Abstract
Patients with inflammatory bowel diseases, such as ulcerative colitis and Crohn's disease, are at increased risk of developing colon cancer, confirming that chronic inflammation predisposes to development of tumors. Moreover, it appears that colon cancers that do not develop as a complication of inflammatory bowel disease are also driven by inflammation, because it has been shown that regular use of nonsteroidal anti-inflammatory drugs (NSAIDs) lowers the mortality from sporadic colon cancer and results in regression of adenomas in familial adenomatous polyposis (FAP) patients, who inherit a mutation in the Apc gene. Colorectal cancer therefore represents a paradigm for the link between inflammation and cancer.
Inflammation is driven by soluble factors, cytokines and chemokines, which can be produced by tumor cells themselves or, more often, by the cells recruited to the tumor microenvironment. Inflammatory cytokines and chemokines promote growth of tumor cells, perturb their differentiation, and support the survival of cancer cells.
Tumor cells become addicted to inflammatory stroma, suggesting that the tumor microenvironment represents an attractive target for preventive and therapeutic strategies. Proinflammatory cytokines, such as TNFα, IL-6 and IL-1β, or transcription factors that are required for signaling by these cytokines, including NF-κB and STATs, are indeed emerging as potential targets for anticancer therapy. TNFα antagonists are in phase I/II clinical trials and have been shown to be well tolerated in patients with solid tumors, and IL-1β antagonists that ameliorate several inflammatory disorders characterized by excessive IL-1β production, will likely follow. Therefore, development of drugs that normalize the tumor microenvironment or interrupt the crosstalk between the tumor and the tumor microenvironment is an important approach to the management of cancer.
Keywords: inflammation, colon cancer, TNF, IL1, NFκB, STAT, TRAIL
Introduction
The link between inflammation and colon cancer
Colorectal cancer is one of the most frequent human neoplasia. The transition from normal epithelium to malignant tumor is driven by alterations in tumor suppressor genes (such as Apc and p53) and oncogenes (e.g. K-ras). In addition, tumors become addicted to proliferative and survival signals from the abnormal tumor microenvironment.
Tumor progression depends on the interaction of tumor cells with components of the tumor microenvironment, including macrophages, B and T cells, mast cells, fibroblasts, myofibroblasts and extracellular matrix. Tumors have the ability to remodel the stroma and to establish a permissive microenvironment for their progression: they secrete factors that recruit inflammatory cells and/or activate stromal cells. In turn, cells in the microenvironment produce soluble factors (cytokines, chemokines, growth factors, proteases) that regulate growth, differentiation and survival of tumor cells and thus aid in tumor progression and promotion. In addition to cytokines, these cells produce elevated levels of reactive oxygen and nitrogen species, which can promote cancer causing genetic alteration in the epithelium. Although chronic inflammation may be involved in all three stages of tumor development: initiation, promotion and progression, it appears to play a major role in tumor promotion and progression.
Inflammatory bowel disease (IBD), which ranks among the top three high risk conditions for colon cancer [1] is a noticeable example of the tight link between inflammation and cancer. The risk for colorectal cancer increases with the duration and extent of the disease, confirming an active role of inflammation in the occurrence of cancer. The regular use of nonsteroidal anti-inflammatory drugs (NSAIDs) also lowers the mortality from sporadic colon cancer and results in regression of adenomas in FAP patients, who inherit a mutation in the Apc gene [2], suggesting that inflammation also contributes to the progression of tumors that are not initiated by inflammation.
Inflammation is driven by soluble factors, cytokines and chemokines, which are produced by tumor cells themselves and by the cells recruited to the tumor microenvironment, such as macrophages and mast cells. Depletion of mast cells [3] or macrophages [4] resulted in a profound remission of Apc-initiated intestinal polyps in mice, confirming the role of immune cells and their soluble factors in both initiation and progression of intestinal cancer. The key role of inflammation in colon cancer has been established by the demonstration that Apc/Min+/− mice with deficient TLR signaling develop reduced number of tumors [5].
The serum levels of several cytokines, including TNFα, IL-8, IL-6 and VEGF, are elevated in colorectal carcinoma (CRC) patients, and some studies have suggested that increased plasma levels of these cytokines may have a prognostic value [6]. Accordingly, cytokines such as TNFα and IL-1β are emerging as potential targets for anticancer therapy. TNFα antagonists are in phase I/II clinical trials and have been shown to be well tolerated in patients with solid tumors [7, 8], and IL-1β antagonists that have been tested in inflammatory disorders characterized by excessive IL-1β production are also available and should be tested for their clinical efficacy in cancer patients [9].
Cytokines secreted by the activated tumor stroma modulate tumor growth and enhance invasiveness of tumor cells by activation of oncogenic signaling pathways in tumor cells, including activation of NF-κB by TNFα and IL-1β, and activation of STAT3 by IL-6 [10]. In addition, activation of oncogenes, such as k-Ras, has been shown to activate NFκB signaling in tumor cells and to trigger the production of several proinflammatory mediators [11–13].
More recently, TNFα [4], Hepatocyte Growth Factor [14], PDGF [15] and FGF19 [16] have been shown to activate Wnt/β–catenin signaling in tumor cells, the oncogenic pathway activated in the majority of colorectal cancers. Signaling through the canonical Wnt pathway results in stabilization and accumulation of β-catenin in the cytoplasm, followed by its nuclear translocation [17]. Finally, in cooperation with members of the TCF/LEF family, β-catenin activates a number of target genes that modulate cell growth and differentiation [18] and stimulates the expansion of cells that have stem cell- like properties. We showed that IL-1β is a potent activator of Wnt signaling in colon cancer cells and that, accordingly, IL-1β and macrophages promote growth and support survival of colon cancer cells [19].
Consistent with the protumorigenic role of inflammation in colon cancer, nonsteroidal antiinflammatory drugs (NSAIDs) exert a strong chemopreventive activity. Several studies have confirmed that regular use of aspirin reduces the relative risk of developing colon cancer by 50% [20]. New findings indicate that aspirin also improves survival of stage I, II and III colorectal cancer patients [21], establishing NSAIDs as therapeutic agents, not only chemopreventive agents. NSAIDs exert their anti-inflammatory (and anti-tumorigenic activity) in large by inhibiting cyclooxygenase 2 (COX-2) [22]. The expression of COX-2 is elevated in 50% of adenomas and in 85% of adenocarcinomas. In human intestinal tumors COX-2 is expressed in epithelial and stromal cells, suggesting that NSAIDs exert their chemopreventive activity by targeting both, tumor cells and cells in the tumor microenvironment. COX-2 is an inducible gene, and proinflammatory cytokines, such as IL-1β and TNFα, and hypoxic environment [23] are potent inducer of COX-2 expression. NF-κB and Wnt signaling have both been shown to regulate the expression of COX-2. Overexpression of COX-2 increased AOM-induced tumor formation [24] and COX-2 deficiency significantly diminished tumorigenesis in mouse models of colon cancer [25, 26], confirming its role in tumorigenesis. Accordingly, selective COX-2 inhibitors have been confirmed to be efficient chemopreventive agents [27], however the enthusiasm about the use of these drugs has been diminished by their negative effect on the cardiovascular system, including increased risk of heart attacks and strokes.
Proinflammatory and protumorigenic activities of COX-2 in CRC are mediated by PGE2, which stimulates growth, angiogenesis and inhibits apoptosis in CRC [28] through activation of a number of oncogenic signaling pathways, including β-catenin/TCF, Ras and the PI3K signaling. Therefore, selective inhibitors of PGE2 may turn out to be potent inhibitors of CRC, but with fewer side effects than nonselective NSAIDs or selective COX-2 inhibitors.
The role of Toll-like receptor (TLR) signaling in inflammation and colon cancer
Intestinal epithelium is in direct contact with a huge number of commensal bacteria. Patients with ulcerative colitis display abnormal immune response to bacterial challenge, accompanied with increased cytokine production [29]. Toll-like receptors (TLRs) which are expressed on epithelial cells recognize both commensal and pathogen associated molecular patterns (PAMPs) from the gut flora. TLRs have a dual role in intestinal inflammation: while signaling through TLRs is required to maintain tolerance to commensal bacterial and for the removal of pathogenic microorganisms, a deregulated TLR mediated immune response can also trigger inflammation and contributes to the link between chronic inflammation and colon cancer [30]. Stimulation of TLRs can directly activate T regulatory (Treg) cells, which can suppress T cell dependent colitis and bacterial mediated intestinal inflammation [31]. TLR5 deficient mice develop spontaneous colitis, demonstrating that in mice flagellin-TLR5 interaction normally curbs intestinal inflammation [32]. TLR2 and TLR4 are upregulated and TLR3 is downregulated in intestinal mucosa of IBD patients [33].
Stimulation of TLR2 or TLR4 on tumor cells by pathogens activates the expression of a plethora or cytokines and chemokines that, in turn, promote growth of tumor cells. Activation of TLR signaling is linked to activation of the NF-κB pathway (Figure 1) and can therefore result in increased survival of tumor cells, leading to chemoresistance. Indeed, TLR4 has been shown to promote colitis associated colon cancer [34] and deficiency of Myd88 (a cofactor for TLR signaling, Figure 1) in IL-10−/− mice [35], or in the ApcMin+/− mice [5] resulted in abrogation of colon cancer, confirming the essential role of TLR/MyD88 signaling for development of CRC.
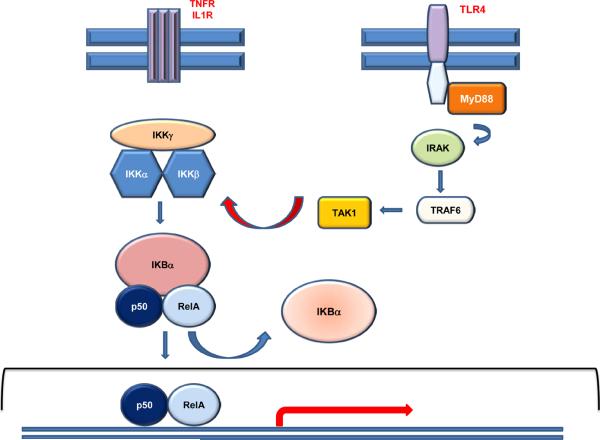
Figure 1. NF-κB signaling.
Binding of TNFα (or IL-1β) to their respective receptors triggers canonical NF-κB signaling. Signaling is initiated by activation of an IκB kinase (IKK) complex consisting of catalytic kinase subunits (IKKα and/or IKKβ) and the regulatory scaffold protein IKKγ. IKK-mediated phosphorylation results in proteasomal degradation of the IκB inhibitor enabling the active NFκB transcription factor subunits to translocate to the nucleus and induce target gene expression. One of the target genes is IκBα gene itself, which re-sequesters NFκB subunits and terminates transcriptional activity, unless a persistent activation signal is present. NF-κB is activated also through TLR signaling via Myd88 dependent pathway. Receptor induced association of MyD88 with the TIR domain allows the formation of a complex, including IRAK1 and TRAF6, resulting in activation of TAK1, which phosphorylates and activates IKKβ. TLR3 can also induce NFκB in a MyD88 independent manner (not shown).
