Summary
MxA is an interferon-induced dynamin-like GTPase with wide-ranging antiviral activity, which hinges upon detection of unique viral structures that differ across virus families. Despite elucidation of its structure, the basis of MxA antiviral specificity remains enigmatic. We used an evolution-guided approach to identify the loop L4 of MxA as a hotspot for recurrent positive selection in primates. Further, we show that single amino acid changes in L4 are necessary and sufficient to explain dramatic differences in species-specific antiviral activity of primate MxA proteins against the orthomyxoviruses Thogoto virus and influenza A virus. Taken together, our findings identify a genetic determinant of MxA target recognition and suggest a model by which MxA achieves antiviral breadth without compromising viral specificity.
Introduction
MxA is a dynamin-like GTPase with antiviral activity against a wide range of RNA and DNA viruses (Haller and Kochs, 2011). The antiviral breadth exhibited by MxA is remarkable because it hinges upon detection of unique viral structures that differ across virus families. For example, differences in resistance and susceptibility between avian (MxA-sensitive) and human (MxA-resistant) influenza virus isolates have been shown to be solely dependent on differences in the nucleoprotein (NP) (Zimmermann et al., 2011). Furthermore, MxA activity against the alphavirus Semliki Forest virus (SFV) is independent of SFV NP or other structural proteins (Landis et al., 1998), and MxA antiviral activity against DNA viruses like hepatitis B virus (HBV) (Li et al., 2012) and African swine fever virus (ASFV) (Netherton et al., 2009) is dependent on unique viral components. However, the evolutionary and molecular basis for MxA antiviral specificity is unknown.
The recently solved crystal structure of human MxA (hsMxA) highlights the difficulty in pinpointing molecular determinants of MxA antiviral specificity. The MxA structure resembles that of other members of the dynamin-like large GTPase superfamily (Gao et al., 2011), consisting of a N-terminal GTPase domain (G domain) and a C-terminal stalk. These two structural domains are linked by a bundle-signaling element (BSE) that is necessary to transfer structural changes during GTP binding and hydrolysis to the stalk structure. Nonetheless, while the crystal structure of MxA illustrated the coupling between different domains, it did not reveal a molecular basis for the long-standing problem of MxA antiviral specificity
In order to determine the basis of MxA specificity for viral recognition, we took an evolution-guided functional approach that capitalizes on the antagonistic arms race between MxA and its viral targets, and the genomic signatures it leaves on host genomes. The rapid accumulation of amino acid replacement changes (dN) relative to synonymous changes (dS), or positive selection (dN/dS > 1), is one hallmark of antagonistic evolution between host and viral genomes. Because positive selection underlies phenotypic adaptation at antiviral interfaces (Daugherty and Malik, 2012; Sawyer et al., 2005), we predict that the hypothetical viral substrate interface(s) of MxA would have evolved with the strongest signature of positive selection.
Here we show that the surface-exposed loop L4, which protrudes from the compact structure of the MxA stalk, bears such a signature of recurrent positive selection. We demonstrate that genetic variation in L4 of primate MxA is a major determinant of its species-specific antiviral activity against the orthomyxoviruses Thogoto virus (THOV) and an avian influenza A virus, and a single positively selected amino acid in L4 is sufficient to alter the antiviral specificity of primate MxA. Moreover, we show that hsMxA L4 can function as a modular determinant of antiviral specificity in the context of the highly divergent mouse ortholog to confer antiviral protection. We propose that, while the architecture of Mx proteins has been evolutionarily conserved, the antiviral specificity determinants have been subject to recurrent arms races with viral substrates. Taken together, our findings identify L4 as a genetic determinant of MxA antiviral specificity, and highlight the power of evolutionary methodologies to delineate host-virus interactions.
Results
Rapid evolution of the unstructured loop L4 in primate MxA
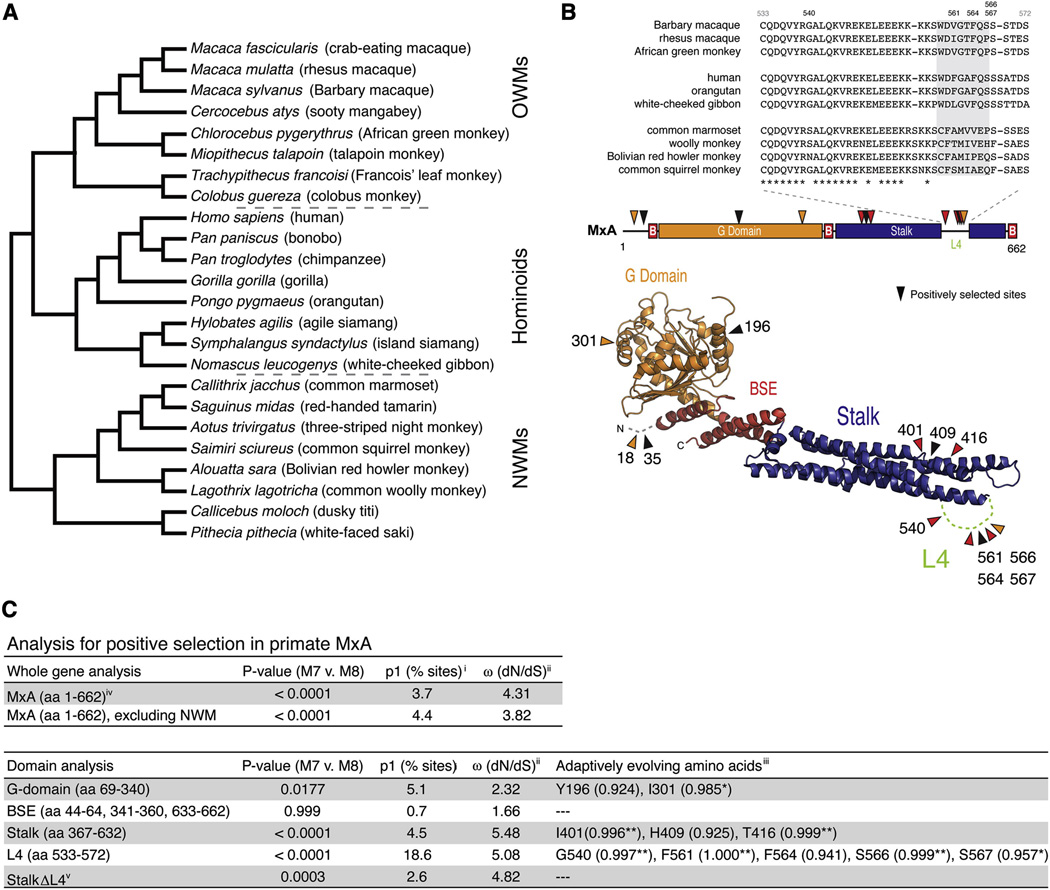
To evaluate how the antiviral specificity of MxA has been shaped during the evolution of primates, we amplified, sequenced and aligned MxA coding regions from 24 primate species representing ~43 million years of evolutionary divergence (Figure 1A, Figure S1 and Table S1). A phylogenetic tree generated from these MxA sequences was congruent with a previously published primate phylogeny (Perelman et al., 2011) confirming that the MxA sequences obtained were orthologous (Figure 1A). Using this dataset, we calculated an average dN/dS value of 0.32 for MxA, which is consistent with purifying selection having acted on the majority of the MxA gene over primate evolution. However, an average dN/dS signature can obscure recurrent positive selection that might have acted on particular codons or protein domains. Indeed, when we compared maximum likelihood codon-based models that disallow positive selection (model M7) to those that allow dN/dS to exceed 1 (model M8), we found strong evidence that MxA has recurrently evolved under positive selection in primates (Figure 1C). While only a few MxA codons (3.7%) have evolved under positive selection, the signature of positive selection associated with them is quite strong (average dN/dS ratio of 4.31). These estimates suggest that although most residues in MxA have evolved under purifying selection to maintain its structure and enzymatic properties, positive selection has acted on MxA at discrete residues likely in response to antagonistic evolution with viruses.
