Abstract
Lymph nodes are compartmentalized organs but whether this feature has a role in T cell differentiation has been unclear. In this issue, Groom et al (Immunity 2012) reveal that spatially separated expression of two CXCR3 ligands guides Th1 cell development.
Generation of correctly polarized CD4+ T cell subsets is critical for successful immune responses. Yet the complete set of requirements for induction of effector T cells within responding lymph nodes (LNs) are not known. Subset-specific chemokine receptors have well established roles in guiding effector T cells to peripheral tissues, but whether these receptors influence early T cell differentiation events has been less clear. In this issue, Groom et al., show that the T helper-1 (Th1) cell-associated chemokine receptor CXCR3, plays a crucial role in promoting cell-cell interactions and guiding intra-lymph node movements of activated T cells that favor differentiation towards an interferon-γ (IFN-γ)-producing Th1 phenotype (Groom et al., 2012). This movement depends on expression of two CXCR3 ligands in distinct LN compartments.
CXCR3 has three ligands, CXCL9, CXCL10 and CXCL11. Their original monikers hint at the Th1 cell connection of this chemokine subfamily: CXCL9 was known as monokine induced by γ-IFN (MIG), CXCL10 as interferon inducible protein 10 (IP10) and CXCL11 as interferon inducible T cell alpha chemoattractant (I-TAC). CXCR3 facilitates recruitment of effector T cells to numerous inflammatory sites, such as the influenza infected lung, the toxoplasma infected brain, and organ allografts where CXCL9 and CXCL10 have been found strongly induced (see reviews cited in (Groom et al., 2012)). The role of CXCL11 has been less well studied, in part because B6 mice lack a functional CXCL11 gene. Despite the emphasis on CXCR3 ligands as inflammatory chemokines, CXCR3 is induced very rapidly on T cells following activation. This observation in conjunction with work demonstrating the presence of CXCR3 ligands in lymphoid organs suggested a role for CXCR3 during priming events that could affect T cell differentiation. Several recent studies have shown that expression of CXCR3 on CD8+ T cells can influence the balance between generation of terminally differentiated effector and long-lived memory cells as well as the functional quality of the latter (Hu et al., 2011; Kohlmeier et al., 2011; Kurachi et al., 2011). However, this effect of CXCR3 seems to be mediated through promoting further exposure of CD8+ T cells to antigen and inflammatory mediators either in lymphoid or peripheral tissues at later stages rather than by influencing early activation events.
To test whether CXCR3 had a cell intrinsic role during CD4+ T cell priming, Groom et al., first made use of an ovalbumin (OVA)-loaded and lipopolysaccharide (LPS) plus polyinosinic-polycytidylic acid (pIC)-exposed dendritic cell (DC) immunization strategy. As well as being a potent means of inducing effector T cell differentiation, this strategy had the advantage of allowing manipulation of chemokine production by the antigen-presenting cells. CXCR3 induction began occurring on the activated T cells by 24 hrs after DC injection and was maximal by 36 hrs. The investigators then asked how CXCR3-deficiency in the T cells affected their activation and differentiation. Early T cell proliferation was found to occur independently of the receptor. However, T cells required CXCR3 for maximal induction of IFN-γ at 3 days post antigen exposure, but not tumor necrosis factor (TNF) or interleukin-2 (IL-2). Whether the CXCR3-deficient T cells had become skewed towards other cytokine producing Th cell subsets was not investigated. The activated DCs expressed considerable amounts of CXCR3 ligands suggesting that they might play a role in further recruitment of activated T cells. Indeed, real-time imaging of lymph nodes showed that fewer of the CXCR3-deficient than wild-type antigen-specific T cells were in stable contacts with the transferred DCs, pointing to the possibility that contact duration might be one mechanism by which CXCR3 signaling reinforces Th1 cell fate. Although many of the DCs expressed high amounts of both CXCL9 and CXCL10, a defect in Th1 cell induction was only seen when Cxcl10−/− but not Cxcl9−/− DCs were used. Whether this reflected a unique ability of CXCL10 to promote DC-T cell conjugates, insufficient production of CXCL9 protein, or a role for CXCL10 single positive rather than CXCL9 and CXCL10 double positive DCs in priming Th1 cell responses remains an area for future investigation. Interestingly, when CXCL10-deficient DCs were used, the Th1 cell defect was greater than observed in the adoptive Cxcr3−/− T cell transfer system suggesting that DC derived CXCL10 recruits other cells that contribute to promoting Th1 cell differentiation. A candidate here is IFN-γ producing NK cells as previous work has shown that DC immunization leads to their recruitment in a CXCR3-dependent manner and contributes to Th1 cell induction (Martin-Fontecha et al., 2004).
Contrasting with the DC transfer model, in a more conventional antigen plus adjuvant immunization system, both CXCL9 and CXCL10 deficient mice showed a reduction in the IFN-γ producing CD4+ T cell response (Groom et al., 2012). This non-redundant requirement for both chemokines raised new questions about how CXCL9 and CXCL10 were functioning. Bone marrow (BM) chimera experiments showed that CXCL10 was important in hematopoietic cells, presumably including DCs but likely also macrophages, while CXCL9 was needed in radiation resistant stromal cells, suggesting it was not acting in the same way as CXCL10.