Inflammation as a prognostic factor
Solid tumors are frequently infiltrated by T cells, B cells, NK cells, mast cells and macrophages. Although epidemiological studies and experimental evidence suggest that inflammation accelerates tumor progression, immune cells can also promote anti-tumor immunity. The nature and the abundance of infiltrating cells and the repertoire of soluble mediators produced by these cells maintain the equilibrium between tumor promoting inflammation and antitumor immunity. Analysis of a large number of CRC patients confirmed that the presence of Th1 cells (and the expression of Th1 specific genes, such as T-bet, IFNγ) in tumors is associated with the absence of metastatic invasion, tumor recurrence and better overall survival [36, 37]. High CD3 density in the center of the tumor or at the invasive margin of the tumor correlated with significantly better survival of CRC patients [36]. Analysis of >400 CRC patients confirmed that infiltration with CD8 T cells correlated with lack of metastasis [37]. Likewise, metastatic tumors have reduced density of CD8 T cells, and CRC with microsatellite instability (MSI), which correlates with good prognosis, are associated with high infiltration of CD8 T cells [38]. Finally, colorectal cancer patients exerting high grade inflammation had better 5 year survival [39].
Consistent with the ability of macrophages to modulate the growth of tumors, to suppress anti tumor response, to promote angiogenesis and to support invasion and metastasis of tumors, increased density of macrophages is associated with poor prognosis in breast, prostate, bladder and cervical cancer [40–45]. There are, however, contrasting reports regarding the prognostic significance of macrophage infiltration in colon cancer [46–48]. Some studies suggested that the location of immune cells determines whether their presence results in suppression or promotion of tumor growth. For example, while high density of macrophages in the central region of the tumors appears to be a poor prognostic factor, the presence of macrophages at the invasive margin correlated with better 5 year survival [39, 47]. It has also been indicated that a high macrophage to cancer cell ratio needs to be achieved for macrophages to exert antitumorigenic activity [47], underscoring, at least in part, an inherent cytotoxic activity of macrophages. Consistent with these findings, depletion of macrophages in rats promoted growth of colon cancer xenografts and impaired survival of experimental animals [48]. Together, these data demonstrate that the activation and polarization state of macrophage is likely to determine whether macrophages will promote or inhibit tumor progression.
Nevertheless, these finding suggested that the analysis of immune infiltration should be an important factor in establishing a prognosis in colorectal cancer. Indeed, the type, density and location of immune cells in colorectal tumors had a prognostic value which was comparable or superior to the established TNM classification [49].
This review is focused on the protumorigenic effects of inflammation in colon cancer. The role of proinflammatory cytokines including TNFα, IL-6, IL-1β and IL-17, and signaling pathways utilized by these cytokines, such as NF-κB, STAT3 and Wnt, will be discussed.
TNFα and NF-κB signaling
Human colon cancers are infiltrated by inflammatory cells, including mast cells and macrophages, which secrete TNFα [3]. Likewise, polyps arising in the ApcΔ468 mice, a genetic model for intestinal cancer, showed infiltration with mast cells. Serum levels of TNFα are elevated in tumor bearing ApcΔ468 mice and depletion of mast cells significantly reduced the levels of TNFα, confirming that mast cells are significant source of TNFα. Most importantly, depletion of mast cells or anti-TNFα treatment significantly suppressed polyposis in the ApcΔ468 mice [3].
TNFα activates oncogenic signaling pathways in epithelial cells, including Wnt and NF-κB (see below), and thereby regulates their growth and survival. The ability of TNF to induce DNA damage in vitro and in vivo is linked to the production of reactive oxygen species, and antioxidants significantly reduced TNFα induced DNA damage. Treatment of cells with TNFα increased chromosomal instability, gene mutations and gene amplifications [50], suggesting a direct mechanism whereby TNFα promotes cancer development.
TNFα plays a pathogenic role in colitis. In the azoxymethane (AOM) and dextran sodium sulfate (DSS) induced mouse model of colitis associated colon cancer, mice lacking TNF-R p55 showed reduced mucosal damage upon AOM/DSS treatment, and subsequently had attenuated formation of intestinal tumors. Accordingly, etanercept, a specific antagonist of TNFα, reduced the number and the size of tumors in the AOM/DSS model, confirming a role of TNFα in inflammation induced intestinal tumorigenesis. More intriguing was the observation by Popivanova [51] that inhibition of TNFα blocks the accumulation of β-catenin mutations, possibly reflecting a mutagenic role of TNFα.
Pharmacological inhibition of TNFα by neutralizing TNFα antibodies is very efficient in the treatment of IBD patients [52] and inhibitors of TNFα have also been tested as potential agents for the treatment of colon cancer. Results from phase I and phase II clinical trials using enbrel or remicade suggest that neutralization of TNFα has some biological activity in colorectal cancer patients [53]. However, anti TNFα medications have profound effects on patient's immune system, resulting in a broad range of infections and have been linked to development of lymphomas and skin and lung cancer. The most remarkable example is perhaps the case of a patient who developed lung cancer while on anti TNFα therapy, and whose lung cancer regressed upon discontinuing anti TNFα therapy [54], underscoring a role of TNFα in immune response and significance of immune system for the immune-surveillance [55, 56].
Binding of TNFα to its receptor triggers canonical NF-κB signaling, which is a major signaling pathway activated by TNFα (Figure 1). Signaling is initiated by activation of an IκB kinase (IKK) complex consisting of catalytic kinase subunits (IKKα and/or IKKβ) and the regulatory scaffold protein IKKγ. IKK-mediated phosphorylation results in proteasomal degradation of the IκB inhibitor enabling the active NF-κB transcription factor subunits to translocate to the nucleus and induce target gene expression. One of the target genes is the IκBα gene itself, which re-sequesters NF-κB subunits and thus terminates its transcriptional activity.
Deletion of IKKβ in intestinal cells, or pharmacological inactivation of IKKβ, resulted in more severe DSS-induced mucosal inflammation and greater ulceration, demonstrating a protective role of IKKβ in acute colitis [57]. Deficiency of IKKβ in intestinal epithelial cells had no effect on the incidence of spontaneous colitis in IL-10 deficient mice, however lack of IKKβ in macrophages or neutrophils significantly attenuated colitis triggered by IL-10 deficiency [57].
Tissue specific inactivation of IKKβ significantly reduced the number of tumors in the AOM/DSS model [58]. Deletion of IKKβ in enterocytes did not reduce inflammation in the AOM/DSS model, but resulted in increased apoptosis and markedly decreased tumor incidence, while deletion of IKKβ in myeloid cells abrogated tumor development through inhibition of proinflammatory cytokines that promote the growth of epithelial cells [58]. This report underscored the importance of NF-κB signaling in both enterocytes and in myeloid cells.
LPS (a component of gram negative bacteria) promotes growth and metastatic spread of CT26 colon cancer cells. Inhibition of NF-κB in intestinal cells has been shown to not only inhibit inflammation- induced tumor growth of colon cancer cells (mediated by TNFα), but also rendered these cells sensitive to LPS-induced TRAIL mediated cell death [59].
Several tumors exert constitutive activation of NF-κB. While persistent activation of NFκB in tumor cells alters their ability to grow and to differentiate, one of the best studied consequences of NF-κB activation is enhanced survival of cancer cells. Indeed, NF-κB directly regulates a plethora of genes that protect tumor cells from apoptosis, including bcl-x, A20, survivin, cIAPs and others. Commonly used chemotherapeutic drugs have been shown to activate NF-κB in tumor cells, which can diminish drug-induced apoptosis through upregulation of the anti-apoptotic genes [60–62]. Selective NF-κB inhibitors should therefore promote the therapeutic efficiency of chemotherapeutic drugs, however long term use of such drugs may lead to immunosuppression. Another impediment is that prolonged pharmacological inhibition of IKKβ resulted in increased IL-1β secretion upon endotoxin challenge [63].
Mice treated with etanercept, a TNFα antagonist, displayed reduced levels of unphosphorylated, active, β-catenin in intestinal tissue [51]. Indeed, TNFα has been shown to promote Wnt signaling in gastric tumor cells [4], and we showed that macrophage-derived TNFα and IL-1β activate Wnt signaling in colon cancer cells [19], establishing a direct connection between inflammation and the major oncogenic signaling pathway in colon cancer. Both TNFα and IL-1β induced phosphorylation of GSK3β in epithelial cells, which caused its inactivation, and thereby increased canonical Wnt signaling and enhanced the expression of Wnt target genes. Consistent with these findings, Apc/Min+/− mice treated with anti-TNFα antibody had significantly reduced levels of c-myc and COX-2 in intestinal mucosa, two prototypical Wnt target genes [64]. While Oguma et all [4] demonstrated that TNFα promotes Wnt signaling in an NF-κB independent manner (gastric cells expressing IκB super-repressor responded to TNFα with enhanced Wnt signaling), we showed that macrophage-derived factors activate Wnt signaling in colon cancer cells through NF-κB signaling [65]. Depletion of macrophages in the ApcΔ716 mice significantly reduced intestinal tumorigenesis [4], confirming the role of macrophage-derived factors for the progression of colon cancer.
IL-1β, NF-κB and Wnt
Interleukin 1β (IL-1β) is a proinflammatory cytokine, produced mainly by activated macrophages. In turn, IL-1β induces the expression of TNFα, IL-6, IL8, IL-17, COX-2 and PGE2, important proinflammatory mediators and promoters of growth of tumor cells [66, 67].
Consistent with the protumorigenic activity of IL-1β, chemically induced tumor formation was shown to be significantly delayed in IL-1β−/− mice. Likewise, IL-1R antagonist (IL-1RA) deficient mice, which are characterized by excessive levels of IL-1β, displayed rapid tumor development and high tumor frequency [67–69]. Injection of IL-1β or LPS (a strong inducer of IL-1β) increased metastasis in the murine B16 model and treatment of mice with IL-1RA reduced metastasis and increased survival rates [69, 70]. Liver metastasis were also reduced in IL-1 KO mice and in the ICE (IL-1β converting enzyme) KO mice, in which processing of IL-1β is inhibited [70], demonstrating that IL-1β promotes tumorigenesis at multiple levels.
IL-1β is regulated at the transcriptional, translational and posttranslational level. Maturation of the inactive IL-1β precursor into secreted biologically active protein is induced by TNFα or LPS and requires caspase 1 activity [71]. NOD2, an intracellular sensor for bacterial muramyldipeptide (MDP) directly activates caspase-1 and thus trigger maturation and secretion of IL-1β [72]. STAT1 deficient cells have defective expression of caspase 1 [73], and accordingly, LPS-induced secretion of IL-1β [74] and tumor induced release of IL-1β [19] are markedly reduced in STAT1 deficient macrophages.
