Abstract
The polyphenol curcumin is the principal flavor and color component of the spice turmeric. Beyond its culinary uses, curcumin is believed to positively impact human health and displays antioxidant, anti-inflammatory, antibacterial, and chemopreventive properties. It also is in clinical trials as an anticancer agent. In aqueous solution at physiological pH, curcumin undergoes spontaneous autoxidation that is enhanced by oxidizing agents. The reaction proceeds through a series of quinone methide and other reactive intermediates to form a final dioxygenated bicyclopentadione product. Several naturally occurring polyphenols that can form quinones have been shown to act as topoisomerase II poisons (i.e., increase levels of topoisomerase II-mediated DNA cleavage). Because several of these compounds have chemopreventive properties, we determined the effects of curcumin, its oxidative metabolites, and structurally related degradation products (vanillin, ferulic acid, and feruloylmethane), on the DNA cleavage activities of human topoisomerase IIα and IIβ. Intermediates in the curcumin oxidation pathway increased DNA scission mediated by both enzymes ~4-5–fold. In contrast, curcumin and the bicyclopentadione, as well as vanillin, ferulic acid, and feruloylmethane, had no effect on DNA cleavage. As found for other quinone-based compounds, curcumin oxidation intermediates acted as redox-dependent (as opposed to interfacial) topoisomerase II poisons. Finally, under conditions that promote oxidation, the dietary spice turmeric enhanced topoisomerase II-mediated DNA cleavage. Thus, even within the more complex spice formulation, oxidized curcumin intermediates appear to function as topoisomerase II poisons.
Turmeric is a common spice that is used in curries and a variety of other Asian cuisines.1-3 It is isolated from the rhizomes of Curcuma longa, which is an herbaceous perennial of the ginger family.3
Curcumin (Figure 1) is the principal flavor and color component of turmeric. Beyond its culinary uses, curcumin is believed to positively impact human health and commonly is used in traditional Chinese herbal medicine and Ayurvedic medicine.2,3 The compound has antioxidant, anti-inflammatory, and antibacterial activities.4,5 Furthermore, it appears to have chemopreventive properties against a variety of human malignancies and currently is in clinical trials as an anticancer agent.4-9
Figure 1.
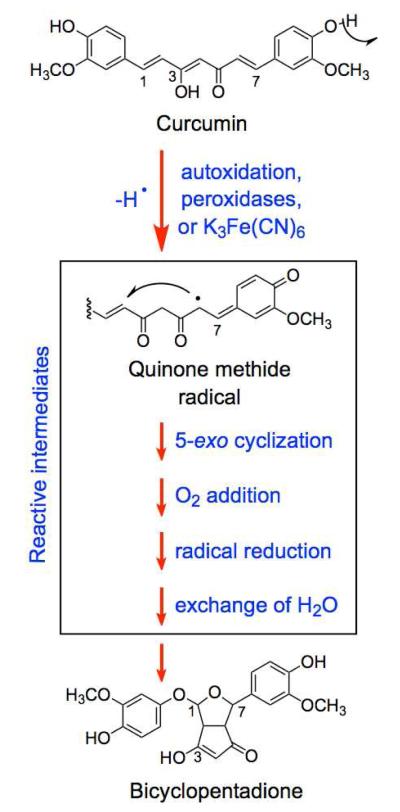
Oxidative transformation of curcumin. Adapted from Griesser et al.14
Curcumin has poor oral bioavailability and is unstable under physiological conditions.10-14 Thus, it has been suggested that curcumin metabolites mediate at least some of the biological effects of the parent compound.15-18 Several metabolic pathways have been proposed. Following oral administration, curcumin often is conjugated to form glucuronides or sulfates. Alternatively, if administered intraperitoneally, it can undergo reductive reactions to form a variety of hydrogenated curcuminoids. However, most studies have concluded that neither the conjugated nor the reduced products of curcumin are biologically active.18
Curcumin also can undergo spontaneous autoxidation in aqueous solutions at physiological pH (Figure 1).14 This reaction gives rise to novel products that have potential for biological activity. Autoxidation of curcumin is stimulated by peroxidases and oxidizing agents and is initiated by hydrogen abstraction from one of the two phenolic hydroxyl moieties.14 Following this abstraction, the reaction is proposed to proceed through several unstable and reactive intermediates, including an electrophilic quinone methide radical.14 The final product of curcumin autoxidation is a dioxygenated bicyclopentadione.14
In addition to oxidation, the heptadienone chain of curcumin can be fragmented into vanillin, ferulic acid, feruloylmethane, and related compounds (see Figure 8).18 These fragmentation products have been shown to display antioxidant and anti-inflammatory properties.18 However, at physiological pH, degradation of the curcumin heptadienone chain appears to be a minor reaction.19
Figure 8.
