Abstract
Background
Acute traumatic coagulopathy (ATC) predicts poor outcome following injury. Females have been demonstrated to be hypercoagulable early in the post trauma period. It remains unclear whether presence of ATC alters gender based outcomes post-injury. This study objective was to characterize the gender dimorphism following severe injury in the presence and absence of ATC.
Methods
Data were obtained from a multicenter prospective cohort study of blunt trauma patients with hemorrhagic shock. ATC was defined as arrival INR >1.5. Cox regression was utilized to determine the independent risks of mortality and multiple organ failure (MOF) associated with gender in subjects with ATC and without (NON-ATC) while controlling for important confounders. The gender mortality differences were characterized over time to determine at what point post-injury any differential risks diverge.
Results
Of 2,007 enrolled subjects, 1,877 had an arrival INR with 439 (23%) having ATC. There was no difference in incidence of ATC across gender (24% vs. 23%, p=0.95). In the ATC group, no difference in ISS, arrival INR, base deficit, temperature or 24 hour blood requirements were found across gender. Cox hazard regression revealed gender was not associated with mortality in NON-ATC patients (HR 0.94, 95%CI 0.6–1.5). Female gender was independently associated with mortality only in the ATC group (HR 2.04, 95%CI 1.1–3.9, p=0.03). These mortality risk differences across gender diverged within the first 24 hours post-injury.
Conclusions
An exaggerated gender dimorphism exists in patients with ATC, with females demonstrating a 2-fold higher independent risk of mortality. These differential mortality risks across gender diverge early post-injury suggesting they may be due to ongoing hemorrhage. Females that present with ATC on admission have a significantly greater risk of poor outcome. Further studies are warranted to explore the mechanisms responsible for gender dimorphism in the setting of ATC.
INTRODUCTION
Acute traumatic coagulopathy (ATC) has become increasingly recognized as an independent determinant of poor outcome following injury.1,2 Evidence suggests that early coagulopathy in trauma occurs as a result of both endogenous coagulopathy immediately following injury as well as acquired coagulopathy associated with resuscitation.3 Systemic activation of the protein C pathway and hyperfibrinolysis associated with severe tissue injury and hypoperfusion promotes endogenous coagulopathy. This tissue injury initiates a cascade of humoral factors, resulting in significant perturbations in the coagulation and inflammatory systems of the body in the immediate post injury period. Large volume resuscitation often leads to hemodilution of circulating clotting factors and derangement of clotting enzyme function from hypothermia, resulting in acquired coagulopathy.
Gender based differential outcomes in the setting of trauma have been well documented.4–8 The mechanisms responsible for this gender dimorphism following injury remain controversial and are postulated to be due to differences in the hormonal response between males and females or other alternative genetic mechanisms.4,9,10 It has been demonstrated that females tend to be hypercoagulable when compared to males following trauma.11 Whether the presences of coagulopathy has an effect or alters the strength of the gender dimorphism post-injury remains less than adequately characterized. The objective of the current analysis was to evaluate the strength of any gender dimorphism in the presence or absence of ATC to determine if their relationship has an effect on outcome. It was hypothesized that females would be relatively protected when compared to males in the setting of coagulopathy given the tendency toward hypercoagulability in the early post trauma period.
METHODS
Data were obtained from the Inflammation and the Host Response to Injury Large Scale Collaborative Program (www.gluegrant.org), supported by the National Institute of General Medical Sciences (NIGMS), which is a multicenter prospective cohort study of blunt injured adults with hemorrhagic shock designed to characterize the genomic and proteomic response following injury.11 Patients admitted to one of seven institutions over a 8 year period (2003–2010) were included in the current analysis. Inclusion criteria for the overall cohort study included: blunt mechanism of injury, presence of pre-hospital or emergency department hypotension (Systolic blood pressure [SBP] < 90 mmHg) or an elevated base deficit (> 6 meq/L), blood transfusion requirement within the first 12hrs, and any body region exclusive of the brain with an abbreviated injury score (AIS) ≥ 2, allowing exclusion of patients with isolated traumatic brain injury. Patients < 18 or > 90 years of age and those with cervical spinal cord injury were also excluded from enrolment. Clinical data were entered and stored in TrialDb, a web-based data collection platform, by trained research nurses.13 Integrity of the data was maintained through ongoing curation and external data review by an independent chart abstractor.
Standard operating procedures were developed and implemented across all institutional centers to minimize variation in post-injury care, including: early goal directed resuscitation, strict glycemic control, venous thromboembolism prophylaxis, appropriate low tidal volume ventilation, ventilator associated pneumonia management, and restrictive transfusion guidelines.14–18 While patients were admitted to the ICU, multiple organ dysfunction scores for renal, hepatic, cardiovascular, metabolic, hematologic, respiratory, and neurological systems were determined daily.19–21 The diagnosis of MOF required a maximum Marshall Multiple Organ Dysfunction score > 5.