IL-1β signals through two receptor chains, IL-1RI and IL-1RII, and is, like TNF, a potent inducer of NF-κB activity. Two adaptor proteins, MyD88 and IRAK, rapidly associate with IL-1R after IL-1β treatment. MyD88 is obligatory for IL-1β signaling as it connects the receptors with a downstream kinase, IRAK [75]. Deficiency in MyD88, which functions downstream of both TLR and IL-1R, has been shown to attenuate polyposis in Apc/Min+/− mice and to increase their survival [5], demonstrating that MyD88 dependent signaling critically contributes to intestinal tumorigenesis.
We showed that colon cancer cells stimulate macrophages to release IL-1β [19] and demonstrated that IL-1β induced NF-κB activation is coupled to inactivation of GSK3β and induction of Wnt signaling in colon cancer cells [65]. The expression of IL-1β target genes such as c-myc, Snail and c-jun was impaired in tumor cells expressing dnTCF4, an inhibitor of Wnt signaling, confirming the significance of Wnt signaling for the biological activity of IL-1β [76]. We showed that macrophages and IL-1β did not induce Wnt signaling in tumor cells expressing dnIκB or dnAKT, demonstrating that they induce Wnt signaling in NF-κB/AKT dependent manner [65] (Figure 2).
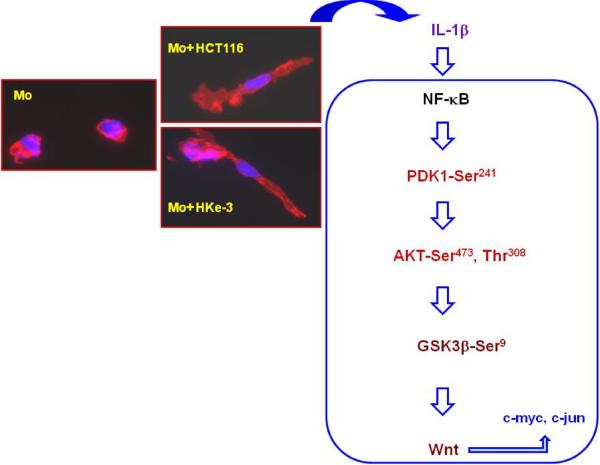
Figure 2. Signaling pathway whereby tumor associated macrophages promote Wnt signaling in tumor cells.
Peripheral blood monocytes (Mo) were cultured with control medium or with conditioned medium from HCT116 or Hke-3 colon cells for 48 hours. As shown here, soluble factor(s) from HCT116 and Hke-3 cells induced maturation of normal peripheral blood monocytes (Mo), demonstrated by phalloidin/DAPI staining, coupled to the release of IL-1β. IL-1β, through activation of NF-κB, induced phosphorylation of PDK1 and AKT, which inactivates GSK3β, leading to enhanced β–catenin/TCF4 transcriptional activity, and increased expression of Wnt target genes in tumor cells, including c-myc and c-jun (adapted from [65]).
Although loss of Apc occurs early in adenoma development, in vivo progression from microadenomas to macroscopic tumors in ApcMin/+ mice is associated with further augmentation of canonical Wnt signaling and increased expression of Wnt target genes [77], demonstrating that the enhancement of Wnt signaling beyond a threshold level, such as we observed in IL1 treated colon cancer cells, might be required for tumor progression and metastatic spread. Indeed, β-catenin translocation is often detected at the invasive front of tumor [78, 79], consistent with the interpretation that stromal tissue at the invasion front provides signals to tumor cells that promote nuclear translocation of β-catenin and thus drive tumor progression.
Consistent with the requirement of STAT1 for the processing of IL-1β, we showed that tumor cells failed to induce IL-1β release from STAT1 deficient macrophages. Accordingly, macrophages with silenced STAT1 expression did not induce Wnt signaling in colon cancer cells and thus failed to promote their growth [19]. We demonstrated that vitamin D3 inhibited tyrosine phosphorylation of STAT1 in macrophages and thus interfered with tumor induced IL-1β release from macrophages [19]. Subsequently, the ability of macrophages to induce Wnt signaling in colon cancer cells and to promote their growth was inhibited upon vitamin D3 treatment. Vitamin D3 is an important chemopreventive agent for colorectal cancer in rodents and in humans [80, 81], and a recent study found that individuals with the highest levels of vitamin D3 had a nearly 40% decrease in colorectal cancer risk compared to those with the lowest levels of vitamin D3 [82]. Our data established that vitamin D3 interrupted a crosstalk between macrophages and tumor cells and thus stalled growth of tumor cells, establishing a new pathway whereby vitamin D3 exerts its chemopreventive activity.
IL-6 and STAT3
IL-6 is secreted by stimulated monocytes, fibroblasts, and endothelial cells, macrophages, T-cells and B-lymphocytes. Colon cancer cells stimulate macrophages to produce IL-6 which activates STAT3 in tumor cells [83]. IL-6 is a growth factor for human colon cancer cells and inhibition of IL-6 signaling interferes with the growth of tumor cells [84, 85]. In addition, IL-6 protects colon cancer cells from Fas-induced apoptosis through upregulation of bcl-x [86]. Recently, tumor derived IL-6 (and IL8) were shown to be responsible for self-seeding of solid tumors by circulating cancer cells, a process whereby circulating tumor cells re-seed the parental tumor and thus promote tumor growth, and enhance angiogenesis and stromal recruitment of primary tumors [87].
Colon cancer patients have increased serum levels of IL-6 compared to healthy controls, and the levels of IL-6 correlate with tumor stage, size, metastasis and patients survival [6]. Serum levels of IL-6 are also increased in Crohn's disease patients [88], consistent with increased production of IL-6 by isolated lamina propria mononuclear cells from IBD patients [89]. In addition, soluble IL-6R (sIL-6R) and circulating complexes of IL-6/sIL-6R were found to be elevated in IBD patients, suggesting that IL-6 can activate cells that lack membranous bound IL-6R [90]. Consistent with active IL-6 signaling, lamina propria T lymphocytes isolated from IBD patients have elevated expression and activity of STAT3 and neutralization of sIL-6R reduced colitis activity [90].
IL-6 −/− mice have reduced tumor number upon AOM/DSS treatment compared to the WT mice [91]. DSS treated IL-6−/− mice showed increased apoptosis and decreased proliferation of intestinal epithelial cells. Consistently, treatment of mice with recombinant IL-6 increased both tumor multiplicity and tumor size in the AOM/DSS model. As we will discuss below, the ability of IL-6 to activate STAT3 in epithelial cells was critical for its protumorigenic activity, as targeted deletion of STAT3 in intestinal enterocytes resulted in markedly attenuated tumorigenesis, associated with increased DSS-induced apoptosis [91, 92]. Consistent with the protumorigenic activity of IL-6, genetic inactivation of IL-6 reduces tumor load in the Apc/Min+/— mice [93].
Binding of IL-6 to the IL-6 receptor activates STAT3, a major oncogenic transcription factor. The first step in IL-6 signaling is binding of lL6 to the cell surface receptors which results in gp130 dimerization and in activation and trans-phosphorylation of Janus kinases (JAK) (Figure 3). This is followed by phosphorylation of STAT3 on conserved tyrosine, subsequent STAT3 dimerization and translocation to the nucleus, where STAT3 dimers bind DNA and modulate the expression of a variety of genes. In addition, the soluble form of the IL-6R (sIL-6R) can also bind IL-6, triggering biological responses upon association with gp130. This type of activation, called “trans-signaling”, results in STAT3 activation in cells that lack cell surface expression of IL-6R. This is significant as the epithelial cells rarely express membrane bound IL-6R, and colon tumors can have decreased expression of membrane-bound IL-6R compared to the normal intestinal tissue. However, the expression of ADAM17, which is responsible for shedding of the IL-6R, is increased in tumors, suggesting that sIL-6 receptor and IL-6 trans-signaling may play a major role in colon cancer [85].
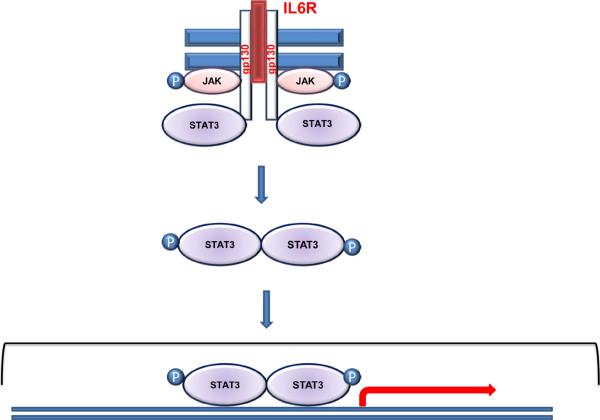
Figure 3. STAT3 signaling.
Binding of IL-6 to the receptor triggers receptor heterodimerization with the gp130. Subsequently, JAKs are activated by phosphorylation which results in the recruitment of STAT3 to the receptor. Phosphorylated STAT3 dimerize and translocate to the nucleus, bind DNA and regulate the expression of its target genes.
While activation of STAT3 in response to IL-6 is transient, tumors often exhibit persistent activation of STAT3. Enhanced expression and activation of both STAT1 and STAT3 have been found in patients with ulcerative colitis and Crohn's disease [94–96], conditions that have been shown to significantly increase the risk of development of colon cancer. It is unknown whether the presence of activated STATs predicts the risk of developing cancer in patients with IBD. A subset of human tumors, including colon tumors, is characterized by elevated levels of unphosphorylated STAT3 [97, 98]. This is important, as STAT3 can regulate the expression of several genes, such as EGFR, bcl-2, cdc2, cyclin A2, E2 and B1, in the absence of dimer formation [99].
Mice with targeted inactivation of STAT3 in intestinal epithelial cells and in macrophages [100] or in macrophages and in neutrophils [101] develop intestinal inflammation. Similarly, inactivation of STAT3 in myeloid and lymphoid cells resulted in spontaneous colitis and development of tumor lesions, including carcinomas [102]. Interestingly, in this model inflammation and proliferation were linked to activation of the mTOR-STAT3 pathway in enterocytes, a situation that is commonly found in IBD patients [102].
Conditional deletion of STAT3 in enterocytes resulted in more severe DSS-induced colitis as these mice displayed reduced proliferation of intestinal cells and increased epithelial injury after DSS challenge [91, 103]. These mice had increased expression of proinflammatory cytokines within the colonic mucosa, including IL-1β, IL-6, and IL-11, and thus displayed an exaggerated inflammatory response to DDS, associated with increased expression of COX-2. Furthermore, intestinal deletion of STAT3 markedly inhibited tumorigenesis in the AOM/DSS model, accompanied with increased apoptosis and reduced expression of STAT3 target genes, including bcl-x [91]. Accordingly, gp130Y757 mice, which contain a mutant gp130 receptor and display enhanced STAT3 activity, show enhanced colitis-induced tumorigenesis [104] and deficiency of SOCS3, a negative regulator of STAT3, is associated with increased tumor numbers [105]. Colonization of Apc/Min+/− mice with the human colonic bacterium enterotoxigenix Bacteroides Fragilis (ETBF), which secretes B. fragilis toxin (BFT), triggered STAT3 activation in intestinal epithelial cells, induced colitis and dramatically increased the number of colonic tumors [106].