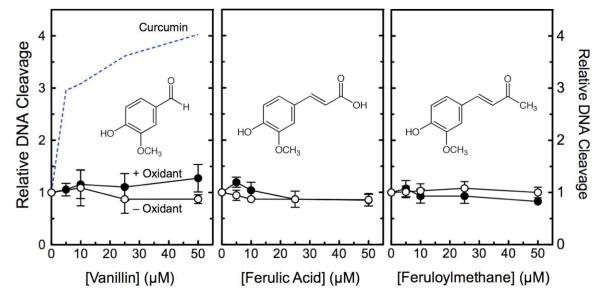
Effects of curcumin degradation products on topoisomerase II-mediated DNA cleavage. The effects of vanillin (left), ferulic acid (middle), or feruloylmethane (right) on the cleavage of negatively supercoiled plasmid DNA by human topoisomerase IIα were determined in the absence (open circles, – Oxidant) or presence (closed circles, + Oxidant) of 50 μM K3Fe(CN)6. Data for curcumin (dashed line from Figure 2) are included in the left panel for comparison. Error bars represent standard deviations for three independent experiments.
Several naturally occurring polyphenols that can form quinones display activity against human type II topoisomerases.20 Many of these, including bioflavonoids such as myricetin (which is common in grapes, berries, and other fruits and vegetables) and catechols such as (−)-epigallocatechin gallate (EGCG, which is the active polyphenol in green tea),21-23 are believed to have chemopreventive properties.24-26 All of these compounds increase levels of DNA cleavage mediated by the type II enzymes.21-23
Type II topoisomerases are enzymes that modulate DNA under- and over-winding and remove knots and tangles from chromosomes.20,27-30 They function by passing an intact double helix through a transient break that they generate in another segment of DNA.20,27-30 In order to maintain genomic integrity during the double-stranded DNA passage event, type II topoisomerases form a covalent intermediate between active site tyrosine residues and the newly generated DNA termini.20,27-30 This requisite intermediate is known as the “cleavage complex”.20 A number of widely used anticancer drugs, such as etoposide and doxorubicin, function by stabilizing cleavage complexes.20,27,29-31 Thus, these “topoisomerase II poisons” convert type II topoisomerases into toxic enzymes that generate DNA strand breaks in treated cells.20,27,29-31 Human cells express two closely related topoisomerase II isoforms, α and β.20,27-30 Both appear to be targets for anticancer drugs.20,27,29-31
Treatment of human cells with curcumin induces DNA cleavage complexes formed by topoisomerase IIα and IIβ.32 Cleavage complex formation is prevented by the addition of an antioxidant, suggesting the importance of oxidative pathways in curcumin activity against the type II enzymes.32
An earlier study found that curcumin could induce topoisomerase II-mediated DNA cleavage in vitro.33 However, since curcumin can undergo oxidation and degradation in aqueous solution, it is not clear whether the parent compound or metabolites (or both) are the topoisomerase II-reactive species. Therefore, we tested the ability of curcumin to poison human type II topoisomerases under conditions in which the compound remains stable or undergoes oxidation. We also examined the activity of vanillin, ferulic acid, feruloylmethane toward the human enzymes. Results indicate that oxidative metabolites of curcumin poison human topoisomerase IIα and IIβ. In contrast, neither the parent compound nor its fragmentation products displayed significant activity toward the human enzymes.
EXPERIMENTAL PROCEDURES
Enzymes and Materials
Human topoisomerase IIα, topoisomerase IIβ, and the mutant topoisomerase IIαC392A/C405A were expressed in Saccharomyces cerevisiae34 and purified as described previously.35,36 Negatively supercoiled pBR322 DNA was prepared from Escherichia coli using a Plasmid Mega Kit (Qiagen) as described by the manufacturer. Curcumin and 4′,4″-dimethylcurcumin were synthesized as described previously.14 The bicyclopentadione oxidative product of curcumin was isolated from autoxidation reactions by high-performance liquid chromatography. Potassium ferricyanide [K3Fe(CN)6] was obtained from Acros and was stored at −20 °C as a 50 mM stock solution in water. Turmeric was obtained from Spice Islands Trading Company and was stored at −20 °C as a 37.5 mg/mL stock solution in 100% DMSO. Vanillin, ferulic acid, and feruloylmethane were obtained from Sigma. All other chemicals were analytical reagent grade. Unless stated otherwise, curcumin and its derivatives were stored at −20 °C as 20 mM stock solutions in 100% DMSO.