Figure 1. Evolution of primate MxA.
A. The phylogeny was built using orthologous MxA sequences from 24 primate species (Figure S1 and Table S1), which is congruent with a previously published primate phylogeny (Perelman et al., 2011).
B. The loop L4 (amino acids 533–572) protein sequence alignment is shown from representative primates (top). Specific codons found to be evolving under positive selection in primate MxA are indicated in a linear schematic (middle) or a model of the MxA crystal structure (bottom) (Gao et al., 2011), where sites with a posterior probability greater than 0.95 and 0.99 are highlighted in orange and red, respectively. In the linear structure “B” depicts the three parts of MxA that form the bundle-signaling element (BSE).
C. Evolutionary analysis for positive selection in primate MxA. P values generated from maximum likelihood ratio tests that fit the data to models of neutral (M7) or adaptive (M8) evolution are given for the entire MxA coding sequence (including or excluding new world monkey (NWM) sequences) or specific domains of the protein as indicated. i. Percentage of codons evolving under positive selection (p1). ii. Associated average dN/dS (ω) of p1 sites. iii. Codons with a high posterior probability (PP > 0.90) that supports the likelihood of a site having a dN/dS > 1 are presented with the PP for each residue in parentheses. Amino acids refer to residues found at indicated positions in hsMxA. iv. Indels removed from alignment. v. StalkΔL4 refers to an analysis of the stalk with amino acids 533–572 removed.
We found no evidence for positively selected sites in the BSE domain of MxA, and limited evidence in the G and N-terminal domains (Figure 1B and C). Instead, the majority of positively selected sites were located in the MxA stalk domain, which has the most significant domain-specific signature of positive selection. Within the stalk domain by far the most striking enrichment of positively selected sites is in the unstructured loop L4 with 18.6% of codons evolving with an average dN/dS value of 5.08, revealing a dramatic history of positive selection in L4 across primates. The overall signal of positive selection in the stalk domain is markedly reduced when L4 residues 533–572 are removed from the analysis (Figure 1C). Interestingly, we observed that there has been an almost complete replacement of the L4 residues between 559 and 566 (Figure 1B, grey box) that distinguish hominoid and old world monkey from new world monkey sequences. However, the new world monkey MxA divergence is not the only cause of a positive selection signature; evolutionary analysis of only hominoid and old world monkey MxA sequences also showed robust evidence of positive selection in MxA even in this less divergent sequence set (Figure 1C). These analyses indicate that significant evolutionary pressures have acted on MxA throughout primate evolution. The cumulative evidence for positive selection in MxA, particularly given the enrichment of sites in L4, strongly suggests that this surface-exposed loop, which is disordered in the recently determined MxA structure (Gao et al., 2011), has been subject to recurrent and strong positive selection during primate evolution.
Loop L4 is the determinant of MxA antiviral specificity against Thogoto virus (THOV)
To determine whether the observed positive selection in primate MxA has functional consequences, MxA coding sequences from 15 representative primate species were subcloned into mammalian expression constructs with a N-terminal HA-epitope. Protein expression was measured by Western blot analysis of cell lysates from transfected 293T cells, and only minor variation in protein expression was observed across the 15 orthologs and hsMxA(T103A), an inactive form carrying a threonine to alanine mutation in the G domain (Ponten et al., 1997) (Figure 2A). We then tested the antiviral function of primate MxA orthologs using the THOV minireplicon system as a readout of MxA antiviral activity. Previous studies have shown that hsMxA severely attenuates the THOV polymerase, and that its activity is sensitive to titration of both hsMxA and NP (Weber et al., 2000), making it an ideal ‘surrogate’ to test the functional consequences of variation across primate MxA orthologs. In this assay, the viral polymerase components (PB2, PB1, PA) and NP are delivered in trans to drive the expression of a firefly luciferase from a viral RNA minigenome in antisense orientation that is flanked by viral 5’ and 3’ UTRs. Firefly luciferase expression is detected 24h-post transfection and normalized to Renilla luciferase levels, serving as a transfection control. Luciferase expression in the absence of MxA is normalized to 100% polymerase activity.