Recently, intestinal deletion of STAT3 in Apc/Min+/− mice revealed that although intestinal deletion of STAT3 reduced the multiplicity of early adenomas, it enhanced growth of tumors and resulted in formation of invasive carcinomas, suggesting that STAT3 can also have anti-oncogenic activity [107]. Consistent with that, in rare carcinomas that develop in the Apc/Min+/− mice, the amount of phosphorylated STAT3 was reduced at the invasive front. The authors suggested that invasive carcinomas develop due to reduced expression of CEACAM1 and enhanced nuclear localization of β-catenin in Apc/Min+/− STAT3ΔIEC mice. Therefore, these data suggest that targeting STAT3 in vivo may have adverse effects on tumor promotion.
STAT3 has been shown to mediate nuclear translocation of β-catenin, a key event in colorectal cancer, and co-expression of nuclear STAT3 and β-catenin was associated with poor survival of colon cancer patients [108]. The nuclear staining for both STAT3 and β-catenin was observed at the invasive front, confirming their regulation by factors from the tumor microenvironment. Dominant negative STAT3 was shown to interfere with β-catenin/TCF transcription and to inhibit proliferation of colon cancer cells. In turn, Wnt signaling has been shown to upregulate STAT3 mRNA [109], suggesting a positive feedback loop between the two pathways. STAT3 directly interacts with RelA, thereby contributing to constitutive activation of NF-κB in tumor cells [110] and NF-B is essential for STAT3 activation in intestinal cells [57], underscoring the intricacy of the interplay between the signaling pathways in tumor cells.
IL-17, a STAT3 regulated cytokine
IL-17 is produced by a subset of T helper cells, which are characterized by the production of IL-17, IL-22 and TNFα (Th17 cells). Paneth cells, intestinal cells with a crucial role in mucosal immunity, also produce IL-17. Accordingly, IL-17 expression was detected at the bottom of the crypts, and colocalized with Paneth cells [111]. IL-17 positive cells constitute a considerable proportion of CD4 cells in the intestinal lamina propria, but are rare in Peyer's patches. The number of Th17 cells was markedly decreased in the large intestine of germ-free mice [112]. The authors demonstrated that development of intestinal Th17 was independent of TLR signaling, but was driven by bacterial derived ATP. Consistently, administration of ATP enhanced Th17 differentiation and aggravated T cell mediated colitis [112].
IL-6 and TGFβ have been shown to be required for Th17 differentiation of murine CD4 cells, while in humans, a combination of TGF-β, IL-1β and IL-23 induces Th17 differentiation from naive T cells. In contrast, interferon gamma (IFNγ) and IL-4, the main stimulators of Th1 and Th2 differentiation, negatively regulate differentiation of Th17 cells. Several tumor derived factors, including IL-1β, TNFα, IL-6 and TGFβ are important promoters of Th17 differentiation. Consistent with this, the density of Th17 cells is increased in the tumor microenvironment [113, 114]. STAT3 is essential for Th17 differentiation [115, 116] and IL-6, a STAT3 activator, together with TGFβ, increased the expression of RORα and RORγ, signature transcription factors for Th17 cells [117]. IL-17, in turn, induces IL-6, which activates STAT3, resulting in upregulation of genes that promote survival, such as bcl-x, in a variety of human cancer cell lines [118].
Although tumor cells express IL-17R and thus respond to IL-17, IL-17 exerts its protumorigenic role also by acting on fibroblasts and endothelial cells in the tumor microenvironment. IL-17 induces VEGF, IL-1β, IL-6 and PGE2 and stimulates IL8 production and the density of Th17 cells was positively correlated with microvessel density in tumors [119] In addition to the effect on the immune system, IL-17 has also been shown to inhibit growth factor-withdrawal induced apoptosis of colon cancer cells, suggesting that it acts as a survival factor in vivo.
IL-17A−/− mice appear to be protected from DSS-induced colitis [120], however neutralizing IL-17A antibodies aggravated DSS-induced colitis [121], underscoring a complex role of IL-17 in intestinal inflammation. Neutralization of IL-17 inhibited enterotoxigenix Bacteroides Fragilis (ETBF)-induced colitis and tumor formation in the Apc/Min+/− mice [106], confirming the role of Th17 in tumor initiation.
It is important to stress, however, that despite numerous reports describing strong protumorigenic activity of IL-17, some reports have presented convincing data describing anti-tumorigenic activity for this cytokine, most likely through enhancement of anti-tumor immunity. Accordingly, tumor growth and metastasis were shown to be enhanced in IL-17 deficient mice [113].
TRAIL and survival of colon cancer cells
Tumor necrosis factor Related Apoptosis Inducing Ligand (TRAIL, also known as Apo2L) belongs to the TNF family of ligands that activate the apoptotic cascade upon engagement of death receptor proteins. TRAIL binds to two agonistic receptors, DR4 and DR5, and to DcR1, DcR2, and osteoprotegerin (OPG), which act as decoy receptors [122]. The expression of OPG is regulated by β-catenin signaling and confers resistance to TRAIL-induced apoptosis [123]. Serum concentrations of OPG are elevated in late-stage colorectal cancer patients and proinflammatory cytokines, including TNFα and IL-1β, have been shown to increase the secretion of OPG from colon cancer cell lines [124]. Mice injected with LPS have elevated levels of OPG, and OPG deficient mice display reduced production of LPS-induced proinflammatory cytokines [125].
TRAIL deficient mice, or neutralization of TRAIL in mice, promotes spontaneous and experimentally induced tumorigenesis [126] confirming its role in tumor surveillance. TRAIL has emerged as a promising candidate to be used in cancer therapy, because it kills selectively cancer cells, while leaving normal cells unharmed [122]. Indeed, in stark contrast to other members of the TNF family, treatment of mice and primates with recombinant TRAIL induced significant regression of tumors without systemic toxicity [127, 128]. Recently, the combination of TRAIL with all trans-retinyl acetate (RAc) has been shown to induce apoptosis selectively in adenomatous polyposis (APC) deficient epithelial cell without harming normal cells and treatment of ApcMin mice with TRAIL and RAc induced apoptosis in intestinal polyps and prolonged animal survival [129]. Although the molecular basis for the tumor selective activity of TRAIL remains, for now, unknown, TRAIL-based therapies are in multiple phase I and phase II clinical trials.
Tumor cells can often develop resistance to apoptosis, which limits the efficiency of therapeutic agents. Resistance to TRAIL has been shown to develop in cells with mutant DR5 [130] or in mismatch repair deficient tumors with Bax mutations [131]. The Raf kinase inhibitor Sorafenib sensitized TRAIL resistant colon cancer line to TRAIL induced apoptosis in vivo by preventing NF-κB dependent expression of two anti-apoptotic genes, IAP2 and MCl-1, offering a new approach to promote the responsiveness of colon cancer cells to TRAIL [132]. Inhibition of NF-κB in the CT26 colon cancer cell line, which responds to LPS with enhanced cell growth and metastasis, resulted in TRAIL mediated cell death upon LPS challenge [59]. LPS induced the expression of TRAIL in both NF-κB proficient and NF-κB deficient CT26 tumor bearing lung tissue, and it has been proposed that selective induction of the TRAIL receptor, DR5, on NF-κB deficient tumor cells, mediates TRAIL-induced apoptosis in these cells [59].
In addition, soluble factors present in the tumor microenvironment have a significant impact on the responsiveness of tumor cells to TRAIL [133, 134]. Epithelial cancer cells from primary colon carcinomas produce IL-4, which increases the expression of antiapoptotic genes, including bcl-x and bcl-2, and thereby inhibits TRAIL induced apoptosis of tumor cells [135]. It appears that colon cancer stem cells, CD133 positive cells, are the main source of IL-4. Neutralization of IL-4 strongly enhanced the therapeutic efficiency of standard chemotherapeutic drugs, including oxaliplatin, 5FU and TRAIL, both in vitro and in vivo [136, 137]. These experiments demonstrated that IL-4 protects CD133 positive cells with stem cell characteristics from undergoing apoptosis [135, 136], establishing a crucial role of IL-4 in the survival of tumor-initiating stem cells.
We recently demonstrated that macrophage-derived IL-1β protects colon cancer cells from TRAIL-induced apoptosis through its ability to induce Wnt signaling in tumor cells [76]. Tumor associated macrophages or recombinant IL1 stabilized Snail in colon cancer cells and thereby inhibited TRAIL-induced apoptosis, demonstrating that factors derived from the tumor microenvironment can alter the response of tumor cells to therapeutic agents [76].
IL-10 and TGF beta (TGFβ): negative regulators of inflammation
IBD is characterized by the loss of balance between proinflammatory and regulatory cytokines. IL-10, a cytokine produced by Th2 cells, B cells, tumor cells and macrophages, is a potent inhibitor of proinflammatory cytokines, including IL-1β, TNFα and IL-6.
Mice deficient for IL-10 develop spontaneous colitis, which requires the presence of luminal bacteria [138], as germ free IL-10−/− mice remain disease free [139]. Enterocolitis in IL-10−/− mice has been shown to be perpetuated by uncontrolled production of proinflammatory cytokines, such as IL-1β, TNFα and IL-6 [140]. AOM treated IL-10−/− mice show enhanced colorectal tumor progression, which is dependent on MyD88 signaling, confirming that signaling through TLR/MyD88 is required for tumor development [35]. While the therapeutic efficiency of IL-10 in mice is encouraging, its efficacy in patients with Crohn's disease is disputed [141].
Another cytokine that is deregulated during inflammation and cancer is TGFβ. Smads are the main signal transducers in TGFβ pathway. Binding of TGFβ to the TGFβR2 activates regulatory Smads (Smad 2, 3, 1, 5, 8), which upon binding to Smad4 translocate to the nucleus and regulate the expression of TGFβ target genes. Mutations in TGFβR2 or Smad4 inactivation are common in several types of carcinomas, including colon cancer. TGFβ inhibits growth of tumor cells, it activates apoptotic cell death or autophagy, and can potently suppress tumor promoting inflammation [142]. Similar to IL-10, TGFβ inhibits IL-6 dependent colon cancer formation [85]. However, in addition to its tumor suppressive activity, TGFβ can also promote tumor progression, and increased levels of TGFβ have been shown to contribute to metastatic spread of tumor cells, at least in part, via its ability to regulate the epithelial mesenchymal transition [142]. TGFβ has therefore a dual role in cell proliferation and cell death. Likewise, TGFβ can promote self renewal of cancer stem cells, or induce their differentiation.
Transgenic overexpression of TGFβ in T lymphocytes significantly delayed development of cancer in the AOM/DSS model and expression of dnTGFRβII increased the number of lesions, confirming a crucial role of TGFβ signaling in colon cancer [84]. Consistently, mucosal T cells from IBD patients display high levels of Smad7, an inhibitor of TGFβ signaling, and intestinal tissue from these patients show reduced levels of phosphorylated Smad3.