Plasmid DNA Cleavage
DNA cleavage reactions were carried out using the procedure of Fortune and Osheroff.37 Topoisomerase II DNA cleavage assays contained 220 nM human topoisomerase IIα, topoisomerase IIβ, or mutant topoisomerase IIαC392A/C405A and 10 nM negatively supercoiled pBR322 in a total of 20 μL of 10 mM Tris–HCl (pH 7.9), 5 mM MgCl2, 100 mM KCl, 0.1 mM EDTA, and 2.5% (v/v) glycerol. Assay buffer contained ~2 μM residual dithiothreitol (DTT) that was carried over from the topoisomerase II storage buffer. Unless stated otherwise, reaction mixtures were incubated at 37 °C for 6 min, and enzyme-DNA cleavage complexes were trapped by the addition of 2 μL of 5% SDS followed by 2 μL of 250 mM EDTA (pH 8.0). Proteinase K (2 μL of a 0.8 mg/mL solution) was added, and samples were incubated at 45 °C for 30 min to digest the enzyme. Samples were mixed with 2 μL of 60% sucrose in 10 mM Tris–HCl (pH 7.9), 0.5% bromophenol blue, and 0.5% xylene cyanol FF, heated at 45 °C for 5 min, and subjected to electrophoresis in 1% agarose gels in 40 mM Tris–acetate (pH 8.3) and 2 mM EDTA containing 0.5 μg/mL ethidium bromide. DNA bands were visualized with longrange ultraviolet light and quantified using an Alpha Innotech digital imaging system. DNA cleavage was monitored by the conversion of supercoiled plasmid DNA to linear molecules.
Assays were carried out in the absence or presence of 0–50 μM curcumin or derivatives (oxidation or degradation) in the absence or presence of 0–50 μM K3Fe(CN)6. Unless stated otherwise, curcumin or a derivative was always the last component added to reaction mixtures. In some cases, assays were carried out in the presence of 250 μM DTT, which was added either before or after establishing topoisomerase II-mediated DNA cleavage complexes.
RESULTS AND DISCUSSION
Oxidative Metabolites of Curcumin Enhance DNA Cleavage Mediated by Human Type II Topoisomerases
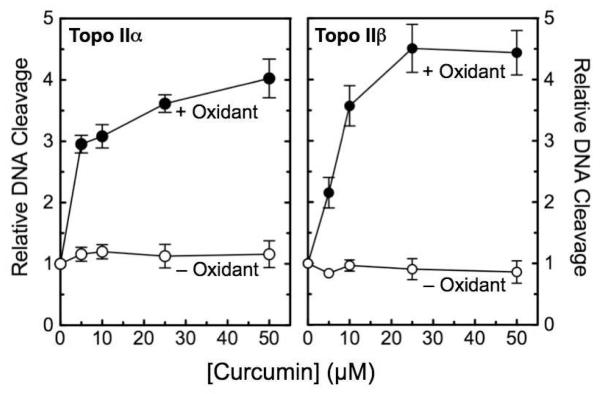
Although curcumin increases levels of DNA cleavage mediated by topoisomerase IIα and IIβ in cultured human cells,32 the ability of the compound to affect enzyme activity in purified systems has not been well characterized. Therefore, the effects of the phytochemical on the human type II enzymes were determined. As seen in Figure 2, curcumin displayed no activity toward either topoisomerase IIα or IIβ. However, in the presence of an oxidizing agent, such as potassium ferricyanide [K3Fe(CN)6], curcumin became a potent topoisomerase II poison. Between 4– and 5–fold DNA cleavage enhancement was observed with human topoisomerase IIα and IIβ, respectively. The activation of curcumin required stoichiometric concentrations of K3Fe(CN)6, and the oxidant had no effect on topoisomerase II-mediated DNA cleavage in the absence of the phytochemical (Figure 3, left).
Figure 2.
Enhancement of topoisomerase II-mediated DNA cleavage by curcumin in the presence of oxidant. The effects of curcumin on the cleavage of negatively supercoiled plasmid DNA by human topoisomerase IIα (left) and topoisomerase IIβ (right) were determined in the absence (open circles, – Oxidant) or presence (closed circles, + Oxidant) of 50 μM K3Fe(CN)6. Error bars represent standard deviations for three independent experiments.
Figure 3.
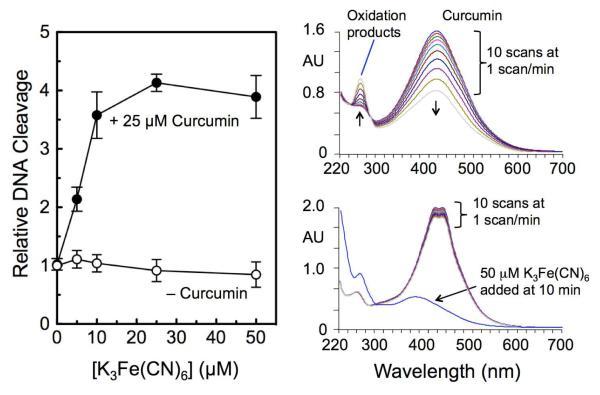
Effects of K3Fe(CN)6 on curcumin oxidation and the DNA cleavage activity of human topoisomerase IIα. Left: The effects of K3Fe(CN)6 on the cleavage of negatively supercoiled plasmid DNA by human topoisomerase IIα were determined in the absence (open circles) or presence (closed circles) of 25 μM curcumin. Error bars represent standard deviations for three independent experiments. Right: Ultraviolet/visible spectroscopic analysis of the loss of curcumin (maximum wavelength at 430 nm) and appearance of oxidized products (peak at 263 nm) in Tris-HCl buffer (pH 7.9) (top) and in topoisomerase II assay buffer (pH 7.9) (bottom). Scans were obtained at a frequency of one per min. K3Fe(CN)6 was added to the reaction in topoisomerase II assay buffer at 10 min.
