Abstract
Hepatoblastoma, the most common pediatric liver cancer, consists of epithelial mixed embryonal/fetal (EMEF) and pure fetal histologic subtypes, with the latter exhibiting a more favorable prognosis. Few embryonal histology markers that yield insight into the biologic basis for this prognostic discrepancy exist. CBP/P-300 interacting transactivator 1 (CITED1), a transcriptional co-activator, is expressed in the self-renewing nephron progenitor population of the developing kidney and broadly in its malignant analog, Wilms tumor (WT). In this current study, CITED1 expression is detected in mouse embryonic liver initially on post-coitum day 10.5 (e10.5), begins to taper by e14.5, and is undetectable in e18.5 and adult livers. CITED1 expression is detected in regenerating murine hepatocytes following liver injury by partial hepatectomy and 3,5-diethoxycarbonyl-1,4-dihydrocollidine. Importantly, while CITED1 is undetectable in normal human adult livers, 36 of 41 (87.8%) hepatoblastoma specimens express CITED1, where it is enriched in EMEF specimens compared to specimens of pure fetal histology. CITED1 overexpression in Hep293TT human hepatoblastoma cells induces cellular proliferation and upregulates the Wnt inhibitors Kringle containing transmembrane protein 1 (KREMEN1) and CXXC finger protein 4 (CXXC4). CITED1 mRNA expression correlates with expression of CXXC4 and KREMEN1 in clinical hepatoblastoma specimens. These data show that CITED1 is expressed during a defined time course of liver development and is no longer expressed in the adult liver but is upregulated in regenerating hepatocytes following liver injury. Moreover, as in WT, this embryonic marker is reexpressed in hepatoblastoma and correlates with embryonal histology. These findings identify CITED1 as a novel marker of hepatic progenitor cells that is re-expressed following liver injury and in embryonic liver tumors.
Introduction
Hepatoblastoma is the most common liver cancer of childhood and is the third most common pediatric intra-abdominal malignancy after neuroblastoma and Wilms tumor (WT), respectively [1]. A variety of hepatoblastoma histologic subtypes exist, with epithelial-type hepatoblastomas being the most common [of hepatoblastoma histologic subtypes exist, with epithelial-type hepatoblastomas being the most common [1]. Epithelial-type hepatoblastomas are subdivided into either epithelial mixed embryonal/fetal (EMEF) tumors, which contain both embryonal and fetal elements, or pure fetal histology tumors, which contain disorganized cells resembling fetal hepatocytes alone [1]. Pure fetal histology tumors have a markedly improved prognosis relative to EMEF tumors; in fact, pure fetal histology hepatoblastomas often are treated by surgical resection alone and do not require the administration of cytotoxic chemotherapy, a necessity in the treatment of all other hepatoblastomas [2].
Like other embryonal tumors, hepatoblastoma is thought to arise from persistence of progenitor cells that escape terminal epithelial differentiation during organogenesis and remain beyond birth. These stem-like cells may undergo subsequent genetic insults that result in neoplastic transformation in the embryonal tumorigenic sequence [3]. In support of this progenitor cell hypothesis, hepatoblastomas have been shown to share both a molecular phenotype and electron micrographic features consistent with hepatic progenitor cells [4,5]. Moreover, components of the Wnt signaling pathway, which plays a complex, but critical, role in promoting embryonic liver development [6], are activated in a large proportion of hepatoblastomas. In fact, hepatoblastoma has the highest reported frequency of activating β-catenin mutations of any cancer [7,8]. These findings indicate that aberrant activation of a common signaling pathway required for normal liver development may also play a role in the pathogenesis of hepatoblastoma.
CBP/P-300 interacting transactivator 1 (CITED1) is a non-DNA binding transcriptional co-activator that along with expression of an additional transcription factor, SIX2, identifies the self-renewing portion of nephron progenitor cells in the metanephric mesenchyme of the developing kidney [9,10]. In the embryonic kidney, CITED1 is expressed only in these progenitor cells during the period of nephrogenesis and is downregulated as these cells undergo differentiation en route to forming mature nephrons [9]. The functional role of CITED1 in kidney development is uncertain because CITED1 null mice do not display altered nephrogenesis [9]. However, previous experiments have shown that CITED1 inhibits Wnt4-dependent transcriptional responses [11]. Because Wnt/β-catenin signaling in the metanephric mesenchyme is a critical regulator of early nephron differentiation [12], it is possible therefore that CITED1 modifies canonical Wnt/β-catenin signaling in these nephron progenitor cells even though genetic studies using CITED1 null mice indicate that its developmental role is functionally redundant [9].
WT is an embryonal tumor that is the most common kidney cancer of childhood and is thought to arise from arrested epithelial differentiation of embryonic kidney nephron progenitor cells [13,14]. Like hepatoblastoma, dysregulation of Wnt signaling and mutation of β-catenin have been implicated in the pathogenesis of WT [15,16]. In this malignant context, we have previously shown that while CITED1 expression is absent in the postnatal kidney, persistent expression of CITED1 is observed in a broad spectrum of WT [17,18]. Moreover, gain-of-function studies indicate that CITED1 enhances cellular proliferation in a putative embryonal tumor cell line and overexpression of a dominant-negative CITED1 construct perturbs embryonal tumor formation in a mouse xenograft model [17,19]. Taken together, these data suggest that CITED1 may play a role in Wilms or embryonal tumorigenesis and that these activities may relate to its ability to block Wnt/β-catenin progenitor cell differentiation in this context.
On the basis of these findings, and given the roles of Wnt signaling in normal liver development and hepatoblastoma, we hypothesized that CITED1 misexpression might also play a role in the pathogenesis of hepatoblastoma. In these studies, therefore, we have characterized for the first time expression of CITED1 in hepatic development and hepatoblastoma. We have shown that CITED1 is expressed in hepatoblasts early in development. CITED1 expression is lost in the adult liver but is strongly expressed in experimental and human hepatoblastoma and also during hepatic regeneration following various forms of hepatic injury. Although the functional role of CITED1 in liver development, hepatoblastoma, and liver regeneration remains to be established, in light of its role as a potential modulator of Wnt signaling and its association with embryonal tumorigenesis in the kidney, these studies suggest that persistent CITED1 expression plays a role in regulating and promoting hepatoblastoma.
Materials and Methods
CITED1LacZ Reporter Mouse
Development of the CITED1LacZ reporter mouse has been previously described [20]. For whole-mount staining for β-galactosidase activity, embryos were isolated in cold phosphate-buffered saline (PBS) and fixed in 0.2%glutaraldehyde in PBS with 2 mM MgCl2 and 5 mM EGTA for 15 minutes. Fixed embryos were equilibrated in β-Gal wash [0.1 M phosphate buffer (pH 7.3) containing 2 mM MgCl2, 5 mM EGTA, 0.02% NP-40, and 0.01% Na deoxycholate] and stained overnight at 37°C in β-Gal wash containing 1 mg/ml X-Gal and 5 mM potassium ferrocyanate and ferricyanate. Embryos were visualized using light microscopy. Timed pregnancies were counted with the morning of vaginal plug appearance as day 0.5.
Hepatoblastoma and Liver Injury Specimens
Forty-one human hepatoblastoma specimens were included in this analysis. A total of 19 paraffin-embedded and snap-frozen hepatoblastoma tissue specimens were obtained from the Children's Oncology Group (COG). Of these COG specimens, 11 were EMEF histology, while 8 were of pure fetal histology. Clinical data were not available or provided from the COG to correlate with patient outcomes. Twenty-two formalin-fixed, paraffin-embedded specimens were available from our institutional repository. Of these 22 institutional specimens, 17 were EMEF histology and 5 were of pure fetal histology. These specimens were only available for immunohistochemistry.
Forty-three paraffin-embedded murine hepatoblastoma specimens, induced chemically from N-nitrosodiethylamine, 2,3,7,8-tetrachlorodibenzo-p-dioxin, were available for analysis [21]. To compare CITED1 reactivation in other injury models, we subjected adult murine livers to two-thirds partial hepatectomy or to exposure for 21 days of a diet containing 0.1% 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC), as previously described [22]. DDC-fed mice were allowed 1 day of recovery following DDC treatment and were sacrificed, and livers were subsequently fixed in 10% formalin and paraffin-embedded for immunohistochemical analysis. Mice undergoing two-thirds partial hepatectomy were sacrificed at 3 and 7 days and livers were similarly prepared for immunohistochemical analysis. All animal husbandry and experiments were conducted in accordance with the standards of and prior approval from the Vanderbilt University Medical Center Institutional Review Board and Institutional Animal Care and Use Committee.
Immunohistochemistry and Immunofluorescence
All tissue samples were subjected to heat-induced antigen retrieval in 10 mM citrate buffer. These 5-µm sections were incubated in affinity-purified rabbit anti-CITED1 (1:50 dilution; Lab Vision Corp, Fremont, CA) antibody for 1 hour at room temperature. Goat anti-rabbit secondary antibody (1:200 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) was applied to tissues at room temperature for 45 minutes. Tissues were visualized with either a Vectastain ABC Kit (Vector Laboratories, Burlingame, CA) or DAKO Envision Kit (DAKO Cytomation, Carpinteria, CA). Immunohistochemistry was coded on an ordinal scale of expression intensity (e.g., 0 = absent, 1 = weak, 2 = strong). Counts of expression were compared overall, in the cytosol, and in the nucleus by an observer blinded to the tissue samples. Proliferating cell nuclear antigen (PCNA) immunostaining was performed similarly using a 1:1000 dilution of mouse anti-PCNA antibody (Santa Cruz).