Concluding remarks
Smoking, obesity, infection and environmental pollutants significant increase the risk of development of many types of human cancers and all of these factors appear to act by instigating inflammation. Mantovani and Pages suggested that the model of cancer formation proposed by Hanahan-Weinberg [143] which comprises six cancer traits (self sufficiency in growth signals, insensitivity to anti-growth signals, evading apoptosis, limitless replicative potential, sustained angiogenesis and tissue invasion and metastasis) has to be updated, and that a seventh hallmark should be immunity and a cancer promoting proinflammatory microenvironment [49, 144]. NF-κB and STAT3, and the proinflammatory cytokines that activate these transcription factors, have emerged as potential targets for cancer therapy. Indeed, anti-inflammatory agents, such as sulindac and vitamin D3, are potent chemopreventive agents. Curcumin inhibits both NF-κB and STAT3 signaling and shows promising results for the treatment of familial adenomatous polyposis (FAP) patients and for the Crohn's disease [145, 146]. We showed that vitamin D3, a potent chemopreventive agent for colorectal cancer, acts on cells in the tumor microenvironment, interrupts the crosstalk between tumor cells and inflammatory cells that infiltrate the tumor, and thereby inhibits the growth and survival of colon cancer cells [19, 76].
Although preclinical data are very encouraging about the effect of drugs that target proinflammatory tumor microenvironment on tumor cell growth and survival, clinical trials will need to be designed to confirm their effectiveness in patients, and to determine whether these drugs will be beneficial as single agents, or they should be used in combination with standard cytotoxic therapy.
Acknowledgments
Work in our lab was supported by CA 111361. I am grateful to Dr. Georg Wisniewski for reading the manuscript and for helpful suggestions.
Abbreviations
- NSAID
Nonsteroidal anti-inflammatory agents
- FAP
Familial Adenomatous Polyposis
- IBD
Inflammatory Bowel Disease
- CRC
Colorectal Cancer
- Apc gene
Adenomatous Polyposis Coli gene
- IL-1RA
IL-1 Receptor Antagonist
- RA
Rheumatoid Arthritis
- COX-2
cyclooxygenase 2
- STAT3
Signal Transducer and Activator of Transcription 3
- TNFα
Tumor Necrosis Factor alpha
- FGF
Fibroblast Growth Factor
- PDGF
Platelet- Derived Growth Factor
- PGE2
Prostaglandin E2
- AOM/DSS
Azoxymethane/Dextran Sodium Sulfate
- LPS
Lipopolysaccharide
- TRAIL
Tumor necrosis factor Related Apoptosis Inducing Ligand
- IL-1β
Interleukin 1β
- IL-1R
Interleukin 1 Receptor
- Myd88
Myeloid differentiation primary response gene 88
- IRAK
Interleukin 1 receptor Associated Kinase
- TLR
Toll-Like Receptor
- GSK3
Glycogen synthase kinase 3
- ICE
IL-1beta Converting Enzyme
- IL-6
Interleukin 6
- IL-6R
Interleukin 6 Receptor
- IL-10
Interleukin 10
- SOCS3
Suppressor of Cytokine Signaling 3
- OPG
Osteoprotegerin
- FLIP
FLICE-like inhibitory protein
REFERENCES
- [1].Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7–17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- [2].Oshima M, Taketo MM. COX selectivity and animal models for colon cancer. Curr Pharm Des. 2002;8:1021–1034. doi: 10.2174/1381612023394953. [DOI] [PubMed] [Google Scholar]
- [3].Gounaris E, Erdman SE, Restaino C, Gurish MF, Friend DS, Gounari F, Lee DM, Zhang G, Glickman JN, Shin K, Rao VP, Poutahidis T, Weissleder R, McNagny KM, Khazaie K. Mast cells are an essential hematopoietic component for polyp development. Proc. Natl. Acad. Sci. U. S. A. 2007;104:19977–19982. doi: 10.1073/pnas.0704620104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Oguma K, Oshima H, Aoki M, Uchio R, Naka K, Nakamura S, Hirao A, Saya H, Taketo MM, Oshima M. Activated macrophages promote Wnt signalling through tumour necrosis factor-alpha in gastric tumour cells. EMBO J. 2008;27:1671–1681. doi: 10.1038/emboj.2008.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rakoff-Nahoum S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 2007;317:124–127. doi: 10.1126/science.1140488. [DOI] [PubMed] [Google Scholar]
- [6].Knupfer H, Preiss R. Serum interleukin-6 levels in colorectal cancer patients--a summary of published results. Int. J. Colorectal Dis. 2009;25:135–140. doi: 10.1007/s00384-009-0818-8. [DOI] [PubMed] [Google Scholar]
- [7].Harrison ML, Obermueller E, Maisey NR, Hoare S, Edmonds K, Li NF, Chao D, Hall K, Lee C, Timotheadou E, Charles K, Ahern R, King DM, Eisen T, Corringham R, DeWitte M, Balkwill F, Gore M. Tumor necrosis factor alpha as a new target for renal cell carcinoma: two sequential phase II trials of infliximab at standard and high dose. J. Clin. Oncol. 2007;25:4542–4549. doi: 10.1200/JCO.2007.11.2136. [DOI] [PubMed] [Google Scholar]
- [8].Madhusudan S, Muthuramalingam SR, Braybrooke JP, Wilner S, Kaur K, Han C, Hoare S, Balkwill F, Ganesan TS. Study of etanercept, a tumor necrosis factor-alpha inhibitor, in recurrent ovarian cancer. J. Clin. Oncol. 2005;23:5950–5959. doi: 10.1200/JCO.2005.04.127. [DOI] [PubMed] [Google Scholar]
- [9].Dinarello CA. Blocking IL-1 in systemic inflammation. J. Exp. Med. 2005;201:1355–1359. doi: 10.1084/jem.20050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ancrile B, Lim KH, Counter CM. Oncogenic Ras-induced secretion of IL6 is required for tumorigenesis. Genes Dev. 2007;21:1714–1719. doi: 10.1101/gad.1549407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004;6:447–458. doi: 10.1016/j.ccr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- [13].Sparmann A, Bar-Sagi D. Ras oncogene and inflammation: partners in crime. Cell Cycle. 2005;4:735–736. doi: 10.4161/cc.4.6.1714. [DOI] [PubMed] [Google Scholar]
- [14].Rasola A, Fassetta M, De Bacco F, D'Alessandro L, Gramaglia D, Di Renzo MF, Comoglio PM. A positive feedback loop between hepatocyte growth factor receptor and beta-catenin sustains colorectal cancer cell invasive growth. Oncogene. 2007;26:1078–1087. doi: 10.1038/sj.onc.1209859. [DOI] [PubMed] [Google Scholar]
- [15].Yang L, Lin C, Liu ZR. P68 RNA helicase mediates PDGF-induced epithelial mesenchymal transition by displacing Axin from beta-catenin. Cell. 2006;127:139–155. doi: 10.1016/j.cell.2006.08.036. [DOI] [PubMed] [Google Scholar]
- [16].Pai R, Dunlap D, Qing J, Mohtashemi I, Hotzel K, French DM. Inhibition of fibroblast growth factor 19 reduces tumor growth by modulating beta-catenin signaling. Cancer Res. 2008;68:5086–5095. doi: 10.1158/0008-5472.CAN-07-2325. [DOI] [PubMed] [Google Scholar]
- [17].Moon RT, Bowerman B, Boutros M, Perrimon N. The promise and perils of Wnt signaling through beta-catenin. Science. 2002;296:1644–1646. doi: 10.1126/science.1071549. [DOI] [PubMed] [Google Scholar]
- [18].Van der Flier LG, Sabates-Bellver J, Oving I, Haegebarth A, De Palo M, Anti M, Van Gijn ME, Suijkerbuijk S, Van de Wetering M, Marra G, Clevers H. The Intestinal Wnt/TCF Signature. Gastroenterology. 2007;132:628–632. doi: 10.1053/j.gastro.2006.08.039. [DOI] [PubMed] [Google Scholar]
- [19].Kaler P, Augenlicht L, Klampfer L. Macrophage-derived IL-1beta stimulates Wnt signaling and growth of colon cancer cells: a crosstalk interrupted by vitamin D(3) Oncogene. 2009 doi: 10.1038/onc.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chan AT, Giovannucci EL, Meyerhardt JA, Schernhammer ES, Wu K, Fuchs CS. Aspirin dose and duration of use and risk of colorectal cancer in men. Gastroenterology. 2008;134:21–28. doi: 10.1053/j.gastro.2007.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chan AT, Ogino S, Fuchs CS. Aspirin use and survival after diagnosis of colorectal cancer. JAMA. 2009;302:649–658. doi: 10.1001/jama.2009.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Brown JR, DuBois RN. COX-2: a molecular target for colorectal cancer prevention. J. Clin. Oncol. 2005;23:2840–2855. doi: 10.1200/JCO.2005.09.051. [DOI] [PubMed] [Google Scholar]
- [23].Kaidi A, Qualtrough D, Williams AC, Paraskeva C. Direct transcriptional up-regulation of cyclooxygenase-2 by hypoxia-inducible factor (HIF)-1 promotes colorectal tumor cell survival and enhances HIF-1 transcriptional activity during hypoxia. Cancer Res. 2006;66:6683–6691. doi: 10.1158/0008-5472.CAN-06-0425. [DOI] [PubMed] [Google Scholar]
- [24].Al-Salihi MA, Terrece Pearman A, Doan T, Reichert EC, Rosenberg DW, Prescott SM, Stafforini DM, Topham MK. Transgenic expression of cyclooxygenase-2 in mouse intestine epithelium is insufficient to initiate tumorigenesis but promotes tumor progression. Cancer Lett. 2009;273:225–232. doi: 10.1016/j.canlet.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chulada PC, Thompson MB, Mahler JF, Doyle CM, Gaul BW, Lee C, Tiano HF, Morham SG, Smithies O, Langenbach R. Genetic disruption of Ptgs-1, as well as Ptgs-2, reduces intestinal tumorigenesis in Min mice. Cancer Res. 2000;60:4705–4708. [PubMed] [Google Scholar]
- [26].Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, Kwong E, Trzaskos JM, Evans JF, Taketo MM. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2) Cell. 1996;87:803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- [27].Fujimura T, Ohta T, Oyama K, Miyashita T, Miwa K. Cyclooxygenase-2 (COX-2) in carcinogenesis and selective COX-2 inhibitors for chemoprevention in gastrointestinal cancers. J Gastrointest Cancer. 2007;38:78–82. doi: 10.1007/s12029-008-9035-x. [DOI] [PubMed] [Google Scholar]