Curcumin undergoes rapid oxidation in aqueous solutions (Figure 3, top right).14 However, the compound was much more stable in the buffer used for topoisomerase II-mediated DNA cleavage assays, and little oxidation was observed over the 6-min course of the reaction (Figure 3, bottom right). The increase in curcumin stability was due largely to the MgCl2 that was included in the assay buffer (data not shown). Complete oxidation of curcumin was observed in assay mixtures shortly after addition of K3Fe(CN)6 (Figure 3, bottom right).
Several control reactions with human topoisomerase IIα were performed to ensure that the DNA cleavage enhancement observed with oxidized curcumin was mediated by the type II enzyme (Figure 4). No DNA scission was seen in the presence of curcumin and K3Fe(CN)6 when the type II enzyme was left out of reactions. Furthermore, topoisomerase IIα-mediated DNA cleavage induced by oxidized curcumin was reversed when the active site Mg2+ ions were chelated with EDTA prior to trapping cleavage complexes with SDS. This reversibility is not consistent with an enzyme-independent reaction. Finally, cleaved plasmid products were covalently linked to topoisomerase II. In the absence of proteinase K, the linear DNA band disappeared and was replaced by a band that remained at the origin of the gel (not shown). These results demonstrate that the DNA scission observed in the presence of curcumin and K3Fe(CN)6 is mediated by the type II enzyme.
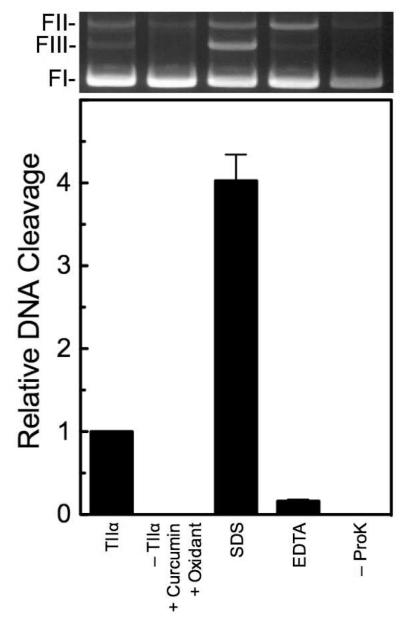
Figure 4.
DNA cleavage induced by oxidized curcumin is reversible and protein-linked. Assay mixtures contained enzyme in the absence of curcumin or oxidant (TIIα), 50 μM curcumin and 50 μM K3Fe(CN)6 in the absence of enzyme [−TIIα + Curcumin + Oxidant], or complete reactions treated with SDS prior to adding EDTA (SDS). To determine whether DNA cleavage induced by oxidized curcumin was reversible, reactions were incubated with EDTA prior to trapping cleavage complexes with SDS (EDTA). To determine whether DNA cleavage induced by oxidized curcumin was protein-linked, proteinase K treatment was omitted (−ProK). Error bars represent standard deviations for three independent experiments. A representative agarose gel is shown at the top. DNA lanes correspond to the bars shown in the graph. The positions of negatively supercoiled (form I, FI), nicked (form II, FII), and linear (form III, FIII) plasmid DNA are indicated.
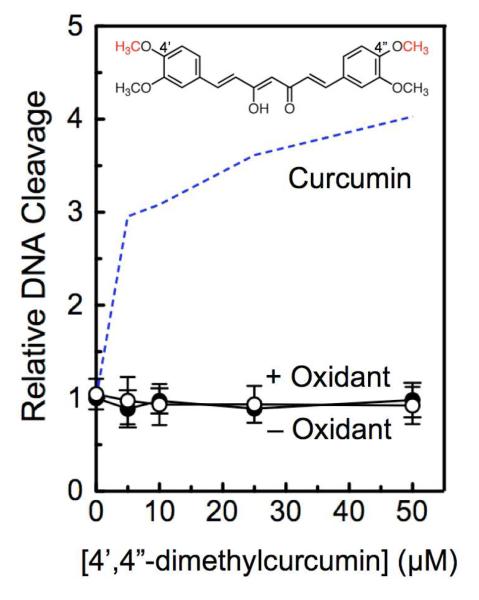
The findings described above provide strong evidence that oxidized metabolites of curcumin, rather than the parent compound, are responsible for the enhancement of topoisomerase II-mediated DNA cleavage. As further evidence supporting this conclusion, the ability of 4′,4″-dimethylcurcumin to poison human topoisomerase IIα was determined. Since the methyl groups protect the 4′- and 4″-hydroxyl moieties from hydrogen abstraction, the compound undergoes oxidation rates that are >1000–fold slower than that of curcumin.14 As seen in Figure 5, no enhancement of topoisomerase II-mediated DNA cleavage was observed in the absence or presence of K3Fe(CN)6.