For immunofluorescence experiments, tissues were similarly treated and quenched using hydrogen peroxide. Mouse fetal heart, kidney, and liver specimens were blocked using mouse-on-mouse block (Vector Laboratories) with 0.5% hydrogen peroxide for 1 hour at room temperature. Sections were then incubated in rabbit anti-CITED1 (1:100; Lab Vision Corp) with mouse-on-mouse block, 10% goat serum, and 0.5% hydrogen peroxide in PBS overnight at 4°C. Anti-mouse HRP (1:750 dilution; KPL, Gaithersburg, MD) was added for 40 minutes at room temperature. For visualization of CITED1, anti-rabbit DyLight 549 (1:600 dilution; Jackson ImmunoResearch, West Grove, PA) or fluorescein isothiocyanate-conjugated (1:100 dilution; Jackson ImmunoResearch) antibodies were added for 2 hours at room temperature. For ureteric bud staining, monoclonal mouse cytokeratin 8 - (Troma-1 antibody, 1:50 dilution; Developmental Studies Hybridoma Bank, Iowa City, IA) was used. TOPRO-3 or 4′,6-diamidino-2-phenylindole, dilactate (DAPI) were used for nuclear staining.
Western Blot Analysis, β-Catenin Sequencing, and Quantitative Real-time. Polymerase Chain Reaction
CITED1 Western blots were performed, as previously described [18]. Immunoblots were performed using affinity-purified rabbit anti-CITED1 (1:1000 dilution; Lab Vision Corp), M2 anti-FLAG antibody (1:1000 dilution; Sigma Chemical Co, St Louis, MO), and mouse anti-β-actin (1:5000 dilution; Sigma Chemical Co). Densitometry was performed using Photoshop (Adobe, San Jose, CA). β-Catenin Western blots were performed similarly using rabbit anti-β-catenin antibody (1:1000 dilution; Cell Signaling Technology, Beverly, MA). Results were normalized to β-actin. MCF7, a breast cancer cell line known to express CITED1, was used for a CITED1-positive control. COS cell lysate was used as a negative control. For developmental specimens (fetal heart, kidneys, and liver), immunoprecipitation Western blot analysis was performed as previously described [17].
To evaluate differences in gene transcription of CITED1 between EMEF and pure fetal histology hepatoblastoma specimens, we isolated and purified total RNA from snap-frozen tissues using RNAzol (Tel-Test Inc, Friendswood, TX) and RNeasy Mini Kits (Qiagen, Germantown, MD). Isolated RNA was quantified using a SpectraMax M5 UV spectrophotometer (Molecular Devices, Sunnyvale, CA). Reverse transcription (RT) of 3 µg of RNA was performed using Superscript II reverse transcriptase (Invitrogen, Grand Island, NY) and oligodT primers (Applied Biosystems, Foster City, CA) to synthesize cDNA suitable for analysis by quantitative real-time-polymerase chain reaction (qRT-PCR; Bio-Rad iCycler; Bio-Rad, Hercules, CA) using iQ SYBR Green Super Mix (Bio-Rad). For Dickkopf-related protein 1 (DKK1), Kringle containing transmembrane protein 1 (KREMEN1), CXXC finger protein 4 (CXXC4), and PCNA, qRT-PCR was performed using TaqMan probe-based gene expression analysis (Applied Biosystems). Changes in mRNA expression were determined by comparison of sample cycle threshold values against a standard curve generated using plasmid or positive control sample cDNA. Results were normalized to the housekeeping gene GAPDH and compared statistically. Primer sequences used for these studies are supplied in Table W1.
To determine the mutational status of the CTNNB1 gene in the hepatoblastoma samples provided by the COG, we amplified cDNA with Taq polymerase using provided primer sequences flanking exons 1 to 5 of the CTNNB1 gene (Table W1). Each reaction for the primers covering exons 2 to 4 comprised 1x PCR buffer (Sigma-Aldrich, St Louis, MO), 200 mM dNTP, 400 nM of each primer, and 0.05 units/ml Taq polymerase (Sigma-Aldrich); each reaction for the primer set of exons 1 to 5 comprised 1x PCR buffer (Promega, Madison, WI), 200 mM dNTP, 300 nM of each primer, 1% DMSO, 1 mM MgCl2, and 2.5 units GoTaq Flexi DNA polymerase (Promega). Cycling conditions were given as follows: 1) initial denaturation at 94°C for 3 minutes; 2) 42 cycles at 94°C for 40 seconds, 54°C for 40 seconds, and 72°C for 50 seconds; and 3) final elongation at 72°C for 10 minutes. PCR products were gel purified using a QIAquick Gel Extraction Kit (Qiagen, Valencia, CA) and subsequently directly sequenced by GeneHunter Corporation (Nashville, TN). Generated sequences and chromatographs were compared to wild-type CTNNB1 using the Basic Local Alignment Search Tool (BLAST; NCBI, Bethesda, MD) to determine the mutational status of each specimen. These methods could not determine the mutational status of four tumors from the COG, leaving a total of 15 tumors analyzed for CTNNB1 mutations. Base-pair references are made with respect to sequence NM_001904 for CTNNB1.
To assess the effect of CITED1 overexpression on Wnt signaling in hepatoblastoma, we isolated and quantified RNA from four consecutive passages of both Hep293TT-pcDNA3 cells and Hep293TT-CITED1 cells using techniques described above. Reverse transcription (RT) was performed with 1 µg of RNA using the RT2 First Strand cDNA Synthesis Kit (SA Biosciences, Frederick, MD). The RT2 Profiler PCR array specific for the Wnt signaling pathway (PAHS-043A) was purchased from SA Biosciences and used according to the manufacturer's instructions. This array provides quantitative analysis of 84 Wnt pathway components and target genes with expression normalized to a panel of five housekeeping genes on a 96-well plate format.
CITED1 Misexpression Studies
CITED1 was misexpressed in the human hepatoblastoma cell line, Hep293TT, using the Fugene HD cell transfection reagent (Promega) [23]. An N-terminal FLAG-tagged CITED1 construct was inserted into pCDNA3 vector (Invitrogen) containing a neomycin-resistance cassette [24]. Subconfluent cells were transfected using 1 µg of plasmid DNA. Seventy-two hours after transfection, 0.3 µg/ml G418 sulfate (Sigma Chemical Co) was added to select for transfected cells. Resulting pooled colonies were isolated and passaged over a 6-week period to foster generation of stable cell lines. Two stable cell lines were generated: 1) empty-vector control cells, Hep293TT-pcDNA, and 2) a CITED1 overexpression cell line, Hep293TT-CITED1. Absence or expression of CITED1 was verified by immunofluorescence and Western blot.
In Vitro Studies: Proliferation and Soft Agar Assays
In vitro effects of CITED1 overexpression on cellular proliferation were assayed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) incorporation using the CellTiter 96 Non-Radioactive Cell Proliferation Assay (Promega). The two aforementioned cell lines were plated in starvation medium (ACL-4 media with 0.2% FBS) [25] for 24 hours before experimentation. The cells were then harvested and 5 x 104 cells were plated in 200 µl of ACL-4 media [25] with 0.2% FBS in four wells for each cell line at each time point tested. MTT was added and quenched as per the manufacturer's instructions. Incorporation was assayed at 0, 24, 48, and 72 hours using spectrophotometry. Absorbances were compared statistically. Results represent four experimental repeats.
In vitro effects of CITED1 overexpression on anchorage-independent growth were determined using soft agar assays. Twenty thousand cells from each described cell line were plated in six separate wells of a six-well plate in SeaPlaque GTG Agarose (Lonza, Rockland, ME) in ACL-4 media (0.8% bottom agar and 0.4% top agar). Agars were covered in 200 µl of ACL-4 media and allowed to incubate for 3 weeks. Colony counts and sizes were assayed using MTT staining and quantified using a GelCount system (Oxford Optronix, Oxford, United Kingdom). Results represent three experimental repeats.