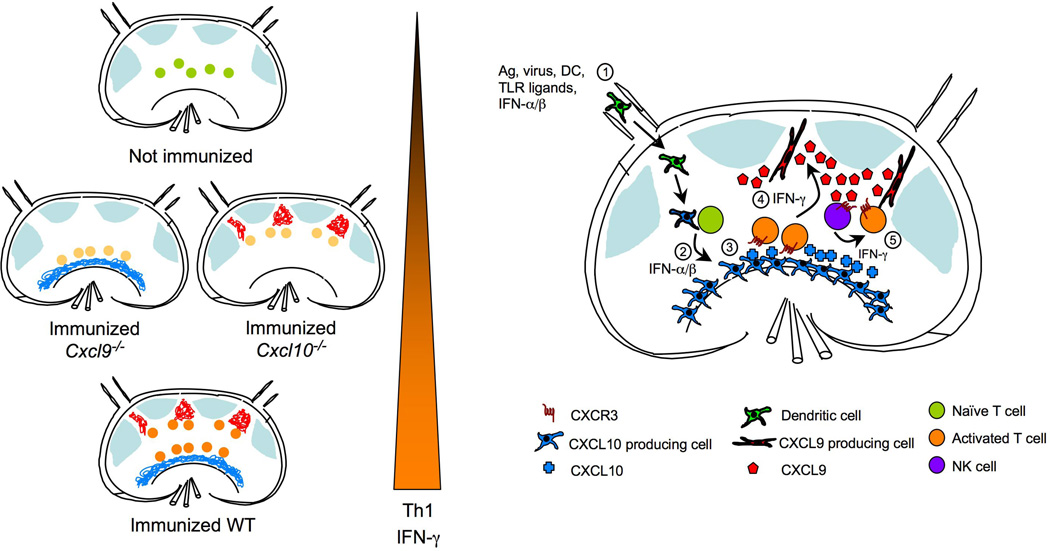
To determine whether CXCL9 and CXCL10 might help organize distinct LN niches, the investigators took the elegant approach of generating a bacterial artificial chromosome (BAC) transgenic reporter mouse line, REX, that ‘paints’ CXCL9 producing cells red (with RFP) and CXCL10 producing cells blue (with BFP) (Groom et al., 2012). Using REX mice they discovered that immunization with an antigen-LPS-pIC cocktail led to induction of CXCL10 in interfollicular regions within 12 hrs but only small amounts of CXCL9 in these regions. At 36 hrs CXCL10 was seen throughout medullary, interfollicular and subcapsular sinus regions; CXCL9 was now strongly induced in interfollicular regions and in some T zone areas but minimally in the medulla. When T cell distribution was examined at 24–36 hrs, wild-type T cells were enriched in interfollicular and medulla regions whereas Cxcr3−/− T cells remained in the T cell zone. In Cxcl10−/− hosts, wild-type T cells remained in the T cell zone but showed some migration toward interfollicular areas, the site of highest CXCL9 expression (Figure 1). In Cxcl9−/− hosts, wild-type T cells again remained in the T cell zone but showed some migration toward the medulla, a site of high CXCL10 expression (Groom et al., 2012).
Figure 1.
Expression of CXCR3 ligands within distinct LN compartments drives optimal Th1 cell-associated IFN-γ production. Left panel is a simplified depiction of re-localization of antigen-specific T cells after activation in relation to LN compartments that express CXCL9 or CXCL10. T cells become fully activated and express highest amounts of IFN-γ only if both CXCL9 and CXCL10 are present. Right panel shows temporal and spatial expression of CXCR3 ligands in response to immunization. 1) Afferent lymphatics bring in activated DC bearing antigen, or soluble antigen, TLR ligands, and type I IFN from infected tissue; 2) While innate stimuli induce expression of CXCL10 in mostly hematopoietic cells in the medullary region, DC activate antigen-specific T cells in the T-zone; 3) Activated T cells upregulate CXCR3 expression, which promotes frequent and prolonged encounters with CXCL10 expressing antigen presenting cells (APC’s) either in the T zone or the medullary region; 4) IFN-γ produced by activated T cells induces CXCL9 expression by unidentified stromal cells in the interfollicular region; 5) CXCL9 may attract other CXCR3 expressing cells, such as NK cells, as well as activated antigen-specific CD4 T cells to the interfollicular areas leading to more exposure to IFN-γ and other cytokines resulting in optimal Th1 cell differentiation.
Differential induction of CXCL10 and CXCL9 by type I IFN and IFN-γ has been well established and most likely forms the basis for the observed sequential expression of these two chemokines in the immunized LN by both myeloid and stromal cells (Groom et al., 2012). Recent work by Sung et al., shows that CXCL10 is induced rapidly in a type I IFN dependent manner following lymphocytic choriomeningitis virus (LCMV) infection whereas CXCL9 induction occurs later and is dependent on IFN-γ and the presence of central memory T cells (Sung et al., 2012). Thus, the early CXCL10 expression observed by Groom et al., is likely secondary to type I IFN induced by LPS and pIC and it led to recruitment of T cells to DCs. After T cell activation, the initial phase of IFN-γ production led to CXCL9 expression in the stromal compartment. Together these chemokines amplified or reinforced the circuit leading to Th1 cell priming but at different LN regions (Figure 1). The observation that the stromal compartment was the major source of CXCL9 might explain why transfer of CXCL9-deficient DC into wild-type mice did not have an impact on T cell IFN-γ production.