Figure 5.
Effects of 4′,4″-dimethylcurcumin on topoisomerase II-mediated DNA cleavage. The effects of 4′,4″-dimethylcurcumin (structure at top, 4′- and 4″-methyl groups highlighted in red) in the absence (open circles, – Oxidant) or presence (closed circles, + Oxidant) of 50 μM K3Fe(CN)6 on the cleavage of negatively supercoiled plasmid DNA by human topoisomerase IIα were determined. Data for oxidized curcumin (dashed line from Figure 2) are included for comparison. Error bars represent standard deviations for three independent experiments.
Upon treatment with an oxidizing agent, curcumin is rapidly converted to a stable bicyclopentadione (Figures 1 and 3). En route to this ultimate oxidation product, the parent compound moves through a series of reactive quinone methide intermediates (Figure 1).14 As discussed below, a number of quinone-based compounds have been shown to poison type II topoisomerases.38-41 Therefore, two experiments were carried out to determine whether the quinone methide intermediates (as opposed to the final bicyclopentadione) are the more likely compounds that poison topoisomerase II in the presence of an oxidant. In the first experiment, curcumin was incubated with K3Fe(CN)6 for 10 min prior to its addition to a topoisomerase IIα-DNA cleavage reaction. Under these conditions, the majority of the parent phytochemical was converted to the bicyclopentadione (Figure 3, bottom right). As seen in Figure 6 (inset), no enhancement of DNA scission was observed. In the second experiment, purified bicyclopentadione was added to DNA cleavage assays in the absence or presence of K3Fe(CN)6 (Figure 6). Virtually no enhancement of topoisomerase IIα-mediated DNA cleavage was observed under either condition. Thus, it appears that the quinone methide metabolites of curcumin are the chemical species that poison topoisomerase II.
Figure 6.
Effects of bicyclopentadione on topoisomerase II-mediated DNA cleavage. The effects of bicyclopentadione (structure shown in Figure 1) in the absence (open circles, – Oxidant) or presence (closed circles, + Oxidant) of 50 μM K3Fe(CN)6 on the cleavage of negatively supercoiled plasmid DNA by human topoisomerase IIα were determined. Inset: curcumin was incubated in the absence (−) or presence (+) of K3Fe(CN)6 for 10 min before addition to topoisomerase IIα-DNA cleavage assay mixtures that contained 50 μM K3Fe(CN)6. Error bars represent standard deviations for three independent experiments.
Oxidative Metabolites of Curcumin Are Redox-Dependent Topoisomerase II Poisons
Compounds that poison type II topoisomerases can be categorized into two broad classes: interfacial vs. redox-dependent. Members of the first group interact with topoisomerase II at the protein-DNA interface (in the vicinity of the active site tyrosine) in a non-covalent manner.20,31,42 Interfacial topoisomerase II poisons include several anticancer drugs (e.g., etoposide) and dietary bioflavonoids (e.g., genistein) and display a number of characteristic properties.20,23,38,39,41,43,44 Primarily, because their actions against topoisomerase II do not depend on redox chemistry, they are unaffected by the presence of reducing agents. Furthermore, these compounds induce similar levels of enzyme-mediated DNA scission whether they are added directly to the binary topoisomerase II-DNA complex or are incubated with the enzyme prior to the addition of the nucleic acid substrate.20,38
Topoisomerase II poisons in the second class (redox-dependent poisons) form covalent adducts with the enzyme at amino acid residues distal to the active site.36,45 The best-characterized members of this group are quinones, such as 1,4-benzoquinone and polychlorinated biphenyl (PCB) metabolites.38,40,41 In contrast to interfacial topoisomerase II poisons, the ability of redox-dependent poisons to form topoisomerase II adducts (and, consequently, increase enzyme-mediated DNA cleavage) requires them to be in an oxidized form. Thus, DNA cleavage enhancement is blocked by the presence of reducing agents.20,23,38,39,41,43,44 However, once final protein adducts are formed, their oxidation state appears to be irrelevant. As a result, if reducing agents are added to assay mixtures after DNA cleavage-ligation equilibria have been established in the presence of a quinone-based poison, they are unable to reverse the cleavage enhancement.20,38,39,41 Finally, while redox-dependent poisons enhance topoisomerase II-mediated DNA cleavage when added to the enzyme-DNA complex, they inactivate topoisomerase II when incubated with the protein prior to the addition of DNA.20,38,39,41
Curcumin requires an oxidant in order for it to increase topoisomerase II-mediated DNA cleavage. Furthermore, many of the proposed metabolites in the oxidation pathway of the compound contain quinones.14 Thus, it seems likely that the active metabolites of curcumin function as redox-dependent (as opposed to interfacial) topoisomerase II poisons. Four approaches were utilized to address this hypothesis.