Statistical Analysis
The incidence of strong [immunohistochemistry (IHC) score of 2 vs 0 or 1] CITED1 expression was compared between EMEF and pure fetal histology specimens overall, in the cytosol, and in the nucleus using Fisher exact test. The monotonic association between CITED1 and PCNA mRNA expression was estimated using the Spearman correlation with 95% confidence intervals estimated by bootstrapping (10,000 replicates). The Wilcoxon rank sum test was used to compare the differences of normalized gene expression between the EMEF and pure fetal histology hepatoblastomas for both qRT-PCR and Western blot and to compare colony size and counts for the soft agar assay for anchorage independent growth. Two-way analysis of variance was used to compare proliferation profiles of Hep293TT-CITED1 to Hep293TT-pcDNA3 cells over time using data generated by the MTT proliferation assay, and this experiment was independently replicated four times. A statistically significant (P < .0001) interaction between treatment and time was detected among all four experiments. Consequently, group comparisons were made at each time point in all four experiments. Although we controlled the overall type I error at 0.05 for all 16 time points (4 time points in each of four experiments) using the method of Holm [26], only one experiment is presented. Statistical analysis from the RT2 Profiler PCR array specific for the Wnt signaling pathway (PAHS-043A) was performed using the manufacturer's statistical software (SA Biosciences). All other analyses were performed using R 2.13.1 (R Development Core Team, Vienna, Austria). Statistical tests resulting in a P value less than .05 were considered statistically significant.
Results
Developmental Domains of CITED1 Expression
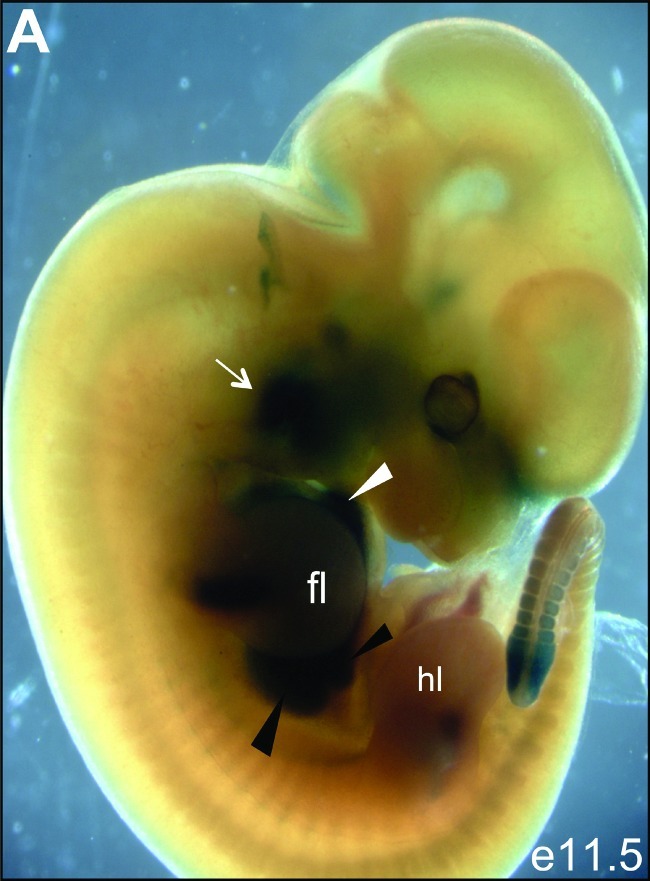
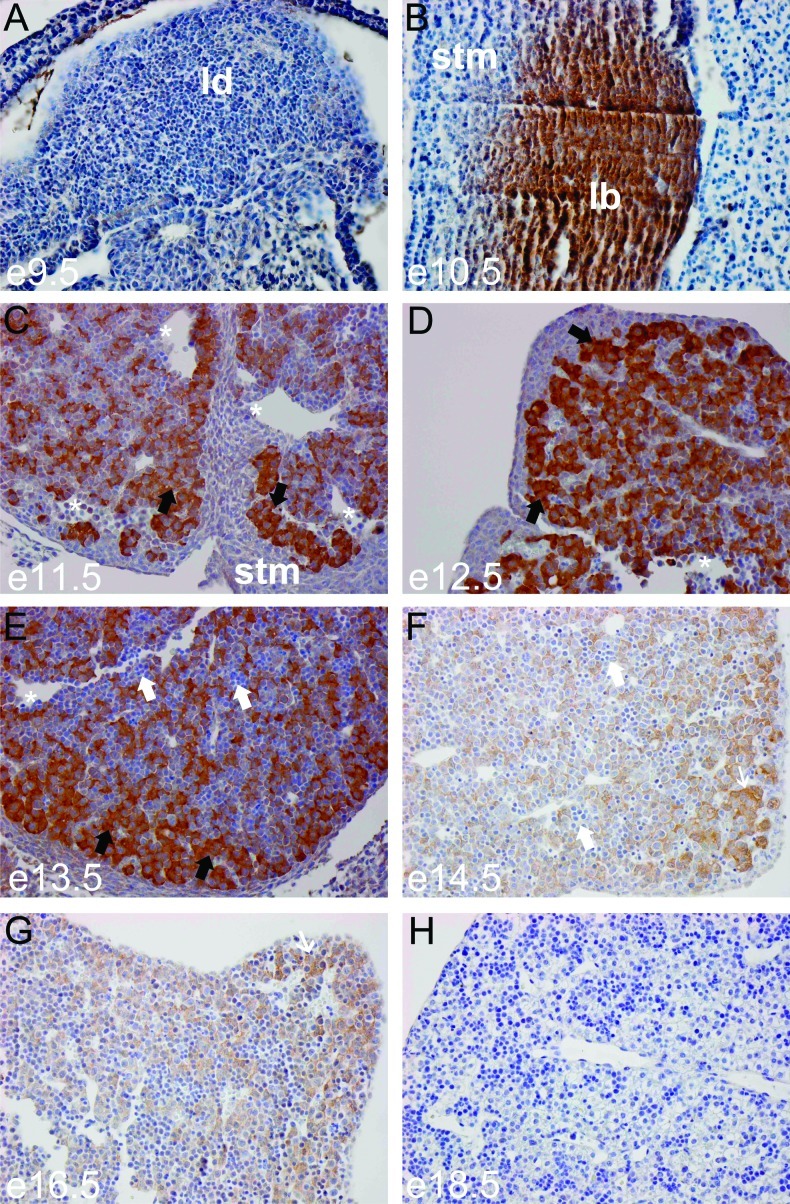
To determine the CITED1 expression domain across development, we inspected whole-mount embryos at e11.5 from CITED1LacZ reporter mice. This analysis demonstrated β-galactosidase expression in the heart, limbs, tail bud, and head mesenchyme (Figure 1) as previously described [27]. There is strong β-galactosidase staining in the fetal liver at this stage of development (Figure 1). Expression in the early metanephric kidney is obscured by the hind limb. To determine more precisely when and where CITED1 is expressed during liver development, we used immunohistochemistry to localize CITED1 protein using an affinity-purified rabbit polyclonal antibody that we have previously shown is highly specific for CITED1 [9]. CITED1 was not detected at e9.5 in the murine liver diverticulum, an out-pouching of the ventral foregut endoderm (Figure 2A). At e10.5, CITED1 expression was detected in the liver bud (Figure 2B). Strong CITED1 expression persisted during maturation of the liver bud from e11.5 to e13.5 (Figure 2, C–E). CITED1 expression then tapered between e14.5 and 16.5, at which point invading hematopoietic cells were detected and the nuclear to cytosolic ratio of the CITED1-positive hepatocytes began to decrease (Figure 2, F and G). CITED1 expression was not detected at e18.5, when the epithelial cells of the fetal liver had a greatly decreased nuclear-to-cytoplasmic ratio typical of maturing fetal hepatocytes (Figure 2H).
Figure 1.
(A) CITED1LacZ reporter mouse embryo at e11.5 gestation reveals expression of CITED1 in the second branchial arch (white arrow), fetal heart (white arrowhead), and fetal liver (black arrowheads). The hind limb obscures the metanephric kidney in this projection. fl = forelimb; hl = hind limb.
Figure 2.
(A) CITED1 is not detected by immunohistochemistry at e9.5 of murine hepatic development, during the period of hepatic specification as the liver diverticulum (ld) forms (hepatoblasts delaminate) from the ventral foregut endoderm. (B) By e10.5, CITED1 is strongly detected in the proliferating hepatic bud and not detected in the adjacent septum transversum mesenchyme (stm). (C) At e11.5, CITED1 is strongly detected in hepatoblasts, which form hepatic cords (black arrows) following their invasion of the septum transversum mesenchyme. Sinusoids are also seen (white asterisks). (D and E) CITED1 remains strongly detected at e12.5 and e13.5. Hepatoblasts, black arrows; hematopoietic cells, white arrows. (F and G) CITED1 expression begins to taper at e14.5 to e16.5, when CITED1-negative invading hematopoietic cells can be seen (white arrows) and the nuclear-to-cytoplasmic ratio of the hepatoblasts begins to decrease (thin white arrow). (H) CITED1 expression is not detected by e18.5, when hepatic cells have a low nuclear-to-cytoplasmic ratio and have the appearance of fetal hepatocytes [original magnification, x400 (A.H)].