For the current secondary analysis all cohort subjects with an initial International Normalized Ratio (INR) were included in the study. Acute traumatic coagulopathy was defined as an arrival INR > 1.5 and patients were stratified into ATC and NON-ATC groups. Demographics, injury characteristics and severity, resuscitation requirements, and outcomes were compared in univariate analysis first across the ATC and NON-ATC groups and then across gender (female vs. male) in both ATC and NON-ATC groups.
The primary outcomes were 30 day in-hospital mortality and multiple organ failure (MOF). Cox proportional hazards regression was utilized to determine the independent risks of mortality and MOF across gender while controlling for differences in age, presence of admission hypotension, admission tachycardia, lowest 24 hour temperature, injury severity score (ISS), maximum abbreviated injury scores (AIS), severe head injury, Glasgow coma score, initial based deficit, APACHE II score, 24 hour resuscitation requirements (blood, plasma, platelet, and crystalloid), early laparotomy or thoracotomy within 48 hours of admission, pre-existing comorbidities, and pre-hospital use of warfarin, aspirin, or anti-platelet medications. Cox regression was first performed for both mortality and MOF on the overall study population using the above covariates with the presence of ATC (yes/no) included in the model and adjusted for. Subsequently, Cox regression was performed using the same regression models stratified by the presence of absence of acute coagulopathy (ATC vs. NON-ATC) to determine whether the strength of the gender dimorphism was altered by the presence of ATC after controlling for important confounders.
To further characterize the relationship between gender and the presences of acute coagulopathy of trauma, Cox-adjusted survival curves were constructed to determine the time course over which any gender differences occurred. Additionally, to account for potential differences in hormonal changes associated with age in females, models were repeated to test for an interaction between gender and menopausal age related subgroups corresponding to premenopausal age (< 48 years old) and postmenopausal age (>52 years old) in females and similar aged males.
Data analysis was conducted using SPSS version 19 (Chicago, IL). For univariate analyses Chi-square tests were used to compare categorical variables, and Mann-Whitney tests were used to compare continuous variables. Continuous data are presented as median (interquartile range [IQR]) unless noted. A p value of ≤ 0.05 was considered significant. The institutional review board of each participating center approved the cohort study, while the institutional review board at the University of Pittsburgh Medical Center approved this current secondary analysis.
RESULTS
Of the 2,007 subjects enrolled in the prospective cohort, 1,877 (94%) had an arrival INR available and represents the study cohort. There were 439 (23%) subjects with an arrival INR > 1.5 making up the ATC group. As expected, patients in the ATC group had higher injury and shock severity, greater resuscitation requirements, and worse outcomes as compared to the NON-ATC group. (Table 1.) However, there was no difference in gender distribution between the ATC and NON-ATC groups. When gender differences were assessed in the ATC group, males and females were similar in demographics, injury and shock severity, presenting coagulopathy, and blood transfusion requirements (Table 2). Males did receive significantly greater plasma, crystalloid, more frequently required early operative intervention, while females also had a significantly lower unadjusted rate of MOF.
Table 1.
Demographics, injury severity, and outcomes by coagulopathy
| Median (IQR) | ATC N=439 (23%) |
NON-ATC N=1438 (77%) |
p |
|---|---|---|---|
| Age | 38 (22–57) | 43 (28–55) | <0.01* |
| Gender (% male) | 66.3 | 66.6 | 0.95 |
| ISS | 38 (27–50) | 29 (22–41) | <0.01* |
| APACHE II | 32 (28–36) | 28 (24–33) | <0.01* |
| Initial base deficit | 9.7 (6.8–13.1) | 7.6 (5.3–10.9) | <0.01* |
| INR | 1.9 (1.7–2.3) | 1.2 (1.1–1.3) | <0.01* |
| 24hr blood requirement | 10.5 (5.7–18.7) | 5 (2.7–9.33) | <0.01* |
| 24hr FFP requirement | 7.2 (3.5–11.8) | 1.6 (0–4.8) | <0.01* |
| 24hr platelet requirement | 0.9 (0–1.8) | 0 (0–0.9) | <0.01* |
| 24hr crystalloid requirement | 14.9 (10.4–20.8) | 12.2 (8.9–16.6) | <0.01* |
| Massive transfusion (%) | 52.2 | 23.4 | <0.01* |
| Early surgery (%) | 53.5 | 40.8 | <0.01* |
| Coumadin (%) | 6.2 | 0.6 | <0.01* |
| ICU days | 9 (4–17) | 12 (5–19) | <0.01* |
| In-hospital mortality (%) | 26.2 | 11.4 | <0.01* |
| MOF (%) | 38.7 | 27.7 | <0.01* |
p < 0.05
Table 2.
Demographics, injury severity, and outcomes by gender in the ATC group
| Median (IQR) | Females N=148 (34%) |
Males N=291 (66%) |
p |
|---|---|---|---|
| Age | 39 (22–61) | 37 (22–56) | 0.32 |
| ISS | 35.5 (27–43) | 38 (29–50) | 0.37 |
| APACHE II | 32 (28–35) | 33 (28–37) | 0.11 |
| Initial base deficit | 9.5 (6.8–13.4) | 9.8 (6.9–13.0) | 0.94 |
| INR | 1.9 (1.7–2.3) | 1.9 (1.7–2.3) | 0.78 |
| 24hr blood requirement | 9.3 (4.7–17.5) | 10.5 (5.8–18.7) | 0.14 |
| 24hr FFP requirement | 6.0 (3.2–10.4) | 7.5 (3.8–12.7a0 | 0.02* |
| 24hr PLT requirement | 0.9 (0–1.8) | 0.9 (0–1.8) | 0.60 |
| 24hr crystalloid requirement | 12.7 (9.3–19.1) | 16.4 (10.9–21.7) | <0.01* |
| Massive Transfusion (%) | 47.3 | 54.6 | 0.16 |
| Early surgery (%) | 46.0 | 57.4 | 0.03* |
| ICU days | 10 (5–18) | 12 (5–19) | 0.33 |
| In-hospital mortality (%) | 29.1 | 24.7 | 0.36 |
| MOF (%) | 31.8 | 42.3 | 0.04* |
p < 0.05
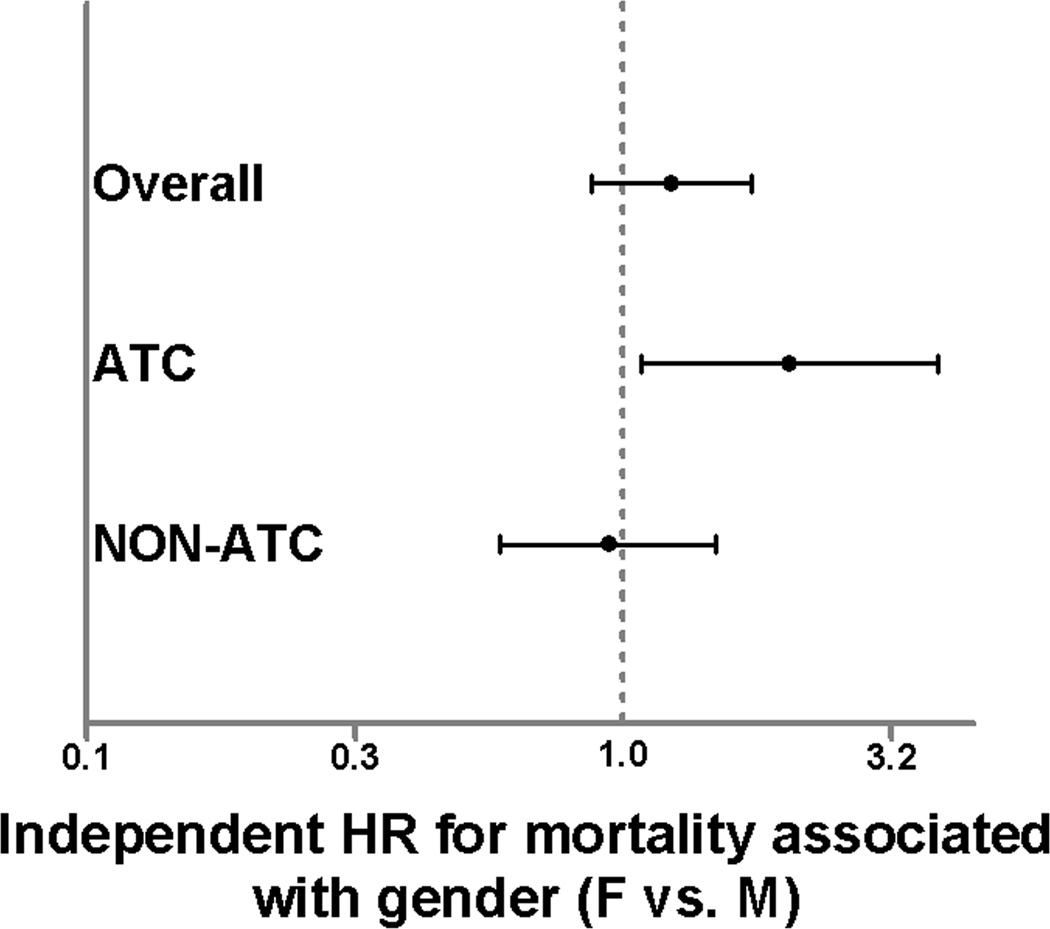
For in-hospital mortality, there was no difference across females and male gender in the overall study cohort (HR 1.23; 95%CI 0.88 – 1.73, p=0.23), however, the presence of acute coagulopathy was a significant independent predictor of mortality (HR 1.46; 95%CI 1.02 – 2.09, p=0.04) suggesting over a 40% higher risk of mortality in those with ATC. When stratified by the presence of absence of ATC, female gender was associated with over a 2-fold higher independent risk of mortality (HR 2.04; 95%CI 1.07 – 3.87, p=0.03) as compared to males in the ATC group. Within the NON-ATC group, female gender was not associated with the risk of mortality (HR 0.94; 95%CI 0.59 – 1.49, p=0.78) relative to males (Fig. 1).
Figure 1.
Hazard ratios from Cox regression for 30 day mortality in the overall study population, ATC group, and NON-ATC group. Hazard ratios represent female relative to male gender. Bars represent 95% confidence intervals. Bars that do not cross 1.0 are considered significant (p<0.05).
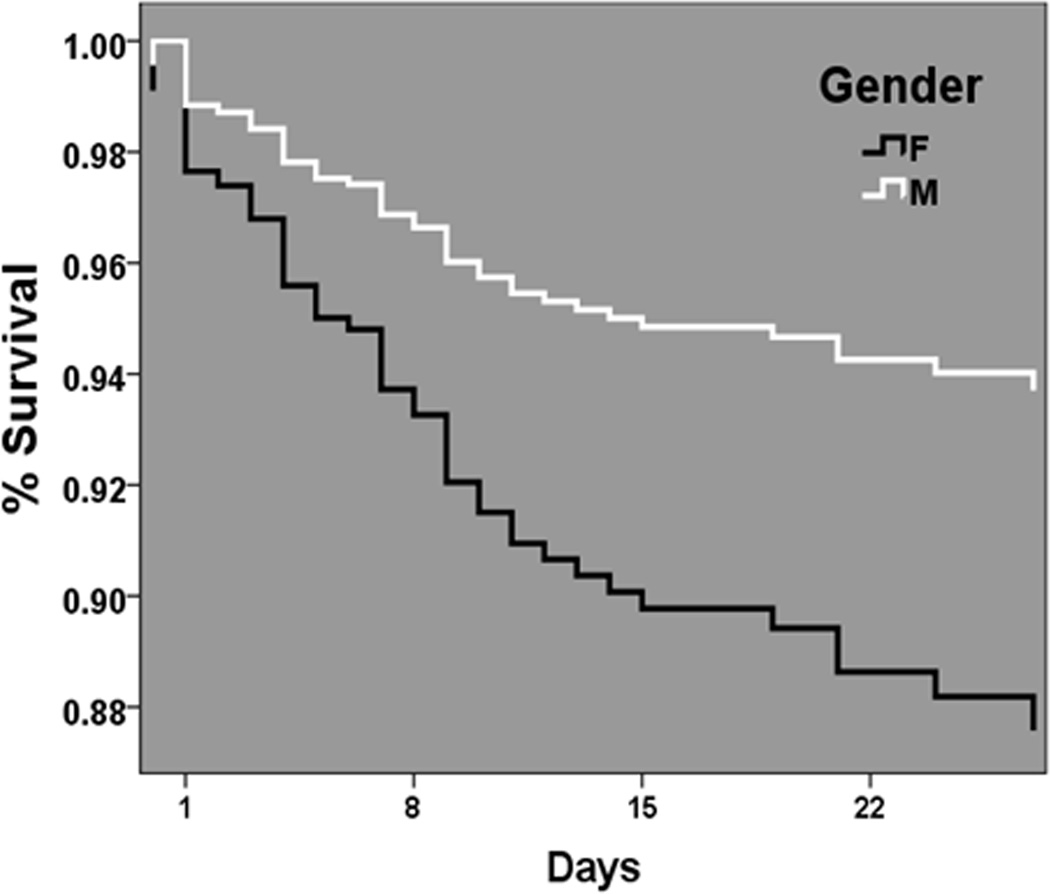
To characterize the timing of these differential outcome risks across gender relative to the presence of coagulopathy, multivariate survival analysis was performed. Cox-adjusted survival curve analysis for the ATC group demonstrated significant separation of male and female curves (p<0.01), occurring early in the first 24 hours post injury with continued separation over the first 30 days post injury (Fig. 2).
Figure 2.
Cox-adjusted survival curve analysis in the ATC group for females (dark line) and males (light line) over 30 days post injury.