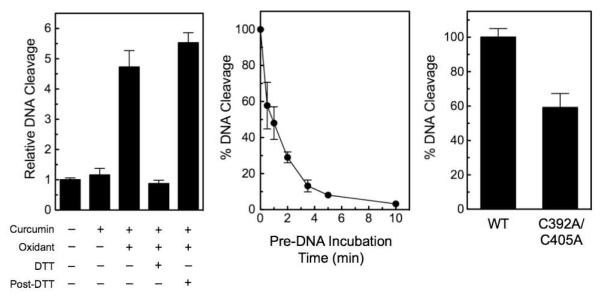
In the first, a 5–fold molar excess of DTT over curcumin and K3Fe(CN)6 was added to assay mixtures prior to the start of reactions. As expected (considering that curcumin requires oxidation for activation), no enhancement of topoisomerase IIα-mediated DNA cleavage was observed (Figure 7, left).
Figure 7.
Oxidized curcumin intermediates act as redox-dependent topoisomerase II poisons. Left: The effects of DTT on the enhancement of topoisomerase IIα-mediated DNA cleavage by oxidized curcumin intermediates were determined. Reaction mixtures contained DNA and human topoisomerase IIα in the absence or presence of 50 μM curcumin, 50 μM K3Fe(CN)6, or 250 μM DTT. In some reactions, DTT was added after the establishment of enzyme-DNA cleavage complexes (Post-DTT). Middle: The effects of oxidized curcumin intermediates on topoisomerase IIα activity when compounds were incubated with the enzyme prior to the addition of DNA. Human topoisomerase IIα was incubated with a combination of 50 μM curcumin and 50 μM K3Fe(CN)6 for 0-10 min prior to the addition of DNA to initiate 6 min cleavage reactions. Right: The effects of oxidized curcumin intermediates on the enhancement of DNA cleavage by human wild-type topoisomerase IIα (WT) and mutant quinone-resistant topoisomerase IIαC392A/C405A (C392A/C405A) were determined. Error bars for all three panels represent standard deviations for three independent experiments.
In the second, DTT was added to reaction mixtures after cleavage complexes had been established. As seen in Figure 7 (left), levels of DNA scission remained high. As discussed above, this finding suggests that the oxidized metabolites of curcumin form covalent topoisomerase II adducts.
In the third, curcumin was incubated with human topoisomerase IIα in the presence of K3Fe(CN)6 prior to the addition of DNA (Figure 7, middle). As predicted for redox-dependent poisons, enzyme activity fell to nearly zero with a t1/2 of 0.9 min. In contrast, the activity of topoisomerase IIα was considerably more stable when the enzyme was incubated with either curcumin (t1/2 = 10.4 min) or K3Fe(CN)6 (t1/2 = >10 min) alone prior to the addition of DNA (not shown).
In the fourth, the ability of oxidized curcumin to stimulate DNA cleavage mediated by topoisomerase IIαC392A/C405A was determined. A previous study established that quinones can adduct human topoisomerase IIα at Cys392 and Cys405 and that topoisomerase IIαC392A/C405A is partially (~2–fold) resistant to redox poisons, such as benzoquinone and PCB quinones, but not to interfacial poisons.36 As seen in Figure 7 (right), the ability of oxidized curcumin to increase DNA cleavage mediated by topoisomerase IIαC392A/C405A was ~60% that of wild-type enzyme.
Taken together, these findings provide strong evidence that the oxidized intermediates of curcumin can be classified as redox-dependent topoisomerase II poisons.
Degradation Products of Curcumin Do Not Poison Topoisomerase II
Under the conditions of the DNA cleavage assays, no significant degradation of curcumin to vanillin, ferulic acid, or feruloylmethane was observed (not shown). However, because some of these compounds display biological activity,18 the effects of vanillin, ferulic acid, and feruloylmethane on topoisomerase IIα-mediated DNA cleavage were determined. As seen in Figure 8, none of the compounds increased levels of DNA scission in the absence or presence of K3Fe(CN)6. Therefore, curcumin fragmentation products do not appear to be interfacial or redox-dependent topoisomerase II poisons.