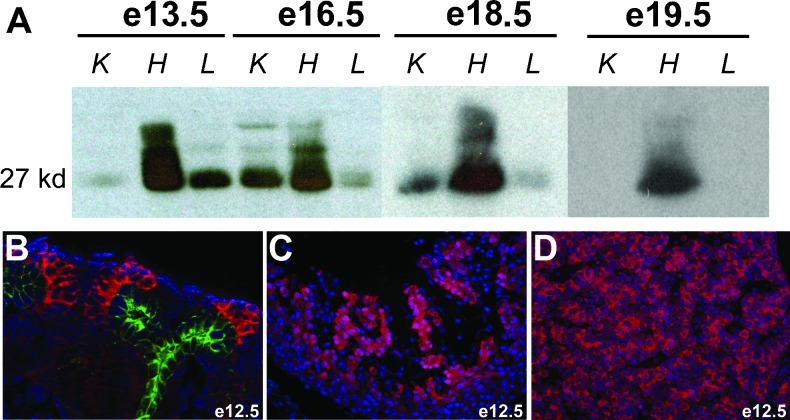
Consistent with immunohistochemical data, CITED1 was detected in the fetal liver strongly by immunoprecipitation Western blot at e13.5 but tapered by e16.5 and was undetectable in e19.5 livers (Figure 3A). Interestingly, these data also show that CITED1 antibodies detect distinct mobility bands in embryonic liver versus heart and kidney. High molecular weight bands detected in embryonic hearts at e13.5 collapse to a single 27-kDa band when lysates are treated with alkaline phosphatase (data not shown). This is consistent with previous studies indicating that CITED1 undergoes regulated phosphorylation events [24]. The functional role of these phosphorylation events is unknown. However, CITED1 immunolocalization studies in e12.5 mouse embryos show cytosolic expression of CITED1 in the metanephric mesenchyme surrounding the ureteric bud tip epithelium (Figure 3B) and in hepatoblasts of the liver bud (Figure 3D) but nuclear expression of CITED1 in the trabeculae of the developing heart (Figure 3C). This suggests that cardiac-specific CITED1 phosphorylation could play a role in regulating CITED1 subcellular localization.
Figure 3.
(A) Immunoprecipitation (IP)-Western blot for CITED1 in e13.5, e16.5, e18.5, and e19.5 murine fetal kidney (K), heart (H), and liver (L). Liver expression of CITED1 is strong at e13.5, but tapers between e16.5 and 18.5, and is no longer detected by e19.5. Time course of CITED1 expression in the kidney and heart is shown for comparison. (B) Cytosolic detection of CITED1 is seen in the e12.5 murine fetal kidney cap mesenchyme (pink) surrounding the ureteric bud tip epithelium, which shows characteristic expression of cytokeratin 8 (green). (C) Nuclear detection of CITED1 in the trabeculae of the developing heart at e12.5. (D) Cytosolic expression of CITED1 in hepatoblasts at e12.5 [original magnification x400 (B and D)].
CITED1 Expression in an Experimental Murine Model of Hepatoblastoma
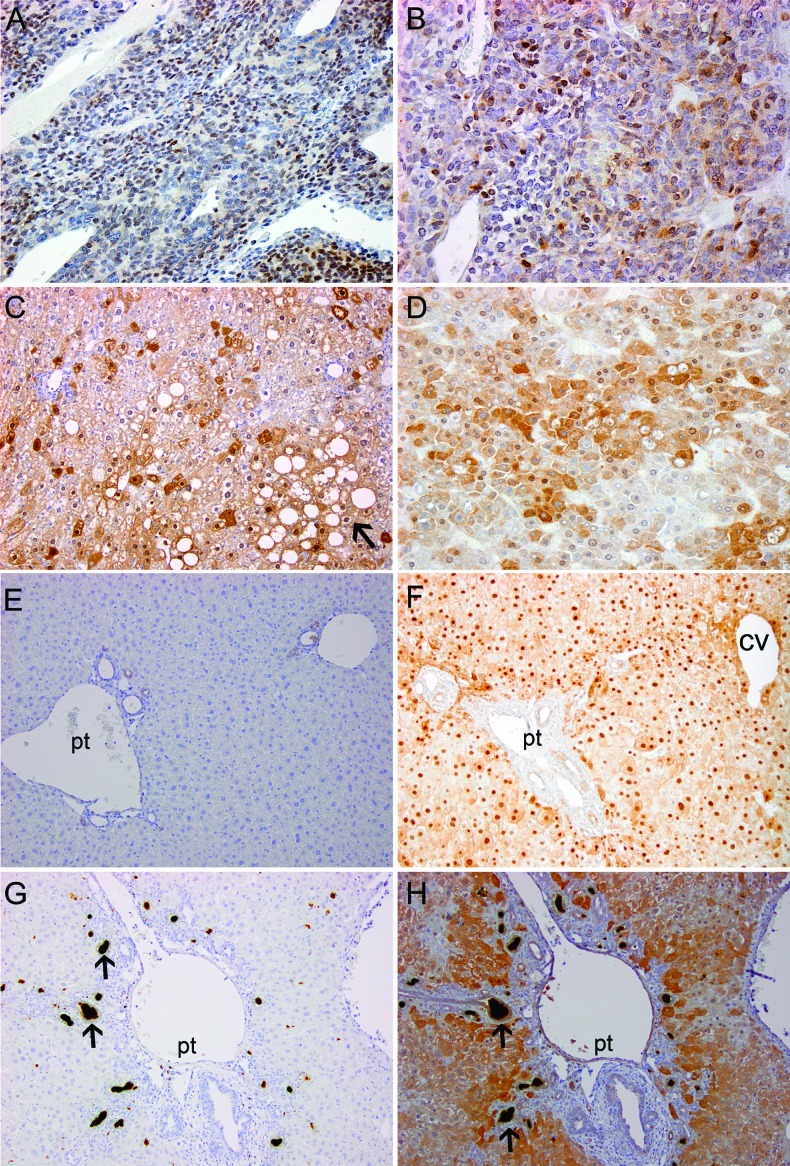
Because of its expression in hepatic development, we next asked whether CITED1 could also be detected in hepatoblastoma, a childhood malignancy that is characterized by cells with an embryonal and abnormal progenitor-like phenotype. We started by testing for the presence of CITED1 in an experimental murine model of hepatoblastoma. Chemically induced mouse hepatoblastomas were investigated for CITED1 expression by immunohistochemistry [21]. CITED1 was detected in 40 of 43 tumors (93% of experimental hepatoblastomas analyzed; Figure 4, A and B). Unexpectedly, CITED1 was also detected in adjacent areas of hepatic parenchymal injury and often coincided with areas of steatosis (Figure 4, C and D). Because we noted intense CITED1 staining in these areas of hepatic parenchymal injury, we wanted also to determine whether CITED1 reactivation occurred in other models of hepatic injury associated with liver regeneration. We tested tissue sections from murine partial hepatectomy specimens and murine livers treated with DDC, an inhibitor of heme biosynthesis that leads to accumulation of porphyrin and induces oval cell proliferation, the proposed progenitor cell of the adult liver [22]. CITED1 reactivation was detected in all cases, particularly surrounding portal triads in both the partial hepatectomy and DDC models and surrounding both portal triads and central veins in the partial hepatectomy model (Figure 4, F and H).
Figure 4.
(A and B) Detection of CITED1 in the N-nitrosodiethylamine, 2,3,7,8-tetrachlorodibenzo-p-dioxin murine model of hepatoblastoma. (C and D) CITED1-positive areas of injured liver in the N-nitrosodiethylamine, 2,3,7,8-tetrachlorodibenzo-p-dioxinmurinemodel often coincided with areas of hepatic steatosis (black arrow); original magnification, x200. (E) Normal, uninjured murine liver is negative for CITED1. (F) Nuclear enrichment of CITED1 is observed by immunohistochemistry at 7 days post-murine partial hepatectomy surrounding portal triads (pt) and central veins (cv). (G) IgG control of DDC induced murine liver injury. Note porphyrin accumulation (black arrows) characteristic of this injury model. (H) CITED1 is detected in DDC injured mouse liver 21 days post-exposure, and expression is enriched surrounding portal triads but not clearly central veins; original magnification, x200 (A–H).
Immunohistochemical Detection of CITED1 in Human Hepatoblastoma
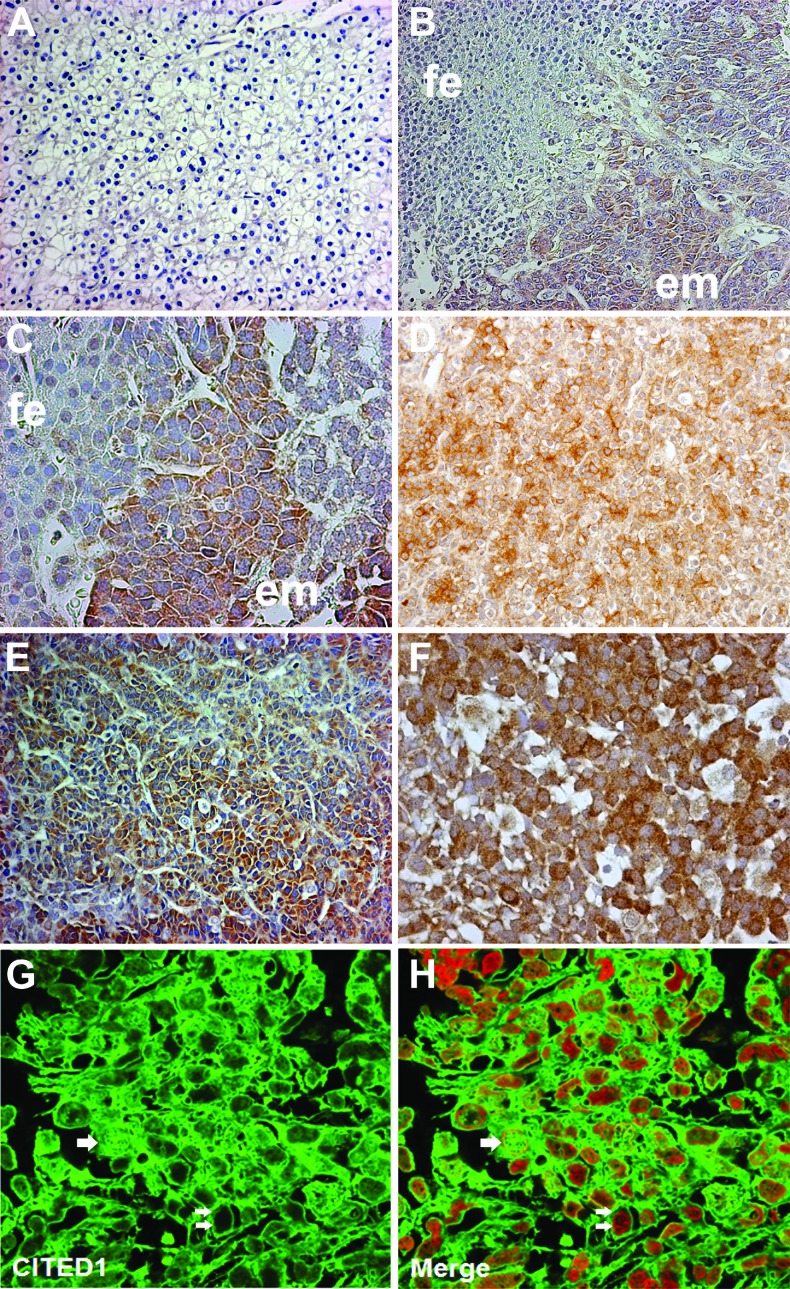
We next tested the presence of CITED1 in human hepatoblastoma specimens. CITED1 was detected by immunohistochemistry in 36 of 41 (87.8%) specimens analyzed. Of note, the six specimens in which CITED1 was not detected were all of pure fetal histology (Figure 5A). CITED1 was observed strongly in embryonal areas of EMEF histology hepatoblastomas (Figure 5, B–F). Overall, CITED1 was strongly expressed in 23 of 28 (82.1%) EMEF histology tumors compared to 1 of 13 pure fetal histology tumors (Fisher exact test; P < .0001). Nuclear expression was strong in 11 (39.3%) of 28 EMEF histology samples, whereas none of the pure fetal samples expressed nuclear CITED1 strongly (Fisher exact test; P < .009). Strong cytosolic expression of CITED1 was expressed in 23 of 28 EMEF histology tumors compared to 1 of 13 pure fetal histology tumors (Fisher exact test; P < .0001). Often, areas of abrupt transition in CITED1 expression correlated with the juxtaposition of embryonal and fetal histology in EMEF histology tumors (Figure 5, B and C). By immunofluorescence, we observed both cytosolic and nuclear expression of CITED1 in hepatoblastoma (Figure 5, G and H).
Figure 5.
(A) Pure fetal histology hepatoblastomas often did not show immunohistochemical detection of CITED1; original magnification, x200. (B) In EMEF tumors, CITED1 expression was more greatly detected in embryonal (em) elements, when compared to fetal (fe) elements; original magnification, x200. (C) High power representation of transition between embryonal and fetal elements; original magnification, x400. (D) An EMEF histology hepatoblastoma exhibiting CITED1 expression in embryonal and fetal elements; original magnification, x200. (E and F) CITED1 is richly detected in the embryonal elements of EMEF histology tumors [original magnification, x200 (E); x400 (F)]. (G) Immunofluorescence shows dysregulated nuclear (single white arrows) and cytosolic (double white arrows) subcellular localization of CITED1 in hepatoblastoma. (H) Merged image of CITED1 (green) and TOPRO3 nuclear stain (red) [original magnification, x400 (G and H)].
Quantification of CITED1 Expression in Pure Fetal and EMEF Histology Hepatoblastomas
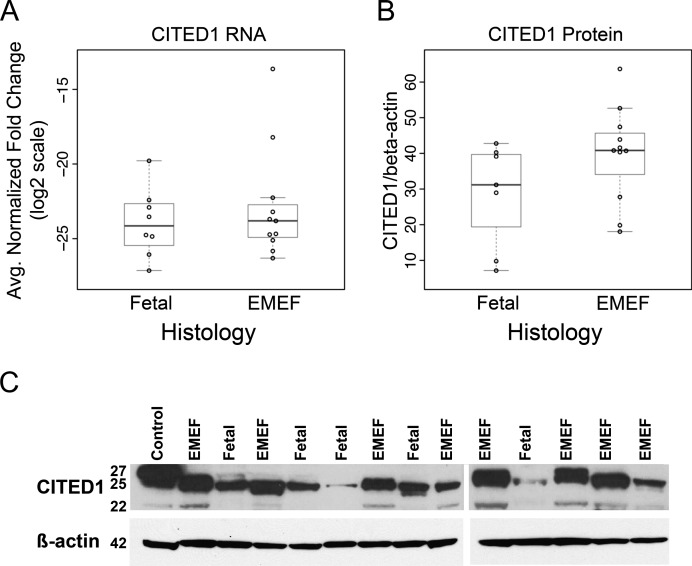
To test the hypothesis that CITED1 expression is greater in more undifferentiated tumors, we performed qRT-PCR and Western blot analysis on hepatoblastomas provided by the COG. While expression of CITED1 mRNA was similar between EMEF and pure fetal histology tumors (Figure 6A), we did detect a marginal increase in CITED1 expression by Western blot for EMEF histology specimens (Wilcoxon rank sum test; P = .085; Figure 6, B and C).
Figure 6.
(A) While the highest CITED1-expressing tumors were EMEF histology, there was no difference in mean CITED1 expression detected by qRT-PCR normalized to β-actin (P = NS). (B) CITED1 expression was marginally higher in EMEF histology specimens by Western blot densitometry normalized to β-actin (P = .085). (C) Example of Western blot demonstrating increased CITED1 expression in hepatoblastoma specimens of EMEF histology. Positive control is MCF7 cell lysate, a breast cancer cell line that is known to express high levels of CITED1.
Functional Significance of CITED1 Overexpression in Human Hepatoblastoma Cells
To investigate the possible function of CITED1 in hepatoblastoma pathogenesis, we established stable Hep293TT human hepatoblastoma cell lines expressing either epitope-tagged CITED1 or empty vector control. The presence and absence of both CITED1 and FLAG expression in these experimental cell lines were confirmed by Western blot (see Figure 8A) and immunofluorescence confirmed expression in all of the selected cells (data not shown).
Figure 8.
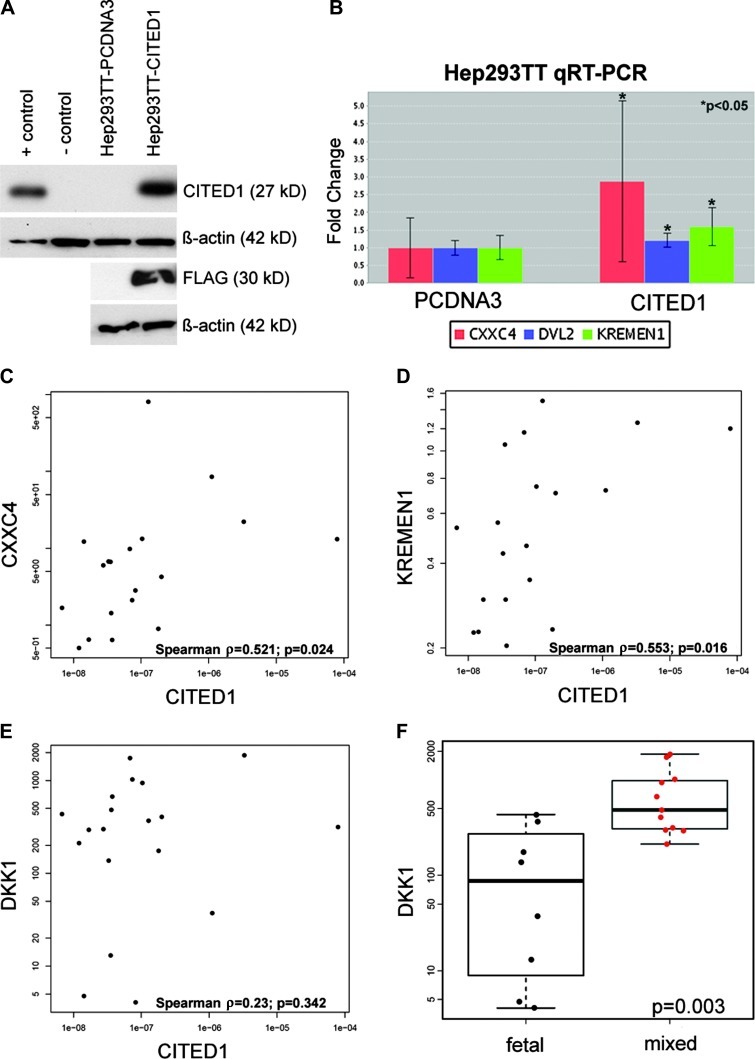
(A) Western blot demonstrates overexpression of CITED1 and detection of epitope-tagged CITED1 (FLAG) in Hep293TT human hepatoblastoma cells transfected with FLAG-tagged wild-type CITED1 (Hep293TT-CITED1) compared to empty vector control plasmid (Hep293TT-pcDNA3). Positive control is MCF7 breast cancer cell line and negative control is COS cell line. (B) qRT-PCR using a Wnt-PCR array revealed a statistically significant increase in fold change of the Wnt inhibitors CXXC4 (log2 fold change = 2.88) and KREMEN1 (log2 fold change = 1.60) and also the Wnt effector DVL2 (log2 fold change = 1.21) after CITED1 overexpression. (C and D) qRT-PCR of human hepatoblastoma specimens reveals a correlation between expression of (C) CITED1 and the Wnt inhibitor CXXC4 (Spearman correlation coefficient = 0.521; P = .024) and (D) CITED1 and the Wnt inhibitor KREMEN1 (Spearman correlation coefficient 0.553; P = .016). (E) No correlation was detected in clinical hepatoblastoma specimens between expression of CITED1 and the KREMEN1 ligand DKK1 (Spearman correlation coefficient = 0.23; P = .342). (F) However, DKK1 was found to be expressed greater in EMEF histology than pure fetal histology hepatoblastoma specimens (P = .003).
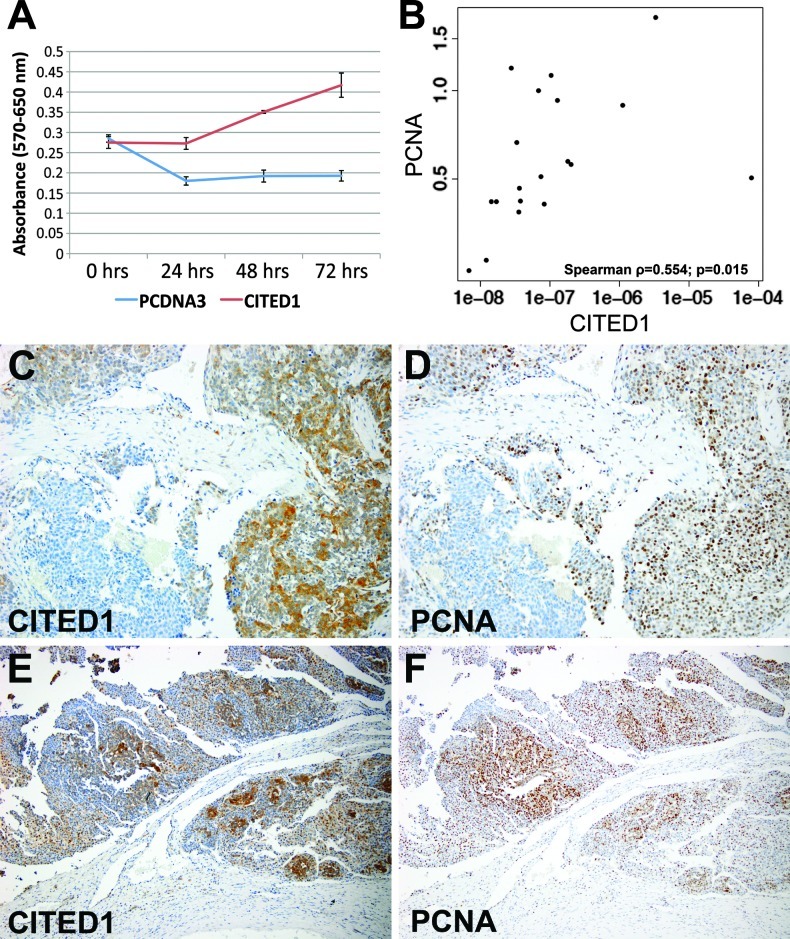
CITED1 overexpression resulted in a statistically significant increase in cell numbers as determined by MTT proliferation assay by 48 and 72 hours after assay initiation in all four experiments; in three of four experiments, this difference was also statistically significant at 24 hours (Figure 7A; Holm-Bonferroni method; P < .05 all time points). Decreased MTT detection in the control cell line initially indicates potential death of control cells in serum-starved conditions. CITED1 overexpression did not, however, significantly affect colony size or colony count when compared to Hep293TT-pcDNA3 empty vector control cells by soft agar assay for anchorage-independent growth (Wilcoxon rank sum test; P = .083 colony size; P = .563 colony count). To validate this association between CITED1 overexpression and increased hepatoblastoma cellular proliferation, we tested for a correlation between increasing CITED1 and PCNA mRNA expression in human hepatoblastoma patient specimens. In the 19 hepatoblastomas provided by the COG, we detected a statistically significant positive correlation between increasing CITED1 and PCNA mRNA expression (Spearman correlation coefficient = 0.554; 95% confidence interval (CI) = 0.061–0.858; P = .015; Figure 7B). CITED1 and PCNA expression localized to similar cellular expression domains by immunohistochemical staining of serial hepatoblastoma tissue sections (Figure 7, C–F).
Figure 7.
(A) An MTT cell proliferation assay in ACL-4 media with 0.2% FBS demonstrates a significant increase in cellular proliferation at 24, 48, and 72 hours with CITED1 overexpression (method of Holm-Bonferroni; P < .05). (B) qRT-PCR of human hepatoblastoma specimens reveals a positive correlation between expression of CITED1 and the proliferative marker PCNA (Spearman correlation coefficient = 0.554; P =.015). (C and D) Serial hepatoblastoma tissue sections immunostained for (C) CITED1 and (D) PCNA demonstrate similar patterns of expression within a given tumor, supporting the correlation between CITED1 expression and cellular proliferation (original magnification, x200). (E and F) Additional serial sections stained for (E) CITED1 and (F) PCNA (original magnification, x100).
Given the potential role of CITED1 as an inhibitor of the Wnt pathway from previous studies and the importance of dysregulated Wnt signaling in hepatoblastoma pathogenesis [28], we tested whether CITED1 overexpression altered Wnt signaling in human hepatoblastoma cells. Using the RT2 Profiler PCR array specific for the Wnt signaling pathway (SA Biosciences), four biologic replicates (passages) of Hep293TT-CITED1 cells showed significant up-regulation of the Wnt inhibitors KREMEN1 and CXXC4 (P = .034 and .043, respectively) and also the Wnt effector DVL2 (P = .042) when compared to Hep293TT-pcDNA3 empty vector control cells (Figure 8B). Complete array data are provided (Tables W2a–W2g). To validate these findings in the context of CITED1 overexpression in human hepatoblastomas in vivo, we tested for a correlation between CITED1 mRNA expression and that of KREMEN1 and CXXC4 in human hepatoblastoma patient specimens. In the 19 hepatoblastomas provided by the COG, we detected a statistically significant positive correlation between increasing CITED1 and CXXC4 mRNA expression (Spearman correlation coefficient = 0.521; 95% CI = 0.061.0.787 P = .024; Figure 7C). Likewise, we detected a significant positive correlation between increasing CITED1 and KREMEN1 mRNA expression (Spearman correlation coefficient = 0.553; 95% CI = 0.143–0.807 P = .016; Figure 7D). Because KREMEN1 is the DKK1 receptor, we also tested whether an association was present between CITED1 and DKK1, which specifically has been shown to become dysregulated in hepatoblastoma [29]. Although no statistically significant correlation was detected between CITED1 and DKK1 expression in these clinical specimens (Spearman correlation coefficient = 0.23; P = .342; Figure 7E), we did note a statistically significant increase in DKK1 expression in mixed-epithelial histology relative to pure fetal histology specimens (Wilcoxon rank sum test; P = .003; Figure 7FFigure 7F).
CITED1 Expression and CTNNB1 Mutational Status
Given the detected correlations between CITED1 expression and components of the Wnt pathway, we determined the mutational status of CTNNB1 in hepatoblastoma specimens provided by the COG by sequencing exons 1 to 5 from cDNA samples reverse transcribed from isolated RNA (Table W3). Ten of 15 (66.7%) hepatoblastoma specimens were found to have a CTNNB1 mutation. Seven of 10 (70%) CTNNB1 mutant hepatoblastoma specimens were EMEF histology, whereas 3 of 10 (30%) were of pure fetal histology (P = .957). Mean CITED1 expression by Western blot densitometry normalized to β-actin was not statistically different between CTNNB1 mutant and wild-type groups (P = .817). However, by Western blot densitometry normalized to β-actin, there was a statistically significant correlation between CITED1 and β-catenin expression (Spearman correlation coefficient = 0.52; P = .033). CITED1 and β-catenin also had similar regions of expression within a given tumor by immunohistochemical staining of serial tumor sections (Figure W1).
Discussion
Our data show that CITED1 is expressed in developing hepatoblasts during maturation of the liver bud and tapers off as these cells differentiate into hepatocytes. Analogous to this embryonic context, CITED1 expression appeared greatest in the undifferentiated embryonal tumor elements of mixed-epithelial hepatoblastoma. Prognostically more favorable pure fetal histology tumors, and even the more differentiated fetal areas of EMEF histology tumors, showed lower expression of CITED1. In addition, as previously described in renal progenitor cells and WT [17], the subcellular localization of CITED1 is dysregulated in hepatoblastoma when compared to its predominantly cytosolic localization in hepatic development. Functionally, CITED1 overexpression in human hepatoblastoma cells increases cellular proliferation but does not affect tumorigenesis in vitro, as measured by soft agar assay of anchorage-independent growth. Proliferation of cells in which CITED1 was overexpressed was often accompanied by apparent cell death in the control group cells, potentially indicating a survival advantage conferred by CITED1 overexpression in low-serum conditions consistent with cell stress. The association between CITED1 activity and cellular proliferation was also apparent in hepatoblastoma clinical specimens. CITED1 was further detected in a nitrosamine and aromatic hydrocarbon-induced murine model of hepatoblastoma; however, we also saw reactivation of CITED1 expression in surrounding areas of hepatic parenchymal injury, providing additional evidence for a possible reactivation of embryonal transcriptional programs in liver injury and regeneration. Reactivation of CITED1 expression associated with hepatic injury and regeneration was confirmed after murine partial hepatectomy and DDC treatment, which both induce oval cell proliferation, a key marker of hepatic regeneration. Taken together, we speculate that CITED1 expression in hepatoblastomas either persists postnatally or becomes reactivated in the neoplastic transformation from progenitor to cancer cell, as inferred from the chemically induced murine model.
CITED1 expression has not been previously reported in human or murine liver development, although the related family member, CITED2, is critical for hepatic development through its interactions with hepatocyte nuclear factor-alpha 4 (HNF-α 4), a required transcription factor for epithelial differentiation in the developing liver [30]. Although expression of CITED1, from our study, appears to adhere to a similar developmental time frame to that reported for CITED2, CITED1 null mice do not exhibit any overt hepaticmorphologic or histologic abnormalities (data not shown), implying functional redundancy of signaling similar to what has been observed in nephrogenesis [9]. While CITED1 null mice do exhibit decreased hepatic mass relative to controls, this is caused by asymmetric intrauterine growth retardation secondary to placental insufficiency [31].
The Wnt signaling pathway has been implicated in maintaining the critical, coordinated balance between differentiation and proliferation in multiple developmental contexts, particularly in the developing liver [6]. Wnt/β-catenin plays a biphasic role in hepatic development, with suppression of signaling until after the period of hepatic specification, followed by increased signaling driving the proliferation of hepatoblasts [6]. Specifically, coordinated control of Wnt signaling has been linked to the transcriptional co-activators, CBP and P300, which are the principal proteins with which CITED1 interacts [32,33]. CITED1 may therefore participate as a co-regulator in the modulation and balance of CBP/β-catenin-mediated transcription critical for stem/progenitor cell maintenance and P300/β-catenin-mediated transcription important in the initiation of cellular differentiation.
Disruption of balanced Wnt signaling is critical in hepatoblastoma pathogenesis, with between 50% and 90% of hepatoblastomas harboring β-catenin mutations [7,8]. Despite activated and pro-proliferative Wnt signaling being a dominant feature in hepatoblastoma, elevation of multiple Wnt antagonists including TrCP, Nkd-1, AXIN2, and DKK1 have been previously reported, possibly signifying a nonfunctional negative feedback loop [29,34]. Because CITED1 is a potential repressor of the Wnt pathway [11], we evaluated the effects in vitro of overexpressing CITED1 on Wnt pathway genes. In these studies, we found that CITED1 overexpression was associated with up-regulation of two key components of Wnt signaling inhibition: KREMEN1 and CXXC4. We validated these findings in clinical hepatoblastoma specimens, which revealed that CITED1 mRNA expression correlated positively with the Wnt components, KREMEN1 and CXXC4, independent of histologic subtype. DKK1 expression (another inhibitor of Wnt signaling) did not correlate with CITED1 mRNA expression but increased in association with mixed (more embryonal) histology. These findings implicate CITED1 as a modulator of Wnt signaling in hepatoblastoma and possibly also liver development. The seemingly paradoxical role of CITED1-promoted increased cellular proliferation and Wnt inhibition in the malignant context implies, analogously, a persistent developmental role, which may be specific to stem-like cells, whether embryonic progenitors or a cancer stem cell. Speculatively, CITED1 may modulate Wnt signaling to simultaneously promote or inhibit differentiation and maintenance/proliferation of the progenitor cell pool in a developing organ.
From these studies, the detection of CITED1 in hepatoblastoma appears to be linked to undifferentiated, embryonal tumor elements. We detected increased CITED1 by immunohistochemistry in EMEF histology hepatoblastomas containing both embryonal and fetal elements when compared to more differentiated, pure fetal histology hepatoblastomas. This observation was not statistically supported by qRT-PCR, whereas a marginally significant increase was detected by Western blot. CITED1 expression appeared to inversely correlate with tumor differentiation, a finding that may have prognostic importance given that pure fetal histology hepatoblastomas exhibit favorable behavior and are able to be treated by surgical resection alone without chemotherapy. Furthermore, we detected an association between the Wnt inhibitor DKK1 mRNA expression and mixed-epithelial histology tumors. Taken together, these results suggest that CITED1 may confer a survival advantage to the more primitive cellular components of hepatoblastoma by enhancing proliferation independent of oncogenic behavior and may further promote maintenance of stem-like properties in this embryonal malignancy by inhibiting Wnt-dependent mechanisms of cellular differentiation.
The role of CITED1 as a marker of the self-renewing stem cell population in kidney development and the promotion of cellular proliferation by CITED1 overexpression in human hepatoblastoma cells in the current study perhaps suggest a role for CITED1 in cancer cell self-renewal. Its role as a marker of hepatoblasts before epithelial transition and its link to undifferentiated tumor elements may implicate CITED1 in a network of gatekeepers that inhibit developmental progenitor or embryonal tumor cell differentiation. Therefore, CITED1 and its associated pathways may provide fertile ground for potential pathway-targeted therapies that abrogate cancer cell self-renewal or promote terminal differentiation of embryonal tumors.
Supplementary Material
Acknowledgments
We acknowledge the excellent services of the COG Biopathology Center, which were instrumental in specimen acquisition and data analysis, and also the NCI-funded Human Tissue Acquisition and Pathology Shared Resource at Vanderbilt (grant P30 CA68485). We also acknowledge Julia Kutaka and Jennifer Westrup Slone for their contributions to the experiments described herein. We were graciously provided the Hep293TT human hepatoblastoma cell line from the laboratory of Tina Chen and Gail Tomlinson and experimental murine hepatoblastoma specimens from the laboratory of Lucy Anderson.
Abbreviations
- EMEF
epithelial mixed embryonal/fetal histologic subtype of hepatoblastoma
- CITED1
CBP/P-300 interacting transactivator 1
- COG
Children's Oncology Group
- CXXC4
CXXC finger protein 4
- DDC
3,5-diethoxycarbonyl-1,4-dihydrocollidine
- DKK1
Dickkopf-related protein 1
- FBS
fetal bovine serum
- KREMEN1
Kringle containing transmembrane protein 1
- MTT assay
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
- PCNA
proliferating cell nuclear antigen
- qRT-PCR
quantitative real-time.polymerase chain reaction
- WT
Wilms tumor
Footnotes
This work was supported by the Section of Surgical Sciences and the Ingram Cancer Center of the Vanderbilt Medical Center, by the National Cancer Institute (NCI) grant 5T32CA106183-06A1 (A.J.M.), and by NCI grant 4R00CA135695-03 (H.N.L.). The authors have no conflicts of interest to disclose.
This article refers to supplementary materials, which are designated by Tables W1 to W3 and Figure W1 and are available online at www.neoplasia.com.
References
- 1.Litten JB, Tomlinson GE. Liver tumors in children. Oncologist. 2008;13:812–820. doi: 10.1634/theoncologist.2008-0011. [DOI] [PubMed] [Google Scholar]
- 2.Malogolowkin MH, Katzenstein HM, Meyers RL, Krailo MD, Rowland JM, Haas J, Finegold MJ. Complete surgical resection is curative for children with hepatoblastoma with pure fetal histology: a report from the Children's Oncology Group. J Clin Oncol. 2011;29:3301–3306. doi: 10.1200/JCO.2010.29.3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scotting PJ, Walker DA, Perilongo G. Childhood solid tumours: a developmental disorder. Nat Rev Cancer. 2005;5:481–488. doi: 10.1038/nrc1633. [DOI] [PubMed] [Google Scholar]
- 4.Ruck P, Xiao JC. Stem-like cells in hepatoblastoma. Med Pediatr Oncol. 2002;39:504–507. doi: 10.1002/mpo.10175. [DOI] [PubMed] [Google Scholar]
- 5.Ruck P, Xiao JC, Kaiserling E. Small epithelial cells and the histogenesis of hepatoblastoma. Electron microscopic, immunoelectron microscopic, and immunohistochemical findings. Am J Pathol. 1996;148:321–329. [PMC free article] [PubMed] [Google Scholar]
- 6.Si-Tayeb K, Lemaigre FP, Duncan SA. Organogenesis and development of the liver. Dev Cell. 2010;18:175–189. doi: 10.1016/j.devcel.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Jeng YM, Wu MZ, Mao TL, Chang MH, Hsu HC. Somatic mutations of β-catenin play a crucial role in the tumorigenesis of sporadic hepatoblastoma. Cancer Lett. 2000;152:45–51. doi: 10.1016/s0304-3835(99)00433-4. [DOI] [PubMed] [Google Scholar]
- 8.Wei Y, Fabre M, Branchereau S, Gauthier F, Perilongo G, Buendia MA. Activation of β-catenin in epithelial and mesenchymal hepatoblastomas. Oncogene. 2000;19:498–504. doi: 10.1038/sj.onc.1203356. [DOI] [PubMed] [Google Scholar]
- 9.Boyle S, Shioda T, Perantoni AO, de Caestecker M. Cited1 and Cited2 are differentially expressed in the developing kidney but are not required for nephrogenesis. Dev Dyn. 2007;236:2321–2330. doi: 10.1002/dvdy.21242. [DOI] [PubMed] [Google Scholar]
- 10.Hendry C, Rumballe B, Moritz K, Little MH. Defining and redefining the nephron progenitor population. Pediatr Nephrol. 2011;26:1395–1406. doi: 10.1007/s00467-010-1750-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plisov S, Tsang M, Shi G, Boyle S, Yoshino K, Dunwoodie SL, Dawid IB, Shioda T, Perantoni AO, de Caestecker MP. Cited1 is a bifunctional transcriptional cofactor that regulates early nephronic patterning. J Am Soc Nephrol. 2005;16:1632–1644. doi: 10.1681/ASN.2004060476. [DOI] [PubMed] [Google Scholar]
- 12.Dressler GR. Advances in early kidney specification, development and patterning. Development. 2009;136:3863–3874. doi: 10.1242/dev.034876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li CM, Guo M, Borczuk A, Powell CA, Wei M, Thaker HM, Friedman R, Klein U, Tycko B. Gene expression in Wilms'tumor mimics the earliest committed stage in the metanephric mesenchymal-epithelial transition. Am J Pathol. 2002;160:2181–2190. doi: 10.1016/S0002-9440(10)61166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davidoff AM. Wilms'tumor. Curr Opin Pediatr. 2009;21:357–364. doi: 10.1097/MOP.0b013e32832b323a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li CM, Kim CE, Margolin AA, Guo M, Zhu J, Mason JM, Hensle TW, Murty VV, Grundy PE, Fearon ER, et al. CTNNB1 mutations and overexpression of Wnt/β-catenin target genes in WT1-mutant Wilms'tumors. Am J Pathol. 2004;165:1943–1953. doi: 10.1016/s0002-9440(10)63246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huff V. Wilms'tumours: about tumour suppressor genes, an oncogene and a chameleon gene. Nat Rev Cancer. 2011;11:111–121. doi: 10.1038/nrc3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lovvorn HN, Westrup J, Opperman S, Boyle S, Shi G, Anderson J, Perlman EJ, Perantoni AO, Wills M, de Caestecker M. CITED1 expression in Wilms'tumor and embryonic kidney. Neoplasia. 2007;9:589–600. doi: 10.1593/neo.07358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy AJ, Pierce J, de Caestecker C, Taylor C, Anderson JR, Perantoni AO, de Caestecker MP, Lovvorn H., III SIX2 and CITED1, markers of nephronic progenitor self-renewal, remain active in primitive elements of Wilms'tumor. J Pediatr Surg. 2011;47:1239–1249. doi: 10.1016/j.jpedsurg.2012.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lovvorn HN, III, Boyle S, Shi G, Shyr Y, Wills ML, Perantoni AO, de Caestecker M. Wilms'tumorigenesis is altered by misexpression of the transcriptional co-activator, CITED1. J Pediatr Surg. 2007;42:474–481. doi: 10.1016/j.jpedsurg.2006.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sado T, Fenner MH, Tan SS, Tam P, Shioda T, Li E. X inactivation in the mouse embryo deficient for Dnmt1: distinct effect of hypomethylation on imprinted and random X inactivation. Dev Biol. 2000;225:294–303. doi: 10.1006/dbio.2000.9823. [DOI] [PubMed] [Google Scholar]
- 21.Beebe LE, Fornwald LW, Diwan BA, Anver MR, Anderson LM. Promotion of N-nitrosodiethylamine-initiated hepatocellular tumors and hepatoblastomas by 2,3,7,8-tetrachlorodibenzo-p-dioxin or Aroclor 1254 in C57BL/6, DBA/2, and B6D2F1 mice. Cancer Res. 1995;55:4875–4880. [PubMed] [Google Scholar]
- 22.Wang X, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Grompe M. The origin and liver repopulating capacity of murine oval cells. Proc Natl Acad Sci USA. 2003;100(suppl 1):11881–11888. doi: 10.1073/pnas.1734199100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen TT, Rakheja D, Hung JY, Hornsby PJ, Tabaczewski P, Malogolowkin M, Feusner J, Miskevich F, Schultz R, Tomlinson GE. Establishment and characterization of a cancer cell line derived from an aggressive childhood liver tumor. Pediatr Blood Cancer. 2009;53:1040–1047. doi: 10.1002/pbc.22187. [DOI] [PubMed] [Google Scholar]
- 24.Shi G, Boyle SC, Sparrow DB, Dunwoodie SL, Shioda T, de Caestecker MP. The transcriptional activity of CITED1 is regulated by phosphorylation in a cell cycle-dependent manner. J Biol Chem. 2006;281:27426–27435. doi: 10.1074/jbc.M602631200. [DOI] [PubMed] [Google Scholar]
- 25.Masuda N, Fukuoka M, Takada M, Kudoh S, Kusunoki Y. Establishment and characterization of 20 human non-small cell lung cancer cell lines in a serum-free defined medium (ACL-4) Chest. 1991;100:429–438. doi: 10.1378/chest.100.2.429. [DOI] [PubMed] [Google Scholar]
- 26.Holm S. A simple sequentially rejective Bonferroni test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 27.Dunwoodie SL, Rodriguez TA, Beddington RS. Msg1 and Mrg1, founding members of a gene family, show distinct patterns of gene expression during mouse embryogenesis. Mech Dev. 1998;72:27–40. doi: 10.1016/s0925-4773(98)00011-2. [DOI] [PubMed] [Google Scholar]
- 28.Armengol C, Cairo S, Fabre M, Buendia MA. Wnt signaling and hepatocarcinogenesis: the hepatoblastoma model. Int J Biochem Cell Biol. 2011;43:265–270. doi: 10.1016/j.biocel.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 29.Wirths O, Waha A, Weggen S, Schirmacher P, Kuhne T, Goodyer CG, Albrecht S, Von Schweinitz D, Pietsch T. Overexpression of human Dickkopf-1, an antagonist of wingless/WNT signaling, in human hepatoblastomas and Wilms'tumors. Lab Invest. 2003;83:429–434. doi: 10.1097/01.lab.0000059926.66359.bd. [DOI] [PubMed] [Google Scholar]
- 30.Qu X, Lam E, Doughman YQ, Chen Y, Chou YT, Lam M, Turakhia M, Dunwoodie SL, Watanabe M, Xu B, et al. Cited2, a coactivator of HNF4α, is essential for liver development. EMBO J. 2007;26:4445–4456. doi: 10.1038/sj.emboj.7601883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Novitskaya T, Baserga M, de Caestecker MP. Organ-specific defects in insulin-like growth factor and insulin receptor signaling in late gestational asymmetric intrauterine growth restriction in Cited1 mutant mice. Endocrinology. 2011;152:2503–2516. doi: 10.1210/en.2010-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teo JL, Kahn M. The Wnt signaling pathway in cellular proliferation and differentiation: a tale of two coactivators. Adv Drug Deliv Rev. 2010;62:1149–1155. doi: 10.1016/j.addr.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 33.Yahata T, de Caestecker MP, Lechleider RJ, Andriole S, Roberts AB, Isselbacher KJ, Shioda T. The MSG1 non-DNA-binding transactivator binds to the p300/CBP coactivators, enhancing their functional link to the Smad transcription factors. J Biol Chem. 2000;275:8825–8834. doi: 10.1074/jbc.275.12.8825. [DOI] [PubMed] [Google Scholar]
- 34.Koch A, Waha A, Hartmann W, Hrychyk A, Schuller U, Wharton KA, Jr, Fuchs SY, von Schweinitz D, Pietsch T. Elevated expression of Wnt antagonists is a common event in hepatoblastomas. Clin Cancer Res. 2005;11:4295–4304. doi: 10.1158/1078-0432.CCR-04-1162. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.