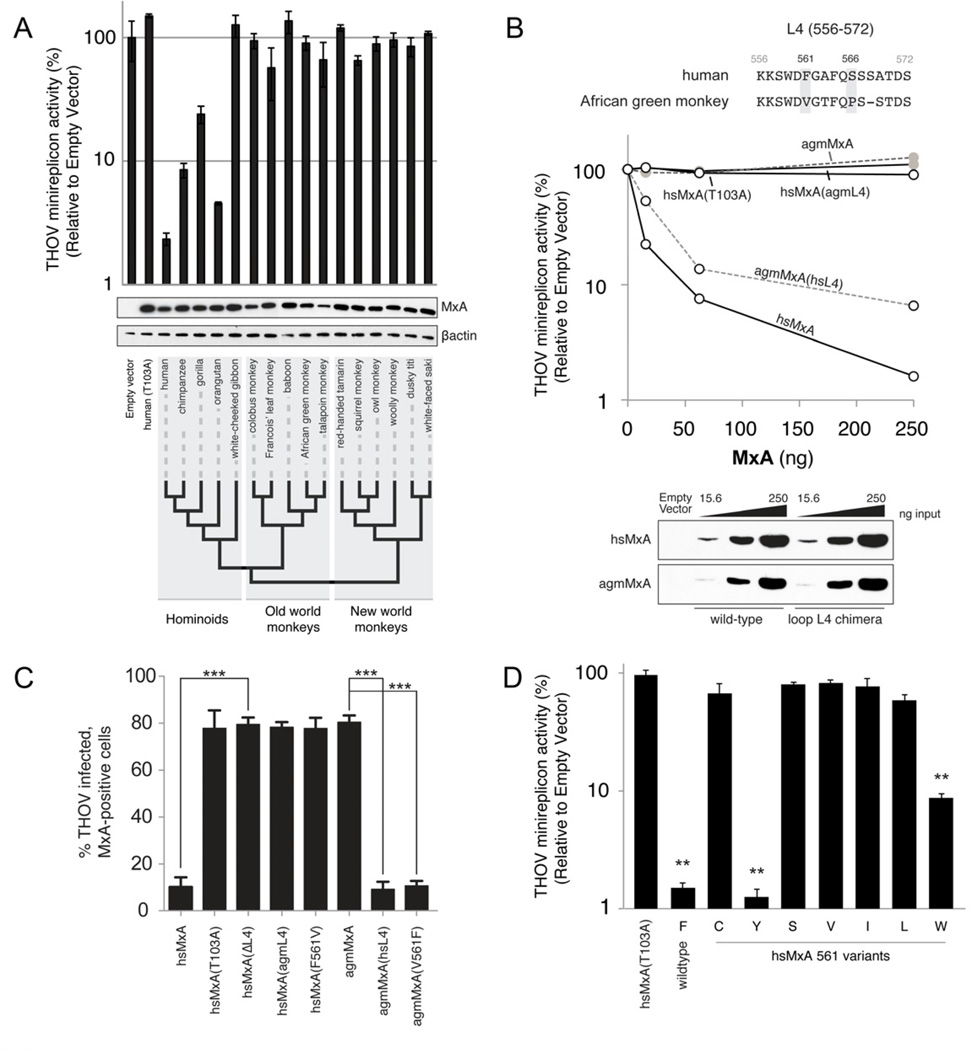
Figure 2. Species-specific antiviral activity results from sequence divergence in loop L4 of primate MxA.
A. THOV minireplicon activity (luciferase levels) was measured 24h post-transfection, and was reported relative to levels observed in the absence of MxA. hsMxA(T103A) is an antivirally inactive mutant of hsMxA. Error bars represent standard deviation across three biological replicates. HA-tagged MxA and βactin protein levels were detected in lysates by Western blot. The phylogenetic relationships of MxA derived from representative species within hominoid, old world monkey and new world monkey lineages are indicated by a cladogram.
B. Differential antiviral activity of human and AGM MxA orthologs is dependent on loop L4. The loop L4 (amino acids 556–572) protein sequence alignment is shown with positively selected sites that differ between hsMxA and agmMxA in grey (top). Dose-responsiveness of the antiviral activity of wild-type MxA and L4 chimeras was determined by co-transfecting increasing amounts of MxA expression constructs with the THOV minireplicon into 293T cells. MxA antiviral activity is measured as described in Figure 2A (middle). The Western blot analysis depicts the expression levels of the wild-type and chimeric MxA proteins in the cell lysates (bottom).
C. Restriction of THOV infectivity by human and AGM MxA is contingent on a single amino acid. Data are presented as percent THOV infected, Mx-positive cells as measured by immunofluorescence for THOV NP and MxA. Error bars represent standard deviation of three biological replicates. ***, p<0.0001 (t-test). See also Figure S2.
D. Antiviral activity of hsMxA 561 variants. Data are presented as described in Figure 2A. **, p<0.001 (t-test) for values compared to hsMxA(T103A).
We found that hsMxA reduces THOV minireplicon activity by ~50-fold when compared to either the inactive mutant hsMxA(T103A) or no MxA (Figure 2A). MxA from great apes reduced THOV primary transcription by 4 to 20-fold. In contrast, MxA proteins from gibbons, old world monkeys and new world monkeys were inactive (less than 2-fold reduction). The observed species-specificity indicates that genetic divergence of primate MxA orthologs bears dramatic functional consequences for MxA antiviral activity against THOV.