Oxidized Turmeric Is a Topoisomerase II Poison
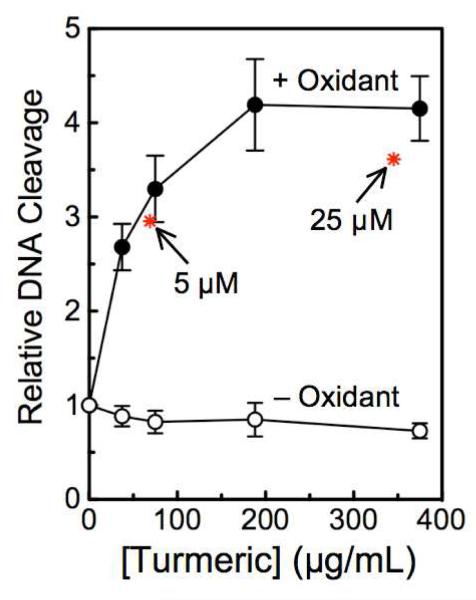
Since curcumin generally is ingested in the form of turmeric,2,3 the powdered spice was dissolved and assessed for its ability to stimulate DNA cleavage mediated by human topoisomerase IIα (Figure 9). In the absence of an oxidant, the spice had no significant effect on enzyme-mediated DNA scission. However, when K3Fe(CN)6 was included in reactions, turmeric stimulated DNA cleavage >4–fold at concentrations of 200-400 μg/mL. As determined by high-performance liquid chromatography, the turmeric solution contained ~2.7% curcumin. On the basis of this value, DNA cleavage data for 5 and 25 μM curcumin [+K3Fe(CN)6] were overlaid for comparison (asterisks). As seen in Figure 9, there is a strong correlation between the activities of turmeric and curcumin. Therefore, even within the more complex spice formulation, oxidized curcumin appears to function as a topoisomerase II poison.
Figure 9.
Effects of turmeric on topoisomerase II-mediated DNA cleavage. The effects of turmeric on the cleavage of negatively supercoiled plasmid DNA by human topoisomerase IIα were determined in the absence (open circles, – Oxidant) or presence (closed circles, + Oxidant) of 50 μM K3Fe(CN)6. The turmeric stock solution was determined to contain ~2.7% curcumin. On the basis of this concentration, DNA cleavage results for 5 and 25 μM oxidized curcumin intermediates (asterisks) are overlaid at the associated turmeric concentrations for comparison. Error bars represent standard deviations for three independent experiments.
Conclusions
Oxidized metabolites of curcumin, even in a solution of turmeric, are redox-dependent poisons of human type II topoisomerases. In contrast, degradation products of the parent compound do not affect topoisomerase II-mediated DNA cleavage.
Curcumin displays a number of medically relevant biological properties, including antioxidant, anti-inflammatory, antibacterial, and chemopreventive activities.4-6,9 The compound also is in cancer chemotherapy trials.7,8 A number of chemopreventive natural products (including genistein and EGCG) and several highly successful anticancer drugs (including etoposide and doxorubicin) are potent topoisomerase II poisons.20-23,27,29-31,43 Coupled with the findings that 1) curcumin enhances DNA cleavage mediated by topoisomerase IIα and IIβ in cultured human cells and 2) cleavage enhancement is abrogated by antioxidant treatment,32 the above findings suggest that at least some of the anticancer activities of curcumin may be mediated by the effects of its oxidized metabolites on the type II topoisomerases.
ACKNOWLEDGEMENTS
We are grateful to Katie J. Aldred, R. Hunter Lindsey, and MaryJean Campbell for critical reading of the manuscript.
Footnotes
This work was supported by National Institutes of Health research grant GM33944 (to N.O.) and by pilot awards from the Vanderbilt Institute of Chemical Biology, the Vanderbilt DDRC (P30DK058404), and the NCI SPORE in GI Cancer (CA095103) (to C.S.). O.N.G. was supported by F31 AT007287 and training grant T32 GM07628 from the National Institutes of Health. A.C.K. was supported by training grant T32 GM065086 from the National Institutes of Health.