- [28].Wang D, Dubois RN. Prostaglandins and cancer. Gut. 2006;55:115–122. doi: 10.1136/gut.2004.047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rahman FZ, Smith AM, Hayee B, Marks DJ, Bloom SL, Segal AW. Delayed resolution of acute inflammation in ulcerative colitis is associated with elevated cytokine release downstream of TLR4. PLoS ONE. 5:e9891. doi: 10.1371/journal.pone.0009891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 10:131–144. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- [31].Maloy KJ, Salaun L, Cahill R, Dougan G, Saunders NJ, Powrie F. CD4+CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J. Exp. Med. 2003;197:111–119. doi: 10.1084/jem.20021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Vijay-Kumar M, Sanders CJ, Taylor RT, Kumar A, Aitken JD, Sitaraman SV, Neish AS, Uematsu S, Akira S, Williams IR, Gewirtz AT. Deletion of TLR5 results in spontaneous colitis in mice. J. Clin. Invest. 2007;117:3909–3921. doi: 10.1172/JCI33084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Himmel ME, Hardenberg G, Piccirillo CA, Steiner TS, Levings MK. The role of T-regulatory cells and Toll-like receptors in the pathogenesis of human inflammatory bowel disease. Immunology. 2008;125:145–153. doi: 10.1111/j.1365-2567.2008.02939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Fukata M, Chen A, Vamadevan AS, Cohen J, Breglio K, Krishnareddy S, Hsu D, Xu R, Harpaz N, Dannenberg AJ, Subbaramaiah K, Cooper HS, Itzkowitz SH, Abreu MT. Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology. 2007;133:1869–1881. doi: 10.1053/j.gastro.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Uronis JM, Muhlbauer M, Herfarth HH, Rubinas TC, Jones GS, Jobin C. Modulation of the intestinal microbiota alters colitis-associated colorectal cancer susceptibility. PLoS ONE. 2009;4:e6026. doi: 10.1371/journal.pone.0006026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- [37].Camus M, Tosolini M, Mlecnik B, Pages F, Kirilovsky A, Berger A, Costes A, Bindea G, Charoentong P, Bruneval P, Trajanoski Z, Fridman WH, Galon J. Coordination of intratumoral immune reaction and human colorectal cancer recurrence. Cancer Res. 2009;69:2685–2693. doi: 10.1158/0008-5472.CAN-08-2654. [DOI] [PubMed] [Google Scholar]
- [38].Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J. Clin. Oncol. 2005;23:609–618. doi: 10.1200/JCO.2005.01.086. [DOI] [PubMed] [Google Scholar]
- [39].Klintrup K, Makinen JM, Kauppila S, Vare PO, Melkko J, Tuominen H, Tuppurainen K, Makela J, Karttunen TJ, Makinen MJ. Inflammation and prognosis in colorectal cancer. Eur. J. Cancer. 2005;41:2645–2654. doi: 10.1016/j.ejca.2005.07.017. [DOI] [PubMed] [Google Scholar]
- [40].Eberhard A, Kahlert S, Goede V, Hemmerlein B, Plate KH, Augustin HG. Heterogeneity of angiogenesis and blood vessel maturation in human tumors: implications for antiangiogenic tumor therapies. Cancer Res. 2000;60:1388–1393. [PubMed] [Google Scholar]
- [41].Goede V, Brogelli L, Ziche M, Augustin HG. Induction of inflammatory angiogenesis by monocyte chemoattractant protein-1. Int. J. Cancer. 1999;82:765–770. doi: 10.1002/(sici)1097-0215(19990827)82:5<765::aid-ijc23>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- [42].Goede V, Fleckenstein G, Dietrich M, Osmers RG, Kuhn W, Augustin HG. Prognostic value of angiogenesis in mammary tumors. Anticancer Res. 1998;18:2199–2202. [PubMed] [Google Scholar]
- [43].Hanada T, Nakagawa M, Emoto A, Nomura T, Nasu N, Nomura Y. Prognostic value of tumor-associated macrophage count in human bladder cancer. Int. J. Urol. 2000;7:263–269. doi: 10.1046/j.1442-2042.2000.00190.x. [DOI] [PubMed] [Google Scholar]
- [44].Lissbrant IF, Stattin P, Wikstrom P, Damber JE, Egevad L, Bergh A. Tumor associated macrophages in human prostate cancer: relation to clinicopathological variables and survival. Int. J. Oncol. 2000;17:445–451. doi: 10.3892/ijo.17.3.445. [DOI] [PubMed] [Google Scholar]
- [45].Salvesen HB, Akslen LA. Significance of tumour-associated macrophages, vascular endothelial growth factor and thrombospondin-1 expression for tumour angiogenesis and prognosis in endometrial carcinomas. Int. J. Cancer. 1999;84:538–543. doi: 10.1002/(sici)1097-0215(19991022)84:5<538::aid-ijc17>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- [46].Etoh T, Shibuta K, Barnard GF, Kitano S, Mori M. Angiogenin expression in human colorectal cancer: the role of focal macrophage infiltration. Clin. Cancer Res. 2000;6:3545–3551. [PubMed] [Google Scholar]
- [47].Forssell J, Oberg A, Henriksson ML, Stenling R, Jung A, Palmqvist R. High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin Cancer Res. 2007;13:1472–1479. doi: 10.1158/1078-0432.CCR-06-2073. [DOI] [PubMed] [Google Scholar]
- [48].Oosterling SJ, van der Bij GJ, Meijer GA, Tuk CW, van Garderen E, van Rooijen N, Meijer S, van der Sijp JR, Beelen RH, van Egmond M. Macrophages direct tumour histology and clinical outcome in a colon cancer model. J. Pathol. 2005;207:147–155. doi: 10.1002/path.1830. [DOI] [PubMed] [Google Scholar]
- [49].Pages F, Galon J, Dieu-Nosjean MC, Tartour E, Sautes-Fridman C, Fridman WH. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2009 doi: 10.1038/onc.2009.416. [DOI] [PubMed] [Google Scholar]
- [50].Yan B, Wang H, Rabbani ZN, Zhao Y, Li W, Yuan Y, Li F, Dewhirst MW, Li CY. Tumor necrosis factor-alpha is a potent endogenous mutagen that promotes cellular transformation. Cancer Res. 2006;66:11565–11570. doi: 10.1158/0008-5472.CAN-06-2540. [DOI] [PubMed] [Google Scholar]
- [51].Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S, Oshima M, Fujii C, Mukaida N. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J. Clin. Invest. 2008;118:560–570. doi: 10.1172/JCI32453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR, de Villiers WJ, Present D, Sands BE, Colombel JF. Infliximab for induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2005;353:2462–2476. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- [53].Brown ER, Charles KA, Hoare SA, Rye RL, Jodrell DI, Aird RE, Vora R, Prabhakar U, Nakada M, Corringham RE, DeWitte M, Sturgeon C, Propper D, Balkwill FR, Smyth JF. A clinical study assessing the tolerability and biological effects of infliximab, a TNF-alpha inhibitor, in patients with advanced cancer. Ann. Oncol. 2008;19:1340–1346. doi: 10.1093/annonc/mdn054. [DOI] [PubMed] [Google Scholar]
- [54].Lees CW, Ironside J, Wallace WA, Satsangi J. Resolution of non-small-cell lung cancer after withdrawal of anti-TNF therapy. N. Engl. J. Med. 2008;359:320–321. doi: 10.1056/NEJMc0800250. [DOI] [PubMed] [Google Scholar]
- [55].Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, Schreiber RD. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc. Natl. Acad. Sci. U. S. A. 1998;95:7556–7561. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- [57].Eckmann L, Nebelsiek T, Fingerle AA, Dann SM, Mages J, Lang R, Robine S, Kagnoff MF, Schmid RM, Karin M, Arkan MC, Greten FR. Opposing functions of IKKbeta during acute and chronic intestinal inflammation. Proc. Natl. Acad. Sci. U. S. A. 2008;105:15058–15063. doi: 10.1073/pnas.0808216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- [59].Luo JL, Maeda S, Hsu LC, Yagita H, Karin M. Inhibition of NF-kappaB in cancer cells converts inflammation- induced tumor growth mediated by TNFalpha to TRAIL-mediated tumor regression. Cancer Cell. 2004;6:297–305. doi: 10.1016/j.ccr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- [60].Cusack JC., Jr. Overcoming antiapoptotic responses to promote chemosensitivity in metastatic colorectal cancer to the liver. Ann. Surg. Oncol. 2003;10:852–862. doi: 10.1245/aso.2003.07.518. [DOI] [PubMed] [Google Scholar]
- [61].Ban JO, Lee HS, Jeong HS, Song S, Hwang BY, Moon DC, Yoon do Y, Han SB, Hong JT. Thiacremonone augments chemotherapeutic agent-induced growth inhibition in human colon cancer cells through inactivation of nuclear factor-{kappa}B. Mol Cancer Res. 2009;7:870–879. doi: 10.1158/1541-7786.MCR-08-0580. [DOI] [PubMed] [Google Scholar]
- [62].Kaler P, Sasazuki T, Shirasawa S, Augenlicht L, Klampfer L. HDAC2 deficiency sensitizes colon cancer cells to TNFalpha-induced apoptosis through inhibition of NF-kappaB activity. Exp. Cell Res. 2008;314:1507–1518. doi: 10.1016/j.yexcr.2008.01.010. [DOI] [PubMed] [Google Scholar]
- [63].Greten FR, Arkan MC, Bollrath J, Hsu LC, Goode J, Miething C, Goktuna SI, Neuenhahn M, Fierer J, Paxian S, Van Rooijen N, Xu Y, O'Cain T, Jaffee BB, Busch DH, Duyster J, Schmid RM, Eckmann L, Karin M. NF-kappaB is a negative regulator of IL-1beta secretion as revealed by genetic and pharmacological inhibition of IKKbeta. Cell. 2007;130:918–931. doi: 10.1016/j.cell.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Rao VP, Poutahidis T, Ge Z, Nambiar PR, Horwitz BH, Fox JG, Erdman SE. Proinflammatory CD4+ CD45RB(hi) lymphocytes promote mammary and intestinal carcinogenesis in Apc(Min/+) mice. Cancer Res. 2006;66:57–61. doi: 10.1158/0008-5472.CAN-05-3445. [DOI] [PubMed] [Google Scholar]
- [65].Kaler P, Godasi BN, Augenlicht L, Klampfer L. The NF-kappaB/AKT-dependent Induction of Wnt Signaling in Colon Cancer Cells by Macrophages and IL-1beta. Cancer Microenviron. 2009 doi: 10.1007/s12307-009-0030-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Duque J, Diaz-Munoz MD, Fresno M, Iniguez MA. Up-regulation of cyclooxygenase-2 by interleukin-1beta in colon carcinoma cells. Cell. Signal. 2006;18:1262–1269. doi: 10.1016/j.cellsig.2005.10.009. [DOI] [PubMed] [Google Scholar]
- [67].Krelin Y, Voronov E, Dotan S, Elkabets M, Reich E, Fogel M, Huszar M, Iwakura Y, Segal S, Dinarello CA, Apte RN. Interleukin-1beta-driven inflammation promotes the development and invasiveness of chemical carcinogen-induced tumors. Cancer Res. 2007;67:1062–1071. doi: 10.1158/0008-5472.CAN-06-2956. [DOI] [PubMed] [Google Scholar]
- [68].Apte RN, Voronov E. Is interleukin-1 a good or bad `guy' in tumor immunobiology and immunotherapy? Immunol. Rev. 2008;222:222–241. doi: 10.1111/j.1600-065X.2008.00615.x. [DOI] [PubMed] [Google Scholar]