Since there is a marked enrichment of positively selected sites in L4, we tested whether divergence in L4 might explain differences in antiviral activity. Therefore, we generated L4 chimeras between hsMxA (active) and the inactive ortholog from African green monkey (agmMxA). Introduction of human L4 into agmMxA (agmMxA(hsL4)) resulted in a dose-dependent rescue of activity against the THOV minireplicon (Figure 2B). Likewise, replacement of hsL4 with that of agmMxA (hsMxA(agmL4)) had the opposite effect, attenuating the block on THOV transcription. Thus, the loop L4, which we identified as evolving under positive selection, is a determinant for the antiviral specificity of MxA.
Next, we tested the hsMxA and agmMxA chimeras in the context of virus infection. Vero (AGM) cells transfected with wildtype hsMxA or agmMxA were infected with THOV. We assessed intracellular viral replication by monitoring the accumulation of viral NP via immunofluorescence. In agreement with the results from the THOV minireplicon, viral infectivity was severely reduced in the presence of hsMxA and agmMxA(hsL4), but not agmMxA and hsMxA(agmL4) (Figure 2C). Additionally, the congruence of antiviral effects seen in the minireplicon assay in human cells (Figure 2B) and viral infection assay in AGM cells (Figure 2C) discounts the formal possibility that L4 effects are due to co-evolution with host-specific cofactors that differ between species. Taken together, our results demonstrate that the difference in antiviral function between hsMxA and agmMxA is the result of sequence variation in L4.
A single amino acid confers MxA antiviral specificity for THOV
HsMxA and agmMxA differ by four amino acids in L4. Of these, we found that two positions, 561 and 566, are evolving under recurrent positive selection (Figure 1B and C). We focused on an analysis of position 561 since the serine residue at position 566 is shared between hsMxA and orthologous MxA proteins inactive against THOV (e.g. Barbary macaque, see Figure S2A). We mutated the phenylalanine at position 561 in hsMxA to the valine found in agmMxA, and also introduced the reciprocal V561F mutation into agmMxA. These single amino acid changes completely recapitulated the L4 phenotype in both hsMxA and agmMxA backgrounds in infected cells and in the THOV minireplicon system (Figure 2C and S2B). Thus, a single amino acid evolving under positive selection in L4 largely determines viral specificity of MxA for THOV.
To understand in greater detail the molecular consequences of changes at residue 561, we tested amino acids present in hominoid and old world monkey species (hsMxA -F561I, -F561L and -F561V) as well as those that could be sampled by a single bp mutation of the hsMxA F561 codon (hsMxA -F561C, -F561Y and -F561S). Only wildtype F561 and F561Y restricted the THOV minireplicon whereas the other mutations dramatically reduced the antiviral activity of hsMxA (Figure 2D). Given the biochemical similarities of phenylalanine and tyrosine, we tested hsMxA-F561W, which also restricted THOV, albeit to a lesser degree (Figure 2D). These results reinforce the concept that MxA antiviral specificity for THOV is conferred by a single amino acid with specific biochemical requirements.
Functional conservation of L4
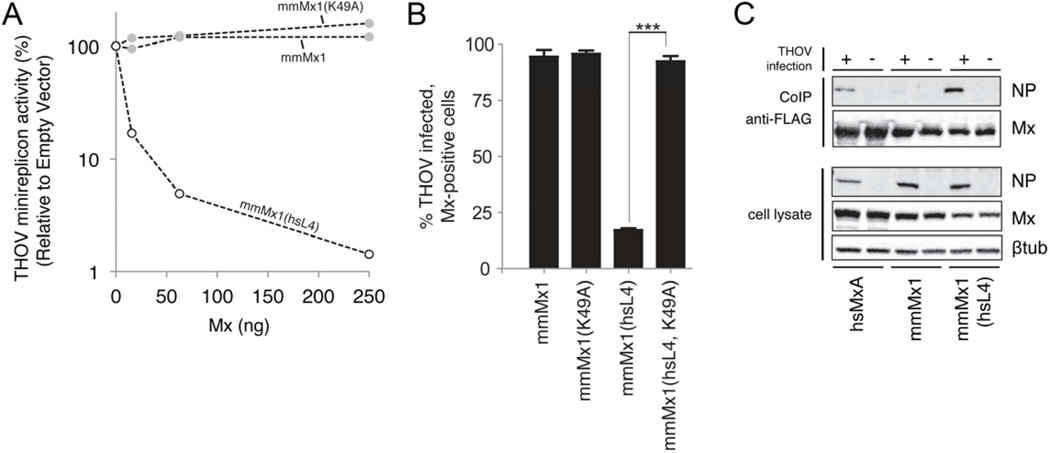
One prediction of the proposed role for L4 in MxA target recognition is that L4-mediated specificity is modular. If that is the case, the antiviral properties of hsMxA might be transferable to a more distantly related Mx protein simply by transplanting the loop L4 of hsMxA. Mouse Mx1 (mmMx1) is relatively inactive against THOV although it does confer some protection in vivo (Haller et al., 1995). We made chimeras replacing the loop L4 of mmMx1 with that from hsMxA, mmMx1(hsL4), and compared it to wildtype mmMx1 in the THOV minireplicon assay. Catalytically-deficient chimeras were also constructed by mutating the K49A in the G domain (Pitossi et al., 1993). We found that mmMx1(hsL4) gained robust activity against THOV (Figure 3A). We repeated these experiments using THOV infection of transfected Vero cells and again found that mmMx1(hsL4) is significantly more potent in blocking THOV than mmMx1 (Figure 3B). Thus, the loop L4 encodes mmMx1 specificity for THOV, which strongly argues that L4 is a modular domain that defines MxA specificity for targeted viral structures.
Figure 3. Loop L4 is a functional module of MxA antiviral activity.
A. Human L4 rescues mouse Mx1 antiviral activity against the THOV minireplicon. mmMx1 expression constructs were co-transfected with the THOV minireplicon system into 293T cells; the experiment was otherwise carried out as in Figure 2B. mmMx1(hsL4) contains an exchange of mmL4 for the L4 of hsMxA. mmMx1(K49A) is a GTPase inactive mutant with no antiviral activity.
B. Human L4 determines the antiviral activity of mmMx1 against THOV infection. Percent infectivity was measured as described in Figure 2C. Error bars represent standard deviation of three biological replicates. ***, p<0.0001.
C. L4 mediates the interaction between MxA and THOV NP. Co-immunoprecipitation of viral NP with hsMxA and mmMx1 proteins was performed with lysates of THOV-infected (+) or uninfected (−) 293T cells transfected with the indicated Flag-tagged Mx expression constructs. Immunoprecipitation was performed using anti-Flag-affinity gel and the precipitated proteins were detected by Western blot with viral NP and MxA-specific antibodies. The lower panel shows the input of viral NP, MxA and β-tubulin in whole cell lysates. See also Figure S3.
L4 influences binding of MxA to THOV NP
We previously identified the viral NP as the target of MxA antiviral action (Zimmermann et al., 2011) and demonstrated interaction of hsMxA with THOV NP by co-immunoprecipitation (Kochs and Haller, 1999). To test if the gain-of-function of the chimeric proteins is accompanied by L4-mediated binding of THOV NP, we monitored the association of NP with Flag-tagged Mx proteins in THOV-infected cells. HsMxA but not mmMx1 associated with THOV NP, as indicated by co-immunoprecipitation (Figure 3C). Furthermore, the mmMx1(hsL4) chimera gained the ability to bind NP (Figure 3C) concomitant with the gain of antiviral function. Accordingly, inserting the agmL4 into hsMxA severely reduced NP association (Figure S3). This indicates that L4 influences hsMxA interaction with and resultant specificity for the THOV NP. On the other hand, we did not observe rescue of NP binding by the reciprocal agmMxA(hsL4) protein (Figure S3). The discrepancy in NP co-immunoprecipitation by agmMxA(hsL4) versus mmMx1(hsL4) proteins may be mediated by presently unidentified residues outside of L4 that differ between the mmMx1 and agmMxA proteins, which could also have some effect (e.g., affinity or stability) on the interaction with THOV NP.
MxA antiviral specificity for influenza A virus is mediated by L4
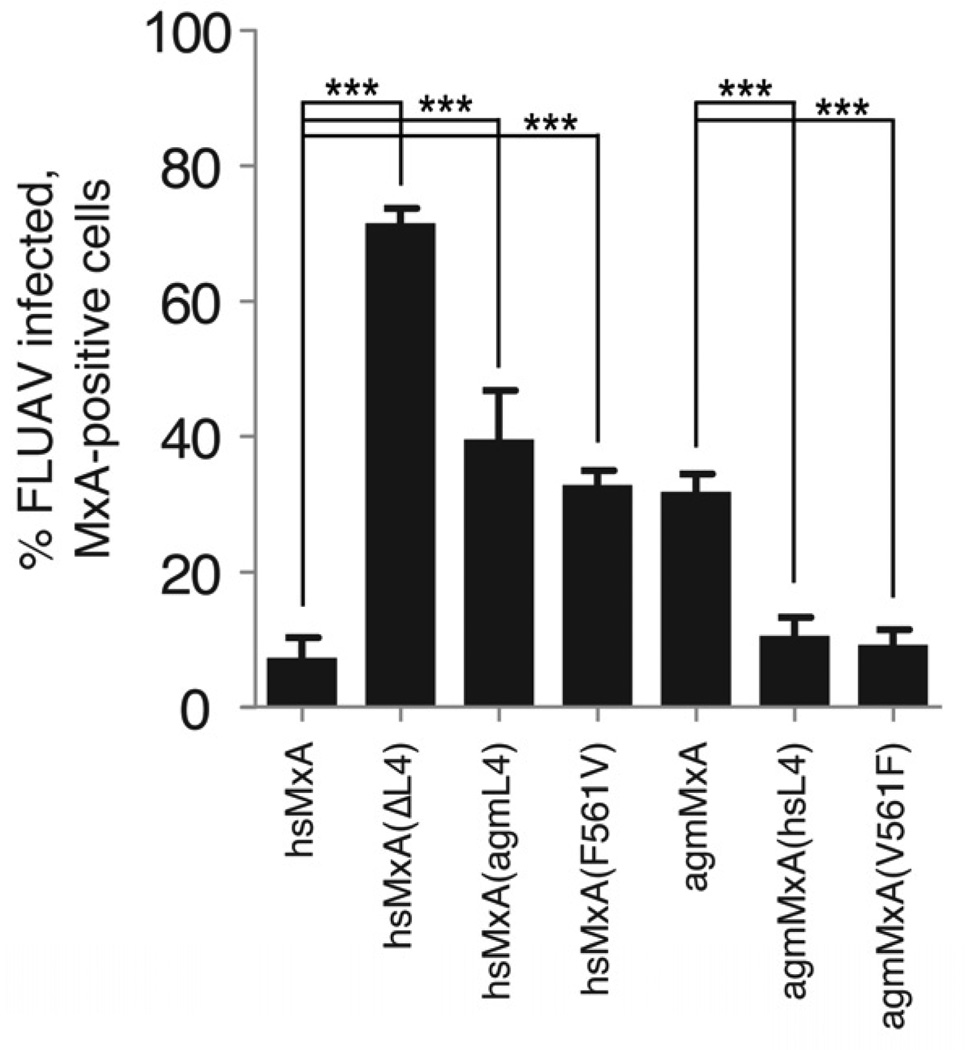
We recently reported that avian influenza A viruses like contemporary H5N1 isolates are sensitive to the antiviral action of hsMxA (Dittmann et al., 2008; Zimmermann et al., 2011). We investigated whether the dramatic positive selection of primate MxA had any consequences on restriction of H5N1 by analyzing the effect of hsMxA and agmMxA in Vero cells infected with avian influenza strain A/Thailand/1/04. Expression of hsMxA blocked virus replication by 10-fold (Figure 4); however agmMxA only had a modest effect on influenza viral replication, when compared to the inactive control hsMxA(delL4). To determine whether differences in L4 could also account for this disparity, we tested L4 chimeric proteins for antiviral activity. We found that adding the L4 region of agmMxA or the single amino acid exchange F561V to hsMxA decreased its restriction of viral replication. Conversely, adding the L4 region of hsMxA or only V561F to agmMxA increased its antiviral activity to the same level as the wildtype hsMxA protein (Figure 4). Taken together, these results are consistent with a generalized function for the loop L4, and F561 in particular, in MxA antiviral specificity for orthomyxoviruses.
Figure 4. The loop L4 mediates functional differences in primate MxA antiviral activity against influenza A virus.
Restriction of A/Thailand/1/04 infection by hsMxA and agmMxA. Data are presented as percent of infected, Mx-positive cells as measured by immuno-fluorescence for viral NP and MxA. Error bars represent standard deviation of three biological replicates. ***, p<0.0001 (t-test).
Discussion
The diversity of viruses that MxA restricts is at odds with the observed specificity in MxA targeting of unique viral structures. Here, we leverage the antagonistic relationship between MxA and its targets using an evolution-guided approach to resolve the biological basis of MxA antiviral specificity. By analyzing signatures of recurrent positive selection characteristic of arms races between host and viral genomes, we identified a cluster of positively selected residues in loop L4 of MxA that predicts its role for antiviral specificity. Using viruses known to be susceptible to hsMxA as ‘surrogates’ to reveal functional consequences of ancient diversifying selection in primate MxA L4, we demonstrate that L4 is indeed a major determinant of primate MxA antiviral activity against the orthomyxoviruses THOV and influenza A. Given that single amino acid mutations confer MxA specificity at a site recurrently altered by positive selection, we propose that the disordered surface-exposed loop L4 mediates MxA antiviral specificity. Although it is possible that this specificity manifests epistatically, the recurrent positive selection and demonstrated influence of L4 on the MxA - THOV NP interaction strongly implicates L4 as a direct interface of MxA target recognition.
The evolutionary signature of positive selection on MxA L4 suggests a mechanism that explains the conundrum of MxA target specificity and antiviral breadth. First, single amino acid changes in the positively selected L4 can have significant consequences for altering MxA antiviral specificity without being affected by other changes in L4 i.e., F561 worked in the context of both human and AGM proteins. These experiments suggest there is relatively little epistasis between different L4 residues. We propose that other positively selected residues in L4 may determine specificity to additional viruses similar to the role that residue 561 plays as a specificity determinant for MxA against THOV. Thus, adaptation to a newly encountered pathogen could occur while not compromising pre-existing substrate specificity or antiviral range.
Second, although bearing the most striking signature of positive selection, loop L4 is not the only adaptive surface that might alter MxA specificity (Figure 1B). For example, great apes share identical loop L4 sequences yet differ in strength of antiviral activity suggesting that L4 is not the only domain in MxA that determines target specificity (Figure 2A). Moreover, the observation that hsL4 is sufficient for MxA interaction with THOV NP in the context of mmMx1 but not agmMxA also points to non-L4 determinants that influence MxA specificity. Other positively selected sites might represent distinct interaction surfaces for different classes of viral pathogens as suggested for avian Mx genes, which display marked variation in an avian-specific region in the distal N-terminus of the protein (Berlin et al., 2008). Thus, independent specificity determinants may help MxA adapt against a wide variety of viruses. Alternatively, these sites may function indirectly, for example by optimizing L4 positioning. Future studies will help elucidate not only the effect of L4 on MxA antiviral breadth but also whether distinct MxA surfaces are used to recognize distinct viral pathogens.
While we expect that further interrogation of diversifying sites in MxA will shed light on MxA antiviral specificity and mechanisms of restriction, it is also interesting to note that the few positively selected sites in MxA are in stark contrast to the strong purifying selection acting on the majority of the MxA protein. An explanation for the dearth of positive selection in the rest of the protein is that evolution is limited by a strictly conserved GTPase architecture. Host-host co-evolutionary interactions with cellular cofactors, such as UAP56 helicase (Wisskirchen et al., 2011), may further constrain MxA evolution. Therefore, flexibility in substrate recognition may have arisen in L4 to accommodate the conservation of the rest of the protein. Indeed, the transfer of hsMxA L4-mediated specificity to rodent Mx1 suggests that while the L4 function is widely conserved, divergence in L4 encodes unique antiviral specificities that reflect a history of lineage-specific arms races against ancient pathogenic viruses in primates.
Recent insights from crystallographic data also help put our findings in biochemical context. Although L4 was unresolved in the hsMxA structure, the resulting structural model situates the loop L4 facing outward from the inner surface of a ring-shaped MxA oligomer in which the G domains are at the periphery and the stalks assemble in the middle in a crisscross pattern (Gao et al., 2011; von der Malsburg et al., 2011). This configuration permits extensive, repetitive contacts between the assembled MxA oligomer and its substrate. The model is particularly appealing for THOV and other negative-sense RNA viruses, because the putative viral substrate NP is repetitively bound to the viral RNA forming a large superhelical structure (Kochs and Haller, 1999; Reichelt et al., 2004). MxA oligomerization and cooperative target binding may also explain the large phenotypic consequences for antiviral activity observed for single amino acid mutations in L4. Even subtle differences in the binding affinity between MxA and NP may largely determine antiviral specificity because their effect is amplified synergistically on a multimeric target like the viral nucleocapsid (Haller et al., 2010).