REFERENCES
- 1.Jaffrey M. Madhur Jaffrey’s spice kitchenfifty recipies introducing Indian spices and aromatic seeds. Clarkson Potter; New York: 1994. [Google Scholar]
- 2.Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “Curecumin”: from kitchen to clinic. Biochem. Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 3.Gupta SC, Sung B, Kim JH, Prasad S, Li S, Aggarwal BB. Multitargeting by turmeric, the golden spice: From kitchen to clinic. Mol. Nutr. Food Res. 2012 doi: 10.1002/mnfr.201100741. Epub. Aug. 13 2012. [DOI] [PubMed] [Google Scholar]
- 4.Satoskar RR, Shah SJ, Shenoy SG. Evaluation of anti-inflammatory property of curcumin (diferuloyl methane) in patients with postoperative inflammation. Int. J. Clin. Pharmacol. Ther. Toxicol. 1986;24:651–654. [PubMed] [Google Scholar]
- 5.Mahady GB, Pendland SL, Yun G, Lu ZZ. Turmeric (Curcuma longa) and curcumin inhibit the growth of Helicobacter pylori, a group 1 carcinogen. Anticancer Res. 2002;22:4179–4181. [PubMed] [Google Scholar]
- 6.Sharma RA, McLelland HR, Hill KA, Ireson CR, Euden SA, Manson MM, Pirmohamed M, Marnett LJ, Gescher AJ, Steward WP. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin. Cancer Res. 2001;7:1894–1900. [PubMed] [Google Scholar]
- 7.Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: from ancient medicine to current clinical trials. Cell. Mol. Life Sci. 2008;65:1631–1652. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, Ng CS, Badmaev V, Kurzrock R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 2008;14:4491–4499. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- 9.Patel VB, Misra S, Patel BB, Majumdar AP. Colorectal cancer: chemopreventive role of curcumin and resveratrol. Nutr. Cancer. 2010;62:958–967. doi: 10.1080/01635581.2010.510259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tonnesen HH, Karlsen J. Studies on curcumin and curcuminoids. VI. Kinetics of curcumin degradation in aqueous solution. Z. Lebensm. Unters. Forsch. 1985;180:402–404. doi: 10.1007/BF01027775. [DOI] [PubMed] [Google Scholar]
- 11.Wang YJ, Pan MH, Cheng AL, Lin LI, Ho YS, Hsieh CY, Lin JK. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997;15:1867–1876. doi: 10.1016/s0731-7085(96)02024-9. [DOI] [PubMed] [Google Scholar]
- 12.Pfeiffer E, Heoehle SI, Solyom AM, Metzler M. Studies on the stability of turmeric constituents. J. Food Engin. 2003;56:257–259. [Google Scholar]
- 13.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol. Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 14.Griesser M, Pistis V, Suzuki T, Tejera N, Pratt DA, Schneider C. Autoxidative and cyclooxygenase-2 catalyzed transformation of the dietary chemopreventive agent curcumin. J. Biol. Chem. 2011;286:1114–1124. doi: 10.1074/jbc.M110.178806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ireson C, Orr S, Jones DJ, Verschoyle R, Lim CK, Luo JL, Howells L, Plummer S, Jukes R, Williams M, Steward WP, Gescher A. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol esterinduced prostaglandin E2 production. Cancer Res. 2001;61:1058–1064. [PubMed] [Google Scholar]
- 16.Anand P, Thomas SG, Kunnumakkara AB, Sundaram C, Harikumar KB, Sung B, Tharakan ST, Misra K, Priyadarsini IK, Rajasekharan KN, Aggarwal BB. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem. Pharmacol. 2008;76:1590–1611. doi: 10.1016/j.bcp.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Shen L, Ji HF. Contribution of degradation products to the anticancer activity of curcumin. Clin. Cancer Res. 2009;15:7108. doi: 10.1158/1078-0432.CCR-09-1749. [DOI] [PubMed] [Google Scholar]
- 18.Shen L, Ji HF. The pharmacology of curcumin: is it the degradation products? Trends Mol. Med. 2012;18:138–144. doi: 10.1016/j.molmed.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Gordon ON, Schneider C. Vanillin and ferulic acid: not the major degradation products of curcumin. Trends Mol. Med. 2012;18:361–363. doi: 10.1016/j.molmed.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deweese JE, Osheroff N. The DNA cleavage reaction of topoisomerase II: wolf in sheep’s clothing. Nucleic Acids Res. 2009;37:738–748. doi: 10.1093/nar/gkn937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Austin CA, Patel S, Ono K, Nakane H, Fisher LM. Site-specific DNA cleavage by mammalian DNA topoisomerase II induced by novel flavone and catechin derivatives. Biochem. J. 1992;282(Pt 3):883–889. doi: 10.1042/bj2820883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bandele OJ, Osheroff N. Bioflavonoids as poisons of human topoisomerase IIα and IIβ. Biochemistry. 2007;46:6097–6108. doi: 10.1021/bi7000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bandele OJ, Osheroff N. (−)-Epigallocatechin gallate, a major constituent of green tea, poisons human type II topoisomerases. Chem. Res. Toxicol. 2008;21:936–943. doi: 10.1021/tx700434v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sang S, Hou Z, Lambert JD, Yang CS. Redox properties of tea polyphenols and related biological activities. Antioxid. Redox Signal. 2005;7:1704–1714. doi: 10.1089/ars.2005.7.1704. [DOI] [PubMed] [Google Scