Abstract
A 12-week-old domestic cat presented at a local veterinary clinic with hypocalcemia and skeletal abnormalities suggestive of rickets. Osteomalacia (rickets) is a disease caused by impaired bone mineralization leading to an increased prevalence of fractures and deformity. Described in a variety of species, rickets is most commonly caused by vitamin D or calcium deficiencies owing to both environmental and or genetic abnormalities. Vitamin D-dependent rickets type 1A (VDDR-1A) is a result of the enzymatic pathway defect caused by mutations in the 25-hydroxyvitamin D3-1-alpha-hydroxylase gene [cytochrome P27 B1 (CYP27B1)]. Calcitriol, the active form of vitamin D3, regulates calcium homeostasis, which requires sufficient dietary calcium availability and correct hormonal function for proper bone growth and maintenance. Patient calcitriol concentrations were low while calcidiol levels were normal suggestive of VDDR-1A. The entire DNA coding sequencing of CYP27B1 was evaluated. The affected cat was wild type for previously identified VDDR-1A causative mutations. However, six novel mutations were identified, one of which was a nonsense mutation at G637T in exon 4. The exon 4 G637T nonsense mutation results in a premature protein truncation, changing a glutamic acid to a stop codon, E213X, likely causing the clinical presentation of rickets. The previously documented genetic mutation resulting in feline VDDR-1A rickets, as well as the case presented in this research, result from novel exon 4 CYP27B1 mutations, thus exon 4 should be the initial focus of future sequencing efforts.
Short Communication
The rigid nature and high compressive strength of bones results from the deposition of dietary calcium, magnesium and phosphate into a collagen matrix. Osteomalacia (rickets) is a disease characterized by the softening of bones resulting in an increased prevalence of fractures and deformities that result from either a lack of dietary calcium, dietary vitamin D3 or improper vitamin D3 conversion. In its native form, vitamin D3 (cholecalciferol) lacks biological activity and is unable to facilitate calcium absorption in the gut. In many species, vitamin D3 is obtained either through diet or conversion in the skin by ultraviolet (UV) light of the steroid 7-deyhdrocholesterol into cholecalciferol. However, in domestic cats vitamin D3 acquisition is restricted to diet, 1 requiring a two-step enzymatic pathway for conversion to the active form of vitamin D3 (calcitriol). In the liver, vitamin D 25-hydroxylase [cytochrome P450 2R (CYP2R1)] catalyzes the initial hydroxylation of vitamin D (cholecalciferol) into 25-hydroxyvitamin D3 (calcidiol). In the kidney, 1-alpha-hydroxylase (CYP27B1) then catalyzes the hydroxylation and metabolic activation of calcidiol into hormonally active 1,25-dihydroxyvitamin D3 (calcitriol). Calcitriol binds and activates the nuclear vitamin D receptor (VDR), with subsequent regulation of calcium homeostasis.
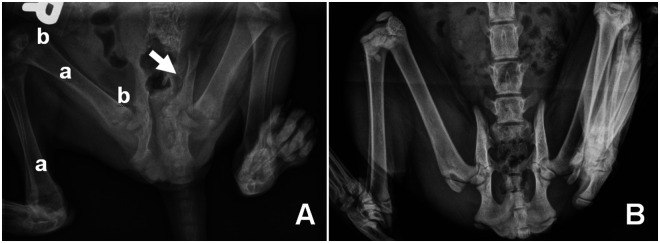
A 3-month-old female Siamese mix was referred to the University of California, Davis Veterinary Medicine Teaching Hospital with clinical signs including lethargy, obstipation, pelvic limb gait abnormality and evidence of generalized pain/sensitivity. Orthogonal radiographic imaging indicated marked osteopenia and radiolucency of the femoral necks, capital and distal physis, distal femur and proximal tibia. Additionally, pelvic asymmetry was observed with displacement of the acetabulum toward the midline (Figure 1). Referring hospital complete blood counts were within normal ranges while blood chemistry values were abnormal, suggestive of metabolic bone disease with secondary hyperparathyroidism. Significant abnormal chemistry findings included: elevated alkaline phosphatase (367 U/l; reference interval (RI) 0–62 U/l) and creatinine phosphokinase (1390 U/l; RI 64–440 U/l) and decreased calcium (5.9 mg/dl; RI 8.2–11.8 mg/dl). Decreased globulin (2.9 g/dl; RI 3.0–5.6 g/dl) and creatinine (0.7 mg/dl; RI 0.8–2.3 mg/dl) were also observed but values were borderline low/normal and not considered significant to the case given radiologic findings. All other values, including phosphorus, were within normal limits. Dietary insufficiency for the observed values was considered unlikely as a co-housed, unaffected sibling exhibited none of the symptoms. Elevated total alkaline phosphatase has been associated with increased osteoblast activity in bone, liver disease or with excesses of exogenous or endogenous glucocorticoids. However, normal complete blood counts in this cat were not consistent with glucocorticoid stimulation and there was no clinical evidence of liver disease. Thus, elevated alkaline phosphatase levels in this cat were most likely to be associated with a bone disorder. For this cat, the most likely differential for the observed hypocalcemia was malabsorption of dietary calcium, thus serum vitamin D metabolites were evaluated. Endocrinology indicated elevated levels of parathyroid hormone (69.50 pmol/l; RI 0.00–4.00 pmol/l), low levels of ionized calcium (0.99 mmol/l; RI 1.20–1.35 mmol/l) but normal levels of calcidiol (134 pmol/l; RI 65–170 pmol/l). Normal calcidiol serum levels indicated dietary vitamin D was sufficient and hydroxylation of cholecalciferol in the liver was normal. However, standard calcidiol levels coupled with low levels of serum calcitriol (7.37 pg/ml; RI 20–40 pg/ml) suggested perturbation of the last hydroxylation step, consistent with VDDR-1A.
Figure 1.
Ventro-dorsal radiograph of VDDR-1A (A) and unaffected age-matched control cat (B). Radiolucency (a), osteopenia (b) and displacement of the left acetabulum (arrow) are indicated. Radiographs were obtained on the same imaging equipment using a standard feline pelvic/spine protocol
Mutations in the genes CYP2R1 and CYP27B1 result in vitamin D-dependent rickets type 1b, and 1a, respectively.2–5 Both types are inherited as autosomal recessive traits. 6 Feline cases of rickets have been characterized and treatment strategies documented, but causal mutations are rarely determined.7–11 In the sole case identifying a genetic defect resulting in feline VDDR-1A, two mutations were identified in feline CYP27B1; G223A and G731del resulting in V75M and a frame-shift causing premature protein truncation, respectively. 5 For the present study, established gene specific primers 5 were used for a sequence level evaluation of CYP27B1 in the affected patient, a co-housed, non-affected sibling and two additional normal controls.
DNA was isolated from whole blood via the DNEasy Tissue Extraction kit (Qiagen), according to manufacturer’s specifications. Six primer sets covering the complete coding regions of the feline CYP27B1 gene 5 were used in the following 25 μl polymerase chain reaction (PCR) reaction: 25 ng of DNA, 2.0 mM MgCl2, 1× ABgene PCR buffer with 0.05% bovine serum albumin, 1.25 mM deoxynucleotide triphosphates (dNTPs), 0.2 mM of each primer and 1 unit of Taq polymerase (ABgene). Each reaction was amplified using the following cycling conditions in a MJ Research DNA engine (MJ Research): 94°C for 3 min initial denaturation, followed by 12 cycles of 45 s at 94°C, 30 s at 64°C and 60 s at 72°C with an annealing temperature decrease of 0.5/cycle. The touchdown cycle was followed by 35 cycles of 45 s at 94°C, 20s at 58°C and 45 s at 72°C. A 10 min 72°C final incubation was added to ensure full product length for all amplicons. PCR products were stored at 4°C.
PCR amplicons were size verified via agarose gel electrophoresis and products were prepared for sequencing with ExoSap-IT exonuclease (USB) according to manufacturer’s protocols. Prepared PCR products were directly sequenced as previously described. 12 Sequence calls were verified by sequencing each amplicon in both the forward and reverse directions. Sequencing products for an individual cat were assembled into a single gene contig using Sequencher analysis software v4.1 (Gene Codes Corporation). Assembled contigs from each cat were aligned with published cat sequence to identify sequence variants.
Sequence was generated for 5682 nucleotides of CYP27B1. Previously, CYP27B1 mutations in exon 2, G223A, which causes a V75M, and an exon 4 G731del, which causes an R244P, were suggested as causative for VDDR-1A in domestic cats. 5 The two previously identified mutations in exons 2 and 4 were not identified in the presenting patient. However, six single nucleotide polymorphisms (SNPs) were found in the patient as compared to the two control samples and an Ensemble reference sequence (Ensfcat00000014705) (Table 1). The six SNPs included a transition and a transversion in the 5’ untranslated region, a transition in exon 2, a transversion in exon 4 and two transversions in intron 7. The unaffected littermate was heterozygous at every polymorphic site. Of the two coding SNPs, C216T in exon 2 is silent whilst G637T in exon 4 results in glutamic acid changing to an ochre stop codon, truncating the terminal 297 amino acids of the protein.
Table 1.
Nucleotide polymorphisms and amino acid changes of feline CYP27B1
| Region | Position * | Ensfcat 00000014705 | DSH controls | Unaffected littermate | Affected patient |
|---|---|---|---|---|---|
| 5’ UTR | T(-996)A | T | T | T/A | A |
| G(-973)A | G | G | A/G | A | |
| Exon 2 | C216T | C | C | C/T | T |
| F72F | F | F | F | F | |
| G223A† | G | G | G | G | |
| V75M | V | V | V | V | |
| Exon 4 | G637T | G | G | G/T | T |
| E213X | E | E | E/X | X | |
| G731del† | G | G | G | G | |
| R244P | R | R | R | R | |
| Intron 7 | C149G | C | C | C/G | G |
| T278A | T | T | T/A | A |
Top is the nucleotide position and change, bottom is the amino acid position and change
Previously identified mutations in CYP27B1
The effects of the truncation dramatically impact protein function. Two of the four α-helices forming the salt bridge stabilized by Asp164 are not translated. Also missing is the heme propionate binding domain found in exon 8, as well as five other α-helices of the protein. Three substrate-binding sites are deleted including the heme binding site. Perhaps most importantly, Thr409, the amino acid demonstrated to form a hydrogen bond with the 25-hydroxyl group of 25-hydroxyvitamin D3, is not present in the altered feline VDDR-1A protein. 13 The cumulative effects of the loss of the 3’ 58% of the protein likely causes a functional disruption of protein activity resulting in low serum levels of calcitriol and the resulting rickets phenotype.
The effects of the two intronic and two 5’ UTR SNPs on transcription are unknown. Interestingly, in both cases of feline VDDR-1A evaluated at the sequence level, coding sequence allelic SNPs were identified in exons 2 and 4, and in both cases the exon 4 SNPs were suggestive as the causative mutations. As the only two sequence level verified cases of feline VDDR-1A have resulted from mutations in exon 4, future sequencing efforts should initiate with exon 4 analysis as the current results coupled with previous sequence level evaluation may indicate exon 4 is subject to increased likelihood of mutation.
Footnotes
Funding: Funding support was provided to LA Lyons from NIH-NCRR R24 RR016094. The study sponsors had no involvement in the study design; in the collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
The authors do not have any potential conflicts of interest to declare.
Accepted: 4 April 2012
References
- 1. How KL, Hazewinkel HA, Mol JA. Dietary vitamin D dependence of cat and dog due to inadequate cutaneous synthesis of vitamin D. Gen Comp Endocrinol 1994; 96: 12–18. [DOI] [PubMed] [Google Scholar]
- 2. Cheng JB, Levine MA, Bell NH, Mangelsdorf DJ, Russell DW. Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proc Natl Acad Sci USA 2004; 101: 7711–7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Takeyama K, Kitanaka S, Sato T, Kobori M, Yanagisawa J, Kato S. 25-Hydroxyvitamin D3 1alpha-hydroxylase and vitamin D synthesis. Science 1997; 277: 1827–1830. [DOI] [PubMed] [Google Scholar]
- 4. Chavez LS, Serda R, Choe S, Davidi L, Harmeyer J, Omdahl JL. Molecular basis for pseudo vitamin D-deficiency rickets in the Hannover pig. J Nutri Biochem 2003; 14: 378–385. [DOI] [PubMed] [Google Scholar]
- 5. Geisen V, Weber K, Hartmann K. Vitamin D-dependent hereditary rickets type I in a cat. J Vet Intern Med 2009; 23: 196–199. [DOI] [PubMed] [Google Scholar]
- 6. Scriver CR. Vitamin D dependency. Pediatrics 1970; 45: 361–363. [PubMed] [Google Scholar]
- 7. Phillips AM, Fawcett AC, Allan GS, Wilkinson M, Fraser DR, Malik R. Vitamin D-dependent non-type 1, non-type 2 rickets in a 3-month-old Cornish Rex kitten. J Feline Med Surg 2011; 13: 526–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. MacKenzie JM, Crawford J, Ghantous S. Successful therapy of vitamin D-dependant rickets in a kitten. J Am Anim Hosp Assoc 2011; 47: 290–293. [DOI] [PubMed] [Google Scholar]
- 9. Tanner E, Langley-Hobbs SJ. Vitamin D-dependent rickets type 2 with characteristic radiographic changes in a 4-month-old kitten. J Feline Med Surg 2005; 7: 307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Henik RA, Forrest LJ, Friedman AL. Rickets caused by excessive renal phosphate loss and apparent abnormal vitamin D metabolism in a cat. J Am Vet Med Assoc 1999; 215: 1644–9. [PubMed] [Google Scholar]
- 11. Schreiner CA, Nagode LA. Vitamin D-dependent rickets type 2 in a four-month-old cat. J Am Vet Med Assoc 2003; 222: 337–339. [DOI] [PubMed] [Google Scholar]
- 12. Grahn RA, Kurushima JD, Billings NC, Grahn JC, Halverson JL, Hammer E, et al. Feline non-repetitive mitochondrial DNA control region database for forensic evidence. Forensic Sci Int Genet 2011; 5: 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yamamoto K, Uchida E, Urushino N, Sakaki T, Kagawa N, Sawada N, et al. Identification of the amino acid residue of CYP27B1 responsible for binding of 25-hydroxyvitamin D3 whose mutation causes vitamin D-dependent rickets type 1. J Biol Chem 2005; 280: 30511–30516. [DOI] [PubMed] [Google Scholar]