How the comparatively diminutive host innate immune repertoire withstands the diversity and rapid evolution of pathogens is a long-standing problem. Taken together, our data provide insight into the molecular nature of antiviral breadth exhibited by one effector of innate immunity, MxA, in response to pathogen diversity. Our work suggests that breadth may be achieved through the evolution of specificity domains that lie within evolutionarily dynamic and structurally ‘flexible’ regions of otherwise conserved antiviral proteins.
Experimental Procedures
Sequencing of primate MxA genes
Primate MxA genes were amplified from RNA isolated from cell lines obtained from Coriell Cell Repositories (Camden, NJ). Gene-specific one-step reverse-transcription polymerase chain reaction (RT-PCR) was conducted using SuperScript III One-Step RT-PCR with Platinum Taq (Invitrogen) to produce complementary DNA (cDNA) using degenerate primers: (F: 5’ – CAAAGAAGGAAGATGGTTSTTTCCGAAGTGG - 3’ and R: 5’ – TTAACCGGGGAACTGGGCRAG – 3’). cDNA was sequenced and assembled using CodonCode Aligner. The following sequences were obtained from Ensembl and were not independently validated: M. mulatta (rhesus macaque): ENSMMUT00000021494 and C. jacchus (marmoset): ENSCJAT00000021974.
MxA sequence analysis
Sequences were aligned in ClustalX and edited to remove indels. ML tests were performed with CODEML using the PAML software suite (Yang, 2007), as previously described (Sawyer et al., 2005). Briefly, sequences were subjected to ML tests using NS sites models disallowing (M7) or allowing (M8) positive selection. For each comparison the models allowing positive selection gave the best fit to the data. The result was consistent under varying models of codon frequency (F61 and F3x4). The same site-specific analyses also provided individual amino acids with high posterior probabilities that are consistent with positive selection.
Plasmids
MxA coding sequence was amplified using AccuPrime Pfx SuperMix (Invitrogen) from cDNA derived from indicated primate species and subcloned into pcDNA3 with an N-terminal hemagglutinin (HA) or Flag epitope. HsMxA and mmMx1 expression plasmids have been described previously (Zimmermann et al., 2011). L4 chimeras were generated by PCR. Point mutations were generated using Quikchange site-directed mutagenesis (Stratagene).
Minireplicon assay
The THOV minireplicon assay was performed in 293T cells in a 96-well format. Briefly, 4.0 ng each of PB2, PB1 and PA, 1.0 ng of NP, all in pCAGGS expression vector, as well as 20 ng pHH21-vNP-FF-Luc (firefly luciferase), 50 ng Tk-luc (Renilla) (Promega) and varying input or a fixed amount of 200 ng MxA plasmids were co-transfected into 293T cells using the reagent TransIT-LT1 (Mirus Bio). At 24h post-transfection, luciferase activity was measured using the Dual-Glo system (Promega). Expression of MxA proteins was detected by Western blot analysis using mouse anti-HA.11 monoclonal antibody 16B12 (Sigma) and rabbit anti-βactin polyclonal antibody AB-8227 (Abcam). Corresponding data from minireplicon assays and Western blots are derived from samples generated from a single master mix. All experiments were done in triplicate.
Transfection, viral infection and co-immunoprecipitation
Vero cells were transfected in 24-well plates with 250 ng MxA-expressing plasmids and nanofectin (PAA) for 24 h and then infected with 10 moi of THOV, strain SiAr126, for 24 h or 5 moi of influenza A virus, strain A/Thailand/1/04, for 5 h. Then, the cells were fixed with 3% paraformaldehyde and MxA and viral NP expression were detected by immunofluorescence analysis using specific antibodies as described previously (Dittmann et al., 2008; Haller et al., 1995).
For immunoprecipitation, 293T cells were transfected in six-well plates with 1 µg of Flag-MxA-encoding plasmids for 24 h and then infected with 10 moi of THOV for additional 24 h. Cells were lysed in buffer, 50 mM Tris, pH 8.0, 150 mM NaCl, 1 mM EDTA, 0.5% NP-40. The supernatants were used for immunoprecipitation of Flag-MxA using Anti-FLAG-M2 affinity gel (Sigma) for 2 h at 4°C. After washing in lysis buffer the precipitated proteins were eluted in SDS-sample buffer at 95°C for 5 min. Co-precipitated MxA and viral NP were detected by Western blotting using monoclonal antibodies specific for MxA and THOV NP (Kochs and Haller, 1999) and β-tubulin (Sigma).
Sequence deposition
All novel primate MxA sequences are deposited into Genbank under accession numbers JX297228-JX297248.
Supplementary Material
Highlights.
The unstructured loop L4 in primate MxA evolves under strong positive selection
Loop L4 is the determinant of MxA antiviral specificity against orthomyxoviruses
A single amino acid confers MxA antiviral specificity for Thogoto virus (THOV)
MxA antiviral specificity for influenza A virus is also mediated by L4
Acknowledgements
We thank Matthew Daugherty, Nisha Duggal, Richard McLaughlin and Maulik Patel for helpful advice. This study was supported by the National Science Foundation Graduate Research Fellowship under grant no. DGE-0718124 (P.S.M.), the Max Planck Research School-IMPRS (C.P.), NIH grant R01 AI30937 (M.E.), NSF CAREER award (H.S.M.), and by the Deutsche Forschungsgemeinschaft, grant Ko 1579/8-1 (G.K.). This work was conducted by Corinna Patzina in partial fulfillment of the requirements for a Ph.D. degree from the Faculty of Biology of the University of Freiburg. HSM is an Early Career Scientist of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no competing conflicts of interest.