- [69].Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, Dinarello CA, Apte RN. IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci U S A. 2003;100:2645–2650. doi: 10.1073/pnas.0437939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Vidal-Vanaclocha F, Fantuzzi G, Mendoza L, Fuentes AM, Anasagasti MJ, Martin J, Carrascal T, Walsh P, Reznikov LL, Kim SH, Novick D, Rubinstein M, Dinarello CA. IL-18 regulates IL-1beta-dependent hepatic melanoma metastasis via vascular cell adhesion molecule-1. Proc. Natl. Acad. Sci. U. S. A. 2000;97:734–739. doi: 10.1073/pnas.97.2.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR, Aunins J. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356:768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- [72].Hsu LC, Ali SR, McGillivray S, Tseng PH, Mariathasan S, Humke EW, Eckmann L, Powell JJ, Nizet V, Dixit VM, Karin M. A NOD2-NALP1 complex mediates caspase-1-dependent IL-1beta secretion in response to Bacillus anthracis infection and muramyl dipeptide. Proc. Natl. Acad. Sci. U. S. A. 2008;105:7803–7808. doi: 10.1073/pnas.0802726105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kumar A, Commane M, Flickinger TW, Horvath CM, Stark GR. Defective TNF-alpha-induced apoptosis in STAT1-null cells due to low constitutive levels of caspases. Science. 1997;278:1630–1632. doi: 10.1126/science.278.5343.1630. [DOI] [PubMed] [Google Scholar]
- [74].Joshi VD, Kalvakolanu DV, Chen W, Zhang L, Kang TJ, Thomas KE, Vogel SN, Cross AS. A role for Stat1 in the regulation of lipopolysaccharide-induced interleukin-1beta expression. J. Interferon Cytokine Res. 2006;26:739–747. doi: 10.1089/jir.2006.26.739. [DOI] [PubMed] [Google Scholar]
- [75].Wesche H, Henzel WJ, Shillinglaw W, Li S, Cao Z. MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity. 1997;7:837–847. doi: 10.1016/s1074-7613(00)80402-1. [DOI] [PubMed] [Google Scholar]
- [76].Kaler P, Galea V, Augenlicht L, Klampfer L. Tumor associated macrophages protect colon cancer cells from TRAIL-induced apoptosis through IL-1beta-dependent stabilization of Snail in tumor cells. PLoS One. 2010;5:e11700. doi: 10.1371/journal.pone.0011700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Oyama T, Yamada Y, Hata K, Tomita H, Hirata A, Sheng H, Hara A, Aoki H, Kunisada T, Yamashita S, Mori H. Further upregulation of {beta}-catenin/Tcf transcription is involved in the development of macroscopic tumors in the colon of Apc Min/+ mice. Carcinogenesis. 2008 doi: 10.1093/carcin/bgn001. [DOI] [PubMed] [Google Scholar]
- [78].Brabletz T, Jung A, Hermann K, Gunther K, Hohenberger W, Kirchner T. Nuclear overexpression of the oncoprotein beta-catenin in colorectal cancer is localized predominantly at the invasion front. Pathol. Res. Pract. 1998;194:701–704. doi: 10.1016/s0344-0338(98)80129-5. [DOI] [PubMed] [Google Scholar]
- [79].Brabletz T, Jung A, Reu S, Porzner M, Hlubek F, Kunz-Schughart LA, Knuechel R, Kirchner T. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc. Natl. Acad. Sci. U. S. A. 2001;98:10356–10361. doi: 10.1073/pnas.171610498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Lamprecht SA, Lipkin M. Chemoprevention of colon cancer by calcium, vitamin D and folate: molecular mechanisms. Nat Rev Cancer. 2003;3:601–614. doi: 10.1038/nrc1144. [DOI] [PubMed] [Google Scholar]
- [81].Lipkin M, Lamprecht SA. Mechanisms of action of vitamin D: recent findings and new questions. J Med Food. 2006;9:135–137. doi: 10.1089/jmf.2006.9.135. [DOI] [PubMed] [Google Scholar]
- [82].Jenab M, Bueno-de-Mesquita HB, Ferrari P, van Duijnhoven FJ, Norat T, Pischon T, Jansen EH, Slimani N, Byrnes G, Rinaldi S, Tjonneland A, Olsen A, Overvad K, Boutron-Ruault MC, Clavel-Chapelon F, Morois S, Kaaks R, Linseisen J, Boeing H, Bergmann MM, Trichopoulou A, Misirli G, Trichopoulos D, Berrino F, Vineis P, Panico S, Palli D, Tumino R, Ros MM, van Gils CH, Peeters PH, Brustad M, Lund E, Tormo MJ, Ardanaz E, Rodriguez L, Sanchez MJ, Dorronsoro M, Gonzalez CA, Hallmans G, Palmqvist R, Roddam A, Key TJ, Khaw KT, Autier P, Hainaut P, Riboli E. Association between pre-diagnostic circulating vitamin D concentration and risk of colorectal cancer in European populations:a nested case-control study. BMJ. 340:b5500. doi: 10.1136/bmj.b5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Herbeuval JP, Lelievre E, Lambert C, Dy M, Genin C. Recruitment of STAT3 for production of IL-10 by colon carcinoma cells induced by macrophage-derived IL-6. J Immunol. 2004;172:4630–4636. doi: 10.4049/jimmunol.172.7.4630. [DOI] [PubMed] [Google Scholar]
- [84].Becker C, Fantini MC, Schramm C, Lehr HA, Wirtz S, Nikolaev A, Burg J, Strand S, Kiesslich R, Huber S, Ito H, Nishimoto N, Yoshizaki K, Kishimoto T, Galle PR, Blessing M, Rose-John S, Neurath MF. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity. 2004;21:491–501. doi: 10.1016/j.immuni.2004.07.020. [DOI] [PubMed] [Google Scholar]
- [85].Becker C, Fantini MC, Wirtz S, Nikolaev A, Lehr HA, Galle PR, Rose-John S, Neurath MF. IL-6 signaling promotes tumor growth in colorectal cancer. Cell Cycle. 2005;4:217–220. [PubMed] [Google Scholar]
- [86].Yuan H, Liddle FJ, Mahajan S, Frank DA. IL-6-induced survival of colorectal carcinoma cells is inhibited by butyrate through down-regulation of the IL-6 receptor. Carcinogenesis. 2004;25:2247–2255. doi: 10.1093/carcin/bgh246. [DOI] [PubMed] [Google Scholar]
- [87].Kim MY, Oskarsson T, Acharyya S, Nguyen DX, Zhang XH, Norton L, Massague J. Tumor self-seeding by circulating cancer cells. Cell. 2009;139:1315–1326. doi: 10.1016/j.cell.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Atreya R, Neurath MF. Involvement of IL-6 in the pathogenesis of inflammatory bowel disease and colon cancer. Clin. Rev. Allergy Immunol. 2005;28:187–196. doi: 10.1385/CRIAI:28:3:187. [DOI] [PubMed] [Google Scholar]
- [89].Reinecker HC, Steffen M, Witthoeft T, Pflueger I, Schreiber S, MacDermott RP, Raedler A. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn's disease. Clin. Exp. Immunol. 1993;94:174–181. doi: 10.1111/j.1365-2249.1993.tb05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Atreya R, Mudter J, Finotto S, Mullberg J, Jostock T, Wirtz S, Schutz M, Bartsch B, Holtmann M, Becker C, Strand D, Czaja J, Schlaak JF, Lehr HA, Autschbach F, Schurmann G, Nishimoto N, Yoshizaki K, Ito H, Kishimoto T, Galle PR, Rose-John S, Neurath MF. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat. Med. 2000;6:583–588. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- [91].Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, Karin M. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Grivennikov S, Karin M. Autocrine IL-6 signaling: a key event in tumorigenesis? Cancer Cell. 2008;13:7–9. doi: 10.1016/j.ccr.2007.12.020. [DOI] [PubMed] [Google Scholar]
- [93].Baltgalvis KA, Berger FG, Pena MM, Davis JM, Muga SJ, Carson JA. Interleukin-6 and cachexia in ApcMin/+ mice. Am J Physiol Regul Integr Comp Physiol. 2008;294:R393–401. doi: 10.1152/ajpregu.00716.2007. [DOI] [PubMed] [Google Scholar]
- [94].Musso A, Dentelli P, Carlino A, Chiusa L, Repici A, Sturm A, Fiocchi C, Rizzetto M, Pegoraro L, Sategna-Guidetti C, Brizzi MF. Signal Transducers and Activators of Transcription 3 Signaling Pathway: An Essential Mediator of Inflammatory Bowel Disease and Other Forms of Intestinal Inflammation. Inflamm Bowel Dis. 2005;11:91–98. doi: 10.1097/00054725-200502000-00001. [DOI] [PubMed] [Google Scholar]
- [95].Mudter J, Weigmann B, Bartsch B, Kiesslich R, Strand D, Galle PR, Lehr HA, Schmidt J, Neurath MF. Activation pattern of signal transducers and activators of transcription (STAT) factors in inflammatory bowel diseases. Am J Gastroenterol. 2005;100:64–72. doi: 10.1111/j.1572-0241.2005.40615.x. [DOI] [PubMed] [Google Scholar]
- [96].Schreiber S, Rosenstiel P, Hampe J, Nikolaus S, Groessner B, Schottelius A, Kuhbacher T, Hamling J, Folsch UR, Seegert D. Activation of signal transducer and activator of transcription (STAT) 1 in human chronic inflammatory bowel disease. Gut. 2002;51:379–385. doi: 10.1136/gut.51.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Kusaba T, Nakayama T, Yamazumi K, Yakata Y, Yoshizaki A, Inoue K, Nagayasu T, Sekine I. Activation of STAT3 is a marker of poor prognosis in human colorectal cancer. Oncol Rep. 2006;15:1445–1451. [PubMed] [Google Scholar]
- [98].Kusaba T, Nakayama T, Yamazumi K, Yakata Y, Yoshizaki A, Nagayasu T, Sekine I. Expression of p-STAT3 in human colorectal adenocarcinoma and adenoma; correlation with clinicopathological factors. J Clin Pathol. 2005;58:833–838. doi: 10.1136/jcp.2004.023416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Yang J, Chatterjee-Kishore M, Staugaitis SM, Nguyen H, Schlessinger K, Levy DE, Stark GR. Novel roles of unphosphorylated STAT3 in oncogenesis and transcriptional regulation. Cancer Res. 2005;65:939–947. [PubMed] [Google Scholar]
- [100].Alonzi T, Newton IP, Bryce PJ, Di Carlo E, Lattanzio G, Tripodi M, Musiani P, Poli V. Induced somatic inactivation of STAT3 in mice triggers the development of a fulminant form of enterocolitis. Cytokine. 2004;26:45–56. doi: 10.1016/j.cyto.2003.12.002. [DOI] [PubMed] [Google Scholar]
