo K, Tsurita G, et al. Id1/Id3 knockdown inhibits metastatic potential of pancreatic cancer. J Surg Res. 2010;161:76–82. doi: 10.1016/j.jss.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 24.Lee KT, Lee YW, Lee JK, Choi SH, Rhee JC, Paik SS, Kong G. Overexpression of Id-1 is significantly associated with tumour angiogenesis in human pancreas cancers. Br J Cancer. 2004;90:1198–1203. doi: 10.1038/sj.bjc.6601684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheung HW, Ling MT, Tsao SW, Wong YC, Wang X. Id-1-induced Raf/MEK pathway activation is essential for its protective role against taxol-induced apoptosis in nasopharyngeal carcinoma cells. Carcinogenesis. 2004;25:881–887. doi: 10.1093/carcin/bgh087. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Ling MT, Wang Q, Lau CK, Leung SC, Lee TK, Cheung AL, Wong YC, Wang X. Identification of a novel inhibitor of differentiation-1 (ID-1) binding partner, caveolin-1, and its role in epithelial-mesenchymal transition and resistance to apoptosis in prostate cancer cells. J Biol Chem. 2007;282:33284–33294. doi: 10.1074/jbc.M705089200. [DOI] [PubMed] [Google Scholar]
- 27.Kunigal S, Ponnusamy MP, Momi N, Batra SK, Chellappan SP. Nicotine, IFN-γ and retinoic acid mediated induction of MUC4 in pancreatic cancer requires E2F1 and STAT-1 transcription factors and utilize different signaling cascades. Mol Cancer. 2012;11:24. doi: 10.1186/1476-4598-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim YW, Kern HF, Mullins TD, Koriwchak MJ, Metzgar RS. Characterization of clones of a human pancreatic adenocarcinoma cell line representing different stages of differentiation. Pancreas. 1989;4:353–362. doi: 10.1097/00006676-198906000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Bruns CJ, Harbison MT, Kuniyasu H, Eue I, Fidler IJ. In vivo selection and characterization of metastatic variants from human pancreatic adenocarcinoma by using orthotopic implantation in nude mice. Neoplasia. 1999;1:50–62. doi: 10.1038/sj.neo.7900005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kinkade R, Dasgupta P, Carie A, Pernazza D, Carless M, Pillai S, Lawrence N, Sebti SM, Chellappan S. A small molecule disruptor of Rb/Raf-1 interaction inhibits cell proliferation, angiogenesis, and growth of human tumor xenografts in nude mice. Cancer Res. 2008;68:3810–3818. doi: 10.1158/0008-5472.CAN-07-6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trombino S, Cesario A, Margaritora S, Granone P, Motta G, Falugi C, Russo P. α7-Nicotinic acetylcholine receptors affect growth regulation of human mesothelioma cells: role of mitogen-activated protein kinase pathway. Cancer Res. 2004;64:135–145. doi: 10.1158/0008-5472.can-03-1672. [DOI] [PubMed] [Google Scholar]
- 32.Tubbs RR, Pettay J, Barry TS, Swain E, Loftus M, Cook JR, Skacel M, Paine G, Roche P, Grogan T. The specificity of interphase FISH translocation probes in formalin fixed paraffin embedded tissue sections is readily assessed using automated staining and scoring of tissue microarrays constructed from murine xenografts. J Mol Histol. 2007;38:159–165. doi: 10.1007/s10735-006-9066-1. [DOI] [PubMed] [Google Scholar]
- 33.van Diest PJ, van Dam P, Henzen-Logmans SC, Berns E, van der Burg ME, Green J, Vergote I. A scoring system for immunohistochemical staining: consensus report of the task force for basic research of the EORTC-GCCG. European Organization for Research and Treatment of Cancer-Gynaecological Cancer Cooperative Group. J Clin Pathol. 1997;50:801–804. doi: 10.1136/jcp.50.10.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang XH, Wu QL, Yu XB, Xu CX, Ma BF, Zhang XM, Li SN, Lahn BT, Xiang AP. Nestin expression in different tumours and its relevance to malignant grade. J Clin Pathol. 2008;61:467–473. doi: 10.1136/jcp.2007.047605. [DOI] [PubMed] [Google Scholar]
- 35.Dasgupta P, Chellappan SP. Nicotine-mediated cell proliferation and angiogenesis: new twists to an old story. Cell Cycle. 2006;5:2324–2328. doi: 10.4161/cc.5.20.3366. [DOI] [PubMed] [Google Scholar]
- 36.Singh S, Pillai S, Chellappan S. Nicotinic acetylcholine receptor signaling in tumor growth and metastasis. J Oncol. 2011;2011:456743. doi: 10.1155/2011/456743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shah AN, Summy JM, Zhang J, Park SI, Parikh NU, Gallick GE. Development and characterization of gemcitabine-resistant pancreatic tumor cells. Ann Surg Oncol. 2007;14:3629–3637. doi: 10.1245/s10434-007-9583-5. [DOI] [PubMed] [Google Scholar]
- 38.Benezra R, Rafii S, Lyden D. The Id proteins and angiogenesis. Oncogene. 2001;20:8334–8341. doi: 10.1038/sj.onc.1205160. [DOI] [PubMed] [Google Scholar]
- 39.Wilson JW, Deed RW, Inoue T, Balzi M, Becciolini A, Faraoni P, Potten CS, Norton JD. Expression of Id helix-loop-helix proteins in colorectal adenocarcinoma correlates with p53 expression and mitotic index. Cancer Res. 2001;61:8803–8810. [PubMed] [Google Scholar]
- 40.Zebedee Z, Hara E. Id proteins in cell cycle control and cellular senescence. Oncogene. 2001;20:8317–8325. doi: 10.1038/sj.onc.1205092. [DOI] [PubMed] [Google Scholar]
- 41.Wong YC, Zhang XM, Ling MT, Wang XH. Inactivation of ID-1 gene induces sensitivity of prostate cancer cells to chemotherapeutic drugs. Adv Exp Med Biol. 2008;617:565–572. doi: 10.1007/978-0-387-69080-3_58. [DOI] [PubMed] [Google Scholar]
- 42.Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 43.Damiano JS. Integrins as novel drug targets for overcoming innate drug resistance. Curr Cancer Drug Targets. 2002;2:37–43. doi: 10.2174/1568009023334033. [DOI] [PubMed] [Google Scholar]
- 44.Lopez-Chavez A, Carter CA, Giaccone G. The role of KRAS mutations in resistance to EGFR inhibition in the treatment of cancer. Curr Opin Investig Drugs. 2009;10:1305–1314. [PubMed] [Google Scholar]
- 45.LoPiccolo J, Blumenthal GM, Bernstein WB, Dennis PA. Targeting the PI3K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resist Updat. 2008;11:32–50. doi: 10.1016/j.drup.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arlt A, Gehrz A, Muerkoster S, Vorndamm J, Kruse ML, Folsch UR, Schafer H. Role of NF-γB and Akt/PI3K in the resistance of pancreatic carcinoma cell lines against gemcitabine-induced cell death. Oncogene. 2003;22:3243–3251. doi: 10.1038/sj.onc.1206390. [DOI] [PubMed] [Google Scholar]
- 47.Trevino JG, Gray MJ, Nawrocki ST, Summy JM, Lesslie DP, Evans DB, Sawyer TK, Shakespeare WC, Watowich SS, Chiao PJ, et al. Src activation of Stat3 is an independent requirement from NF-γB activation for constitutive IL-8 expression in human pancreatic adenocarcinoma cells. Angiogenesis. 2006;9:101–110. doi: 10.1007/s10456-006-9038-9. [DOI] [PubMed] [Google Scholar]
- 48.Summy JM, Trevino JG, Lesslie DP, Baker CH, Shakespeare WC, Wang Y, Sundaramoorthi R, Metcalf CA, III, Keats JA, Sawyer TK, et al. AP23846, a novel and highly potent Src family kinase inhibitor, reduces vascular endothelial growth factor and interleukin-8 expression in human solid tumor cell lines and abrogates downstream angiogenic processes. Mol Cancer Ther. 2005;4:1900–1911. doi: 10.1158/1535-7163.MCT-05-0171. [DOI] [PubMed] [Google Scholar]
- 49.Summy JM, Trevino JG, Baker CH, Gallick GE. c-Src regulates constitutive and EGF-mediated VEGF expression in pancreatic tumor cells through activation of phosphatidyl inositol-3 kinase and p38 MAPK. Pancreas. 2005;31:263–274. doi: 10.1097/01.mpa.0000178280.50534.0c. [DOI] [PubMed] [Google Scholar]
- 50.Martinez-Garcia E, Irigoyen M, Gonzalez-Moreno O, Corrales L, Teijeira A, Salvo E, Rouzaut A. Repetitive nicotine exposure leads to a more malignant and metastasis-prone phenotype of SCLC: a molecular insight into the importance of quitting smoking during treatment. Toxicol Sci. 2010;116:467–476. doi: 10.1093/toxsci/kfq138. [DOI] [PubMed] [Google Scholar]