Whether the contribution of stromal CXCL9 to Th1 cell induction is all through effects on activated T cell positioning or through effects on NK cell (Martin-Fontecha et al., 2004) or monocyte (Janatpour et al., 2001) recruitment remains to be fully addressed. More precise identification of the CXCL9 producing stromal cells and their cytokine production profile should further this endeavor. Since IFN-γ is undoubtedly the single most important cytokine in reinforcing a Th1 cell-associated program (Mullen et al., 2001), the CXCL9+ stromal cells, which likely include gp38+ fibroblastic reticular cells (Sung et al., 2012), may provide a niche to concentrate the newly activated Th1 cells with other IFN-γ producing cells, such as NK cells and γδT cells (Kastenmuller et al., 2012), thus increasing Th1 cell exposure to this cytokine. Why this may occur in the interfollicular but not the medullary areas of the LN could be due to lack of access of some cell types to the latter region. Such a model may explain why both CXCR3 ligands are necessary for optimal Th1 cell-associated IFNγ production.
Is this type of chemokine-guided effector differentiation unique to Th1 cells? Probably not, as a recent study provided evidence for CXCR5 directed positioning of cells in interfollicular regions during Th2 cell-priming in response to a nematode, broadening the earlier finding that CXCR5 contributes to follicular helper T cell differentiation (Leon et al., 2012). Politics aside, clearly much more work is needed, using colorful reporters and temporal analyses like those of Groom and coworkers, if we are to assemble a complete cellular and molecular picture of the lymphoid niches supporting effector T cell priming.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Groom JR, Richmond J, Murooka TT, Sorensen EW, Sung JH, Bankert K, von Andrian UH, Moon JJ, Mempel TR, Luster AD. CXCR3 Chemokine Receptor-Ligand Interactions in the Lymph Node Optimize CD4(+) T Helper 1 Cell Differentiation. Immunity. 2012 doi: 10.1016/j.immuni.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JK, Kagari T, Clingan JM, Matloubian M. Expression of chemokine receptor CXCR3 on T cells affects the balance between effector and memory CD8 T-cell generation. Proc. Natl. Acad. Sci. U.S.A. 2011;108:E118–E127. doi: 10.1073/pnas.1101881108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janatpour MJ, Hudak S, Sathe M, Sedgwick JD, McEvoy LM. Tumor Necrosis Factor-dependent Segmental Control of MIG Expression by High Endothelial Venules in Inflamed Lymph Nodes Regulates Monocyte Recruitment. J. Exp. Med. 2001;194:1375–1384. doi: 10.1084/jem.194.9.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastenmuller W, Torabi-Parizi P, Subramanian N, Lammermann T, Germain RN. A spatially-organized multicellular innate immune response in lymph nodes limits systemic pathogen spread. Cell. 2012;150:1235–1248. doi: 10.1016/j.cell.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlmeier JE, Reiley WW, Perona-Wright G, Freeman ML, Yager EJ, Connor LM, Brincks EL, Cookenham T, Roberts AD, Burkum CE, et al. Inflammatory chemokine receptors regulate CD8(+) T cell contraction and memory generation following infection. J. Exp. Med. 2011;208:1621–1634. doi: 10.1084/jem.20102110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurachi M, Kurachi J, Suenaga F, Tsukui T, Abe J, Ueha S, Tomura M, Sugihara K, Takamura S, Kakimi K, Matsushima K. Chemokine receptor CXCR3 facilitates CD8(+) T cell differentiation into short-lived effector cells leading to memory degeneration. J. Exp. Med. 2011;208:1605–1620. doi: 10.1084/jem.20102101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon B, Ballesteros-Tato A, Browning JL, Dunn R, Randall TD, Lund FE. Regulation of T(H)2 development by CXCR5+ dendritic cells and lymphotoxin-expressing B cells. Nat. Immunol. 2012;13:681–690. doi: 10.1038/ni.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, Sallusto F. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat. Immunol. 2004;5:1260–1265. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- Mullen AC, High FA, Hutchins AS, Lee HW, Villarino AV, Livingston DM, Kung AL, Cereb N, Yao TP, Yang SY, Reiner SL. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science. 2001;292:1907–1910. doi: 10.1126/science.1059835. [DOI] [PubMed] [Google Scholar]
- Sung JH, Zhang H, Moseman EA, Alvarez D, Iannacone M, Henrickson SE, de la Torre JC, Groom JR, Luster AD, von Andrian UH. Chemokine guidance of central memory T cells is critical for antiviral recall responses in lymph nodes. Cell. 2012;150:1249–1263. doi: 10.1016/j.cell.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]