- [101].Takeda K, Clausen BE, Kaisho T, Tsujimura T, Terada N, Forster I, Akira S. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39–49. doi: 10.1016/s1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- [102].Deng L, Zhou JF, Sellers RS, Li JF, Nguyen AV, Wang Y, Orlofsky A, Liu Q, Hume DA, Pollard JW, Augenlicht L, Lin EY. A Novel Mouse Model of Inflammatory Bowel Disease Links Mammalian Target of Rapamycin-Dependent Hyperproliferation of Colonic Epithelium to Inflammation-Associated Tumorigenesis. Am. J. Pathol. 2009 doi: 10.2353/ajpath.2010.090622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Grivennikov SI, Karin M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2009 doi: 10.1016/j.cytogfr.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Bollrath J, Phesse TJ, von Burstin VA, Putoczki T, Bennecke M, Bateman T, Nebelsiek T, Lundgren-May T, Canli O, Schwitalla S, Matthews V, Schmid RM, Kirchner T, Arkan MC, Ernst M, Greten FR. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15:91–102. doi: 10.1016/j.ccr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- [105].Rigby RJ, Simmons JG, Greenhalgh CJ, Alexander WS, Lund PK. Suppressor of cytokine signaling 3 (SOCS3) limits damage-induced crypt hyper-proliferation and inflammation-associated tumorigenesis in the colon. Oncogene. 2007;26:4833–4841. doi: 10.1038/sj.onc.1210286. [DOI] [PubMed] [Google Scholar]
- [106].Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F, Housseau F, Pardoll DM, Sears CL. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat. Med. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Musteanu M, Blaas L, Mair M, Schlederer M, Bilban M, Tauber S, Esterbauer H, Mueller M, Casanova E, Kenner L, Poli V, Eferl R. Stat3 Is a Negative Regulator of Intestinal Tumor Progression in Apc(Min) Mice. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.11.049. [DOI] [PubMed] [Google Scholar]
- [108].Kawada M, Seno H, Uenoyama Y, Sawabu T, Kanda N, Fukui H, Shimahara Y, Chiba T. Signal transducers and activators of transcription 3 activation is involved in nuclear accumulation of beta-catenin in colorectal cancer. Cancer Res. 2006;66:2913–2917. doi: 10.1158/0008-5472.CAN-05-3460. [DOI] [PubMed] [Google Scholar]
- [109].Hao J, Li TG, Qi X, Zhao DF, Zhao GQ. WNT/beta-catenin pathway up-regulates Stat3 and converges on LIF to prevent differentiation of mouse embryonic stem cells. Dev. Biol. 2006;290:81–91. doi: 10.1016/j.ydbio.2005.11.011. [DOI] [PubMed] [Google Scholar]
- [110].Lee H, Herrmann A, Deng JH, Kujawski M, Niu G, Li Z, Forman S, Jove R, Pardoll DM, Yu H. Persistently activated Stat3 maintains constitutive NF-kappaB activity in tumors. Cancer Cell. 2009;15:283–293. doi: 10.1016/j.ccr.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Takahashi N, Vanlaere I, de Rycke R, Cauwels A, Joosten LA, Lubberts E, van den Berg WB, Libert C. IL-17 produced by Paneth cells drives TNF-induced shock. J. Exp. Med. 2008;205:1755–1761. doi: 10.1084/jem.20080588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, Yagita H, Ishii N, Evans R, Honda K, Takeda K. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- [113].Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH, Chang A, Coukos G, Liu R, Zou W. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Gounaris E, Blatner NR, Dennis K, Magnusson F, Gurish MF, Strom TB, Beckhove P, Gounari F, Khazaie K. T-regulatory cells shift from a protective anti-inflammatory to a cancer-promoting proinflammatory phenotype in polyposis. Cancer Res. 2009;69:5490–5497. doi: 10.1158/0008-5472.CAN-09-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, Rudensky AY. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326:986–991. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, Tato C, Yoshimura A, Hennighausen L, O'Shea JJ. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc. Natl. Acad. Sci. U. S. A. 2006;103:8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- [118].Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J. Exp. Med. 2009;206:1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- [120].O'Connor W, Jr., Kamanaka M, Booth CJ, Town T, Nakae S, Iwakura Y, Kolls JK, Flavell RA. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol. 2009;10:603–609. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Ogawa A, Andoh A, Araki Y, Bamba T, Fujiyama Y. Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin. Immunol. 2004;110:55–62. doi: 10.1016/j.clim.2003.09.013. [DOI] [PubMed] [Google Scholar]
- [122].Wang S. The promise of cancer therapeutics targeting the TNF-related apoptosis-inducing ligand and TRAIL receptor pathway. Oncogene. 2008;27:6207–6215. doi: 10.1038/onc.2008.298. [DOI] [PubMed] [Google Scholar]
- [123].De Toni EN, Thieme SE, Herbst A, Behrens A, Stieber P, Jung A, Blum H, Goke B, Kolligs FT. OPG is regulated by beta-catenin and mediates resistance to TRAIL-induced apoptosis in colon cancer. Clin. Cancer Res. 2008;14:4713–4718. doi: 10.1158/1078-0432.CCR-07-5019. [DOI] [PubMed] [Google Scholar]
- [124].Pettersen I, Bakkelund W, Smedsrod B, Sveinbjornsson B. Osteoprotegerin is expressed in colon carcinoma cells. Anticancer Res. 2005;25:3809–3816. [PubMed] [Google Scholar]
- [125].Maruyama K, Takada Y, Ray N, Kishimoto Y, Penninger JM, Yasuda H, Matsuo K. Receptor activator of NF-kappa B ligand and osteoprotegerin regulate proinflammatory cytokine production in mice. J. Immunol. 2006;177:3799–3805. doi: 10.4049/jimmunol.177.6.3799. [DOI] [PubMed] [Google Scholar]
- [126].Takeda K, Smyth MJ, Cretney E, Hayakawa Y, Kayagaki N, Yagita H, Okumura K. Critical role for tumor necrosis factor-related apoptosis-inducing ligand in immune surveillance against tumor development. J. Exp. Med. 2002;195:161–169. doi: 10.1084/jem.20011171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, Smith C, Smolak P, Goodwin RG, Rauch CT, Schuh JC, Lynch DH. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat. Med. 1999;5:157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- [128].Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, DeForge L, Koumenis IL, Lewis D, Harris L, Bussiere J, Koeppen H, Shahrokh Z, Schwall RH. Safety and antitumor activity of recombinant soluble Apo2 ligand. J. Clin. Invest. 1999;104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Zhang L, Ren X, Alt E, Bai X, Huang S, Xu Z, Lynch PM, Moyer MP, Wen XF, Wu X. Chemoprevention of colorectal cancer by targeting APC-deficient cells for apoptosis. Nature. 464:1058–1061. doi: 10.1038/nature08871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].El-Deiry WS. Insights into cancer therapeutic design based on p53 and TRAIL receptor signaling. Cell Death Differ. 2001;8:1066–1075. doi: 10.1038/sj.cdd.4400943. [DOI] [PubMed] [Google Scholar]
- [131].LeBlanc H, Lawrence D, Varfolomeev E, Totpal K, Morlan J, Schow P, Fong S, Schwall R, Sinicropi D, Ashkenazi A. Tumor-cell resistance to death receptor--induced apoptosis through mutational inactivation of the proapoptotic Bcl-2 homolog Bax. Nat Med. 2002;8:274–281. doi: 10.1038/nm0302-274. [DOI] [PubMed] [Google Scholar]
- [132].Ricci MS, Kim SH, Ogi K, Plastaras JP, Ling J, Wang W, Jin Z, Liu YY, Dicker DT, Chiao PJ, Flaherty KT, Smith CD, El-Deiry WS. Reduction of TRAIL-induced Mcl-1 and cIAP2 by c-Myc or sorafenib sensitizes resistant human cancer cells to TRAIL-induced death. Cancer Cell. 2007;12:66–80. doi: 10.1016/j.ccr.2007.05.006. [DOI] [PubMed] [Google Scholar]
- [133].McMillin DW, Delmore J, Weisberg E, Negri JM, Geer DC, Klippel S, Mitsiades N, Schlossman RL, Munshi NC, Kung AL, Griffin JD, Richardson PG, Anderson KC, Mitsiades CS. Tumor cell-specific bioluminescence platform to identify stroma-induced changes to anticancer drug activity. Nat. Med. 16:483–489. doi: 10.1038/nm.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Meads MB, Gatenby RA, Dalton WS. Environment-mediated drug resistance: a major contributor to minimal residual disease. Nat Rev Cancer. 2009;9:665–674. doi: 10.1038/nrc2714. [DOI] [PubMed] [Google Scholar]
- [135].Todaro M, Lombardo Y, Francipane MG, Alea MP, Cammareri P, Iovino F, Di Stefano AB, Di Bernardo C, Agrusa A, Condorelli G, Walczak H, Stassi G. Apoptosis resistance in epithelial tumors is mediated by tumor-cell-derived interleukin-4. Cell Death Differ. 2008;15:762–772. doi: 10.1038/sj.cdd.4402305. [DOI] [PubMed] [Google Scholar]
- [136].Todaro M, Alea MP, Di Stefano AB, Cammareri P, Vermeulen L, Iovino F, Tripodo C, Russo A, Gulotta G, Medema JP, Stassi G. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell. 2007;1:389–402. doi: 10.1016/j.stem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- [137].Todaro M, Perez Alea M, Scopelliti A, Medema JP, Stassi G. IL-4-mediated drug resistance in colon cancer stem cells. Cell Cycle. 2008;7:309–313. doi: 10.4161/cc.7.3.5389. [DOI] [PubMed] [Google Scholar]
- [138].Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- [139].Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, Rennick DM, Sartor RB. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect. Immun. 1998;66:5224–5231. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Berg DJ, Davidson N, Kuhn R, Muller W, Menon S, Holland G, Thompson-Snipes L, Leach MW, Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Lindsay JO, Ciesielski CJ, Scheinin T, Hodgson HJ, Brennan FM. The prevention and treatment of murine colitis using gene therapy with adenoviral vectors encoding IL-10. J. Immunol. 2001;166:7625–7633. doi: 10.4049/jimmunol.166.12.7625. [DOI] [PubMed] [Google Scholar]
- [142].Ikushima H, Miyazono K. TGFbeta signalling: a complex web in cancer progression. Nat Rev Cancer. 10:415–424. doi: 10.1038/nrc2853. [DOI] [PubMed] [Google Scholar]
- [143].Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- [144].Mantovani A. Cancer: Inflaming metastasis. Nature. 2009;457:36–37. doi: 10.1038/457036b. [DOI] [PubMed] [Google Scholar]
- [145].Cruz-Correa M, Shoskes DA, Sanchez P, Zhao R, Hylind LM, Wexner SD, Giardiello FM. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2006;4:1035–1038. doi: 10.1016/j.cgh.2006.03.020. [DOI] [PubMed] [Google Scholar]
- [146].Holt PR, Katz S, Kirshoff R. Curcumin therapy in inflammatory bowel disease: a pilot study. Dig. Dis. Sci. 2005;50:2191–2193. doi: 10.1007/s10620-005-3032-8. [DOI] [PubMed] [Google Scholar]