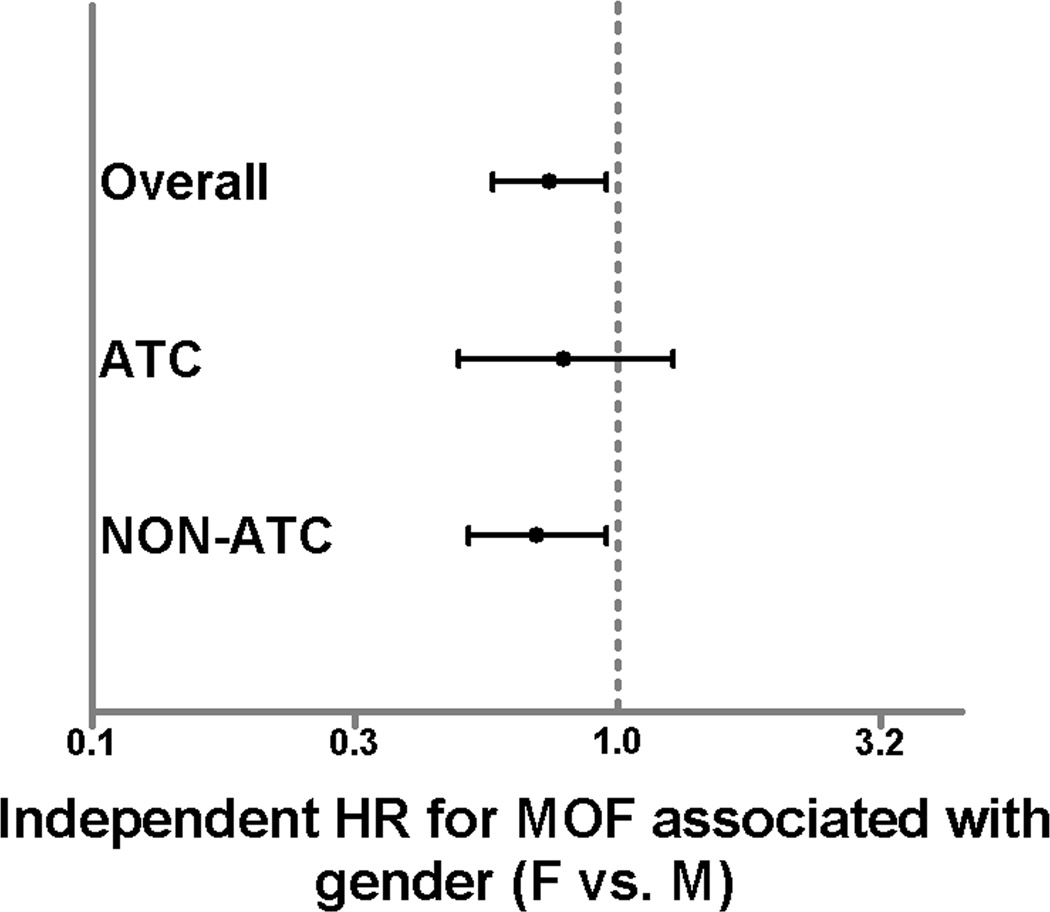
Female gender was independently associated with a significantly lower risk of MOF in the overall study cohort (HR 0.74; 95%CI 0.58 – 0.95, p=0.02), as has been previously demonstrated in this study population.4 As expected, the presence of ACT was associated with an increased risk of MOF (HR 1.35; 95%CI 1.05 – 1.73, p=0.02). Interestingly, when the regression models were stratified by the presence or absence of ATC, the protection afforded to females from the development of MOF remained statistically significant and robust in the NON-ACT group (HR 0.70; 95%CI 0.52 – 0.95, p=0.02). When assessed in the ATC group, female gender was no longer associated with a lower risk of MOF (HR 0.79; 95%CI 0.50 – 1.28, p=0.34), suggesting the protection afforded to females from the development of MOF is less robust or does not exist in patients with ATC (Fig. 3).
Figure 3.
Hazard ratios from Cox regression for 28 day MOF in the overall study population, ATC group, and NON-ATC group. Hazard ratios represent female relative to male gender. Bars represent 95% confidence intervals. Bars that do not cross 1.0 are considered significant (p<0.05).
Interactions were tested between gender and menopause related age subgroups in the Cox regression models to see if the associated differences in mortality risk across gender in the ATC group were related to the age of the female patient. These interactions were not significant in the overall cohort (HR 0.83; 95%CI 0.42 – 1.63, p=0.59), ATC group (HR 0.95; 95%CI 0.27 – 3.38, p=0.94), or NON-ATC group (HR 0.86; 95%CI 0.36 – 2.06, p=0.73). Similarly, gender and age interactions for MOF were not significant in the overall cohort (HR 1.17; 95%CI 0.72 – 1.90, p=0.53), ATC group (HR 1.33; 95%CI 0.56 – 3.11, p=0.52), or NON-ATC group (HR 1.13; 95%CI 0.62 – 2.07, p=0.69). These results suggest that the overall higher mortality risk for females in the ATC group were relatively uniform across age and unrelated to any potential sex hormone levels relative to menopause in females.
DISCUSSION
Several groups have documented the relative hypercoagulability of non-injured females when compared to males at baseline, which is most often attributed to the effects of estrogen.23–25 Schreiber and colleagues used thromboelastography (TEG) to show females were relatively hypercoagulable in the setting of trauma; a finding that developed in the first 24 hours from injury.11 Additionally, Engels et al. found that female gender was protective against development of early coagulopathy of trauma in multivariate analysis.26 An analysis of massive transfusion patients showed that males had an associated mortality benefit from high plasma and platelet to blood ratios but not females.27 These authors and others have suggested that the hypercoagulability of females post injury may result in improvements in hemostasis following traumatic hemorrhage.11,27
Although studies have shown females to be less likely to develop ATC than their male counterparts, it is unclear whether gender influences outcomes when ATC already is present. The results of the current analysis demonstrate an exaggerated gender dimorphism exists in injured patients with ATC. Female gender in the ATC group was associated with an over 2-fold increased risk of mortality after controlling for important confounders. Further, the protective effect of female gender seen in the overall cohort and the NON-ATC group from the development of MOF no longer existed when examined in the ATC group. These findings suggest that although females may be hypercoagulable compared to males following injury, females with ATC have an increased risk of adverse outcomes. These differential mortality risks across gender diverge early post-injury suggesting they may be due to ongoing hemorrhage. Rowell and colleagues, in their study of gender differences in the setting of massive transfusion found that females receiving a high ratio of blood products had a higher mean INR than females with low ratios with no improvement in survival based on ratio group.27 Although no gender comparisons were made specifically for patients with ATC, this may indirectly hint at the difficulties encountered in coagulopathic female trauma patients.
It remians unclear what mechanisms are responsible for these findings. A large amount of experimental animal studies have substantiated the protective role of female sex hormones following trauma and shock.28–31 Evidence for sex hormone mediated differences in human trauma outcomes has been largely supported by studies demonstrating benefits in premenopausal aged females. Mostafa et al. showed a lower incidence of MOF, mortality, and shorter intensive care unit stays in females less than 45 years old, but not in those 45 years or older.7 A large National Trauma Databank study demonstrated improved survival in females aged 13 to 64 when presenting with hypotension and an ISS ≥ 16.32 In a study of more than 4,000 trauma patients, premenopausal aged females demonstrated lower lactate levels and less blood requirements despite higher injury severity.33 Similarly, analysis of sex hormone levels in trauma patients have shown higher estradiol to progesterone levels correlate with higher survival, fibrin cross linking, and lower partial thromboplastin times, but not related to differences in INR or platelet counts.9 Despite this large amount of prior literature, the mechanisms responsible for the gender dimorphism post-injury remain controversial and less than adequately characterized.34
As the interaction between gender and age specific subgroups across the spectrum of menopause did not show significant associations with mortality or MOF, it is difficult with the current cohort of patients to attribute the differences in outcome observed in this study solely to sex hormone differences between males and females. Sperry and colleagues examining the same dataset previously also demonstrated a female gender protective association with MOF and nosocomial infection that were not age related.4 George et al. demonstrated a protective association of female gender and mortality in blunt trauma patients aged 50 and older but not premenopausal aged females in a large national cohort.35 Beyond sex hormones, genetic differences based on sex chromosomes may play a role. As males possess only one X chromosome, they can only produce a single phenotypic response related to genetic polymorphisms located on the X chromosome. Females, alternatively, undergo inactivation of one X chromosome in each cell leading to functional mosaicism with a broadened response of X chromosome related polymorphisms. Many genes involved in inflammation and the cellular response to injury are located on the X chromosome and may influence the gender dimorphisms evident following trauma.36
The gender related adverse outcomes observed when ATC was present on admission may represent a more severe derangement of the coagulation system in females to overcome their innate hypercoagulability early in the post injury period. Thus, relative to their male counterparts, when females reach the ATC threshold, their outcomes are significantly worse. Importantly, these findings do not appear to be related directly in injury severity, as males and females had similar injury severity across several measures in the ATC group. There is evidence which suggests that endogenous estrogen, regardless of gender, is a marker of severe injury and mortality, with serum estradiol performing as well as or better than the Trauma and Injury Severity Score and ISS to predict death following trauma.37
This study has several limitations for consideration. The current study is a secondary data analysis of the Host Response to Injury cohort, which was not designed to address the specific issues analyzed here. This limits potential confounders that may be controlled for as well as the outcomes available for analysis. The current dataset was prospectively collected from several centers, and although standardized protocols were in place to minimize between center variations in care, it is likely that variation between trauma center practices existed over the study period. A small proportion of subjects was missing initial INR data and was excluded. Further, authors have shown that routine coagulation parameters fail to correlate with more detailed and sophisticated measurements of coagulation status such as TEG analysis, which was not available in this dataset.11 Finally, this dataset did not include measurements of sex hormones following injury, and any inference of sex hormone status is based on average estimates of age across the spectrum of menopause.38
Despite these limitations, the cohort was designed to be homogenous at baseline as evidenced by gender comparisons across coagulopathy groups, and a large number of patients were available, strengthening the power of analysis. Despite limitations of an arrival INR as the determinant of ATC in this analysis, this test is rapidly and widely available in trauma centers, with differences in outcome observed based on standard coagulation parameters in prior studies.1,2
It is clear that our understanding of both the cellular mechanisms of ATC and biology underlying gender dimorphisms following injury continue to increase. This is the first study to our knowledge that demonstrates adverse outcomes associated with female gender in the setting of trauma. Females demonstrated a significantly higher mortality when ATC was present on admission to the trauma center. This risk emerged early in the post injury period and may be a result of ongoing hemorrhage. This may argue for more aggressive hemostatic resuscitation in females presenting with ATC in the setting of traumatic hemorrhage; however further study is needed to determine whether early intervention can mitigate this increased risk. Finally, the apparent protective association of female gender against MOF was lost in the setting of ATC. These findings underscore the need for further investigation into the complex relationship of coagulation and gender following injury.
Acknowledgments
Funding/Support: NIH NIGMS U54 GM062119-1 and NIH NIGMS K23GM093032-1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This paper was presented as an oral presentation at the annual meeting of the Western Trauma Association in Vail, Colorado, February 26th – Mar 2nd, 2012.
REFERENCES
- 1.Brohi K, Singh J, Heron M, et al. Acute traumatic coagulopathy. J Trauma. 2003;54:1127–1130. doi: 10.1097/01.TA.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- 2.MacLeod JB, Lynn M, McKenney MG, et al. Early coagulopathy predicts mortality in trauma. J Trauma. 55:39–44. doi: 10.1097/01.TA.0000075338.21177.EF. [DOI] [PubMed] [Google Scholar]
- 3.Cohen MJ, West M. Acute traumatic coagulopathy: From endogenous acute coagulopathy to systemic acquired coagulopathy and back. J Trauma. 2011;70:S47–S49. doi: 10.1097/TA.0b013e31821a5c24. [DOI] [PubMed] [Google Scholar]
- 4.Sperry JL, Nathens AB, Frankel HL, et al. Characterization of the gender dimorphism after injury and hemorrhagic shock: Are hormonal differences responsible? Crit Care Med. 2008;36:1838–1845. doi: 10.1097/CCM.0b013e3181760c14. [DOI] [PubMed] [Google Scholar]
- 5.George RL, McGwin G, Jr, Metzger J, et al. The association between gender and mortality among trauma patients as modified by age. J Trauma. 2003;54:464–471. doi: 10.1097/01.TA.0000051939.95039.E6. [DOI] [PubMed] [Google Scholar]
- 6.George RL, McGwin G, Jr, Windham ST, et al. Age-related gender differential in outcome after blunt or penetrating trauma. Shock. 2003;19:28–32. doi: 10.1097/00024382-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Mostafa G, Huynh T, Sing RF, et al. Gender-related outcomes in trauma. J Trauma. 2002;53:430–434. doi: 10.1097/00005373-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Choudhry MA, Schwacha MG, Hubbard WJ, et al. Gender differences in acute response to trauma-hemorrhage. Shock. 2005;24:S101–S106. doi: 10.1097/01.shk.0000191341.31530.5e. [DOI] [PubMed] [Google Scholar]
- 9.Gee AC, Sawai RS, Differding J, et al. The influence of sex hormones on coagulation and inflammation in the trauma patient. Shock. 2008;29:334–341. doi: 10.1097/shk.0b013e3181506ee5. [DOI] [PubMed] [Google Scholar]
- 10.Sperry JL, Friese RS, Frankel HL, et al. Male Gender is Associated With Excessive IL-6 Expression Following Severe Injury. J Trauma. 2008;64:572–579. doi: 10.1097/TA.0b013e3181650fdf. [DOI] [PubMed] [Google Scholar]
- 11.Schreiber MA, Differding J, Thorborg P, et al. Hypercoagulability is most prevalent early after injury and in female patients. J Trauma. 2005;58:475–480. doi: 10.1097/01.ta.0000153938.77777.26. [DOI] [PubMed] [Google Scholar]
- 12.Maier RV, Bankey PE, McKinley B, et al. Inflammation and the host response to injury, a large-scale collaborative project: patient-oriented research core–standard operating procedures for clinical care [foreword] J Trauma. 2005;59:762–763. [PubMed] [Google Scholar]
- 13.Brandt CA, Deshpande AM, Lu C, et al. TrialDB: A web-based Clinical Study Data Management System. AMIA Annu Symp Proc. 2003:794. [PMC free article] [PubMed] [Google Scholar]
- 14.Moore FA, McKinley BA, Moore EE, et al. Inflammation and the Host Response to Injury, a large-scale collaborative project: patient-oriented research core—standard operating procedures for clinical care. III. Guidelines for shock resuscitation. J Trauma. 2006;61(1):82–89. doi: 10.1097/01.ta.0000225933.08478.65. [DOI] [PubMed] [Google Scholar]
- 15.Minei JP, Nathens AB, West M, et al. Inflammation and the Host Response to Injury, a Large-Scale Collaborative Project: patient-oriented research core--standard operating procedures for clinical care. II. Guidelines for prevention, diagnosis and treatment of ventilator-associated pneumonia (VAP) in the trauma patient. J Trauma. 2006;60(5):1106–1113. doi: 10.1097/01.ta.0000220424.34835.f1. [DOI] [PubMed] [Google Scholar]
- 16.Nathens AB, Johnson JL, Minei JP, et al. Inflammation and the Host Response to Injury, a large-scale collaborative project: Patient-Oriented Research Core--standard operating procedures for clinical care. I. Guidelines for mechanical ventilation of the trauma patient. J Trauma. 2005;59(3):764–769. [PubMed] [Google Scholar]
- 17.West MA, Shapiro MB, Nathens AB, et al. Inflammation and the host response to injury, a large-scale collaborative project: Patient-oriented research core-standard operating procedures for clinical care. IV. Guidelines for transfusion in the trauma patient. J Trauma. 2006;61(2):436–439. doi: 10.1097/01.ta.0000232517.83039.c4. [DOI] [PubMed] [Google Scholar]
- 18.Harbrecht BG, Minei JP, Shapiro MB, et al. Inflammation and the host response to injury, a large-scale collaborative project: patient-oriented research core-standard operating procedures for clinical care: VI. Blood glucose control in the critically ill trauma patient. J Trauma. 2007;63(3):703–708. doi: 10.1097/TA.0b013e31811eadea. [DOI] [PubMed] [Google Scholar]
- 19.Carrico CJ, Meakins JL, Marshall JC, et al. Multiple-organ-failure syndrome. Arch Surg. 1986;121:196–208. doi: 10.1001/archsurg.1986.01400020082010. [DOI] [PubMed] [Google Scholar]
- 20.Marshall JC. Organ dysfunction as an outcome measure in clinical trials. Eur J Surg Suppl. 1999;(584):62–67. doi: 10.1080/11024159950188583. [DOI] [PubMed] [Google Scholar]
- 21.Marshall JC, Cook DJ, Christou NV, et al. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23:1638–1652. doi: 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 23.Gorton HJ, Warren ER, Simpson NA, et al. Thromboelastography identifies sex-related differences in coagulation. Anesth Analg. 2000;91:1279–1281. doi: 10.1097/00000539-200011000-00042. [DOI] [PubMed] [Google Scholar]
- 24.Roeloffzen WW, Kluin-Nelemans HC, Mulder AB, et al. In normal controls, both age and gender affect coagulability as measured by thromboelastography. Anesth Analg. 2010;100:987–994. doi: 10.1213/ANE.0b013e3181d31e91. [DOI] [PubMed] [Google Scholar]
- 25.Scarpelini S, Rhind SG, Nascimento B, et al. Normal range values for thromboelastography in healthy adult volunteers. Braz J Med Biol Res. 2009;42:1210–1217. doi: 10.1590/s0100-879x2009001200015. [DOI] [PubMed] [Google Scholar]
- 26.Engels PT, Rezende-Neto JB, Al Mahroos M, et al. The Natural History of Trauma-Related Coagulopathy: Implications for Treatment. J Trauma. 2011;71:S448–S455. doi: 10.1097/TA.0b013e318232e6ac. [DOI] [PubMed] [Google Scholar]
- 27.Rowell SE, Barbosa RR, Allison CE, et al. Gender-based differences in mortality in response to high product ratio massive transfusion. J Trauma. 2011;71:S375–S379. doi: 10.1097/TA.0b013e318227f1aa. [DOI] [PubMed] [Google Scholar]
- 28.Jarrar D, Wang P, Knoferl MW, et al. Insight into the mechanism by which estradiol improves organ functions after trauma-hemorrhage. Surgery. 2000;128:246–252. doi: 10.1067/msy.2000.107376. [DOI] [PubMed] [Google Scholar]
- 29.Knoferl MW, Angele MK, Schwacha MG, et al. Preservation of splenic immune functions by female sex hormones after traumahemorrhage. Crit Care Med. 2002;30:888–893. doi: 10.1097/00003246-200204000-00029. [DOI] [PubMed] [Google Scholar]
- 30.Knoferl MW, Angele MK, Diodato MD, et al. Female sex hormones regulate macrophage function after trauma-hemorrhage and prevent increased death rate from subsequent sepsis. Ann Surg. 2002;235:105–112. doi: 10.1097/00000658-200201000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choudhry MA, Schwacha MG, Hubbard WJ, et al. Gender differences in acute response to trauma-hemorrhage. Shock. 2005;24:S101–S106. doi: 10.1097/01.shk.0000191341.31530.5e. [DOI] [PubMed] [Google Scholar]
- 32.Haider AH, Crompton JG, Chang DC, et al. Evidence of hormonal basis for improved survival among females with trauma-associated shock: An analysis of the National Trauma Databank. J Trauma. 2010;69:537–540. doi: 10.1097/TA.0b013e3181efc67b. [DOI] [PubMed] [Google Scholar]
- 33.Deitch EA, Livingston DH, Lavery RF, et al. Hormonally active women tolerated shock-trauma better than do men: a prospective study of over 4000 trauma patients. Ann Surgery. 2007;246:447–453. doi: 10.1097/SLA.0b013e318148566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sperry JL, Minei JP. Gender dimorphism following injury: making the connection from bench to bedside. J Leukoc Biol. 2008;83:499–506. doi: 10.1189/jlb.0607360. [DOI] [PubMed] [Google Scholar]
- 35.George RL, McGwin G, Metzger J, et al. The association between gender and mortality among trauma patients as modified by age. J Trauma. 2003;54:464–471. doi: 10.1097/01.TA.0000051939.95039.E6. [DOI] [PubMed] [Google Scholar]
- 36.Spolarics Z. The X-files of inflammation: Cellular mosaicism of X-linked polymorphic genes and the female advantage in the host response to injury and infection. Shock. 2007;27:597–604. doi: 10.1097/SHK.0b013e31802e40bd. [DOI] [PubMed] [Google Scholar]
- 37.Dossett LA, Swenson BR, Heffernan D, et al. High levels of endogenous estrogens are associated with death in the critically injured adult. J Trauma. 2008;64:580–585. doi: 10.1097/TA.0b013e31816543dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nichols HB, Trentham-Dietz A, Hampton JM, et al. From menarche to menopause: Trends among US women born from 1912 to 1969. Am J Epidemiol. 2006;164:1003–1011. doi: 10.1093/aje/kwj282. [DOI] [PubMed] [Google Scholar]