References
- Berlin S, Qu L, Li X, Yang N, Ellegren H. Positive diversifying selection in avian Mx genes. Immunogenetics. 2008;60:689–697. doi: 10.1007/s00251-008-0324-0. [DOI] [PubMed] [Google Scholar]
- Daugherty MD, Malik HS. Rules of engagement: molecular insights from host-virus arms races. Annual Review of Genetics. 2012 doi: 10.1146/annurev-genet-110711-155522. In press. [DOI] [PubMed] [Google Scholar]
- Dittmann J, Stertz S, Grimm D, Steel J, García-Sastre A, Haller O, Kochs G. Influenza A virus strains differ in sensitivity to the antiviral action of Mx-GTPase. J. Virol. 2008;82:3624–3631. doi: 10.1128/JVI.01753-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, von der Malsburg A, Dick A, Faelber K, Schröder GF, Haller O, Kochs G, Daumke O. Structure of myxovirus resistance protein a reveals intra- and intermolecular domain interactions required for the antiviral function. Immunity. 2011;35:514–525. doi: 10.1016/j.immuni.2011.07.012. [DOI] [PubMed] [Google Scholar]
- Haller O, Frese M, Rost D, Nuttall PA, Kochs G. Tick-borne thogoto virus infection in mice is inhibited by the orthomyxovirus resistance gene product Mx1. J. Virol. 1995;69:2596–2601. doi: 10.1128/jvi.69.4.2596-2601.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller O, Gao S, von der Malsburg A, Daumke O, Kochs G. Dynamin-like MxA GTPase: structural insights into oligomerization and implications for antiviral activity. J. Biol. Chem. 2010;285:28419–28424. doi: 10.1074/jbc.R110.145839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller O, Kochs G. Human MxA protein: an interferon-induced dynamin-like GTPase with broad antiviral activity. J. Interferon Cytokine Res. 2011;31:79–87. doi: 10.1089/jir.2010.0076. [DOI] [PubMed] [Google Scholar]
- Kochs G, Haller O. GTP-bound human MxA protein interacts with the nucleocapsids of Thogoto virus (Orthomyxoviridae) J. Biol. Chem. 1999;274:4370–4376. doi: 10.1074/jbc.274.7.4370. [DOI] [PubMed] [Google Scholar]
- Landis H, Simon-Jödicke A, Klöti A, Di Paolo C, Schnorr JJ, Schneider-Schaulies S, Hefti HP, Pavlovic J. Human MxA protein confers resistance to Semliki Forest virus and inhibits the amplification of a Semliki Forest virus-based replicon in the absence of viral structural proteins. J. Virol. 1998;72:1516–1522. doi: 10.1128/jvi.72.2.1516-1522.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Zhang L, Chen L, Feng W, Xu Y, Chen F, Liu X, Chen Z, Liu W. MxA inhibits hepatitis B virus replication by interaction with core protein HBcAg. Hepatology. 2012;56:803–811. doi: 10.1002/hep.25608. [DOI] [PubMed] [Google Scholar]
- Netherton CL, Simpson J, Haller O, Wileman TE, Takamatsu HH, Monaghan P, Taylor G. Inhibition of a large double-stranded DNA virus by MxA protein. J. Virol. 2009;83:2310–2320. doi: 10.1128/JVI.00781-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perelman P, Johnson WE, Roos C, Seuánez HN, Horvath JE, Moreira MA, Kessing B, Pontius J, Roelke M, Rumpler Y, et al. A molecular phylogeny of living primates. PLoS Genet. 2011;7 doi: 10.1371/journal.pgen.1001342. e1001342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitossi F, Blank A, Schröder A, Schwarz A, Hüssi P, Schwemmle M, Pavlovic J, Staeheli P. A functional GTP-binding motif is necessary for antiviral activity of Mx proteins. J. Virol. 1993;67:6726–6732. doi: 10.1128/jvi.67.11.6726-6732.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponten A, Sick C, Weeber M, Haller O, Kochs G. Dominant-negative mutants of human MxA protein: domains in the carboxy-terminal moiety are important for oligomerization and antiviral activity. J. Virol. 1997;71:2591–2599. doi: 10.1128/jvi.71.4.2591-2599.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichelt M, Stertz S, Krijnse-Locker J, Haller O, Kochs G. Missorting of LaCrosse virus nucleocapsid protein by the interferon-induced MxA GTPase involves smooth ER membranes. Traffic. 2004;5:772–784. doi: 10.1111/j.1600-0854.2004.00219.x. [DOI] [PubMed] [Google Scholar]
- Sawyer SL, Wu LI, Emerman M, Malik H. Positive selection of primate TRIM5alpha identifies a critical species-specific retroviral restriction domain. Proc. Natl. Acad. Sci. U S A. 2005;102:2832–2837. doi: 10.1073/pnas.0409853102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Malsburg A, Abutbul-Ionita I, Haller O, Kochs G, Danino D. Stalk domain of the dynamin-like MxA GTPase protein mediates membrane binding and liposome tubulation via the unstructured L4 loop. J. Biol. Chem. 2011;286:37858–37865. doi: 10.1074/jbc.M111.249037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, Haller O, Kochs G. MxA GTPase blocks reporter gene expression of reconstituted Thogoto virus ribonucleoprotein complexes. J. Virol. 2000;74:560–563. doi: 10.1128/jvi.74.1.560-563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisskirchen C, Ludersdorfer TH, Müller DA, Moritz E, Pavlovic J. Interferon-induced antiviral protein MxA interacts with the cellular RNA helicases UAP56 and URH49. J. Biol. Chem. 2011;286:34743–34751. doi: 10.1074/jbc.M111.251843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol. Biol. Evolution. 2007;24:1586–1591. doi: 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]
- Zimmermann P, Mänz B, Haller O, Schwemmle M, Kochs G. The viral nucleoprotein determines Mx sensitivity of influenza A viruses. J. Virol. 2011;85:8133–8140. doi: 10.1128/JVI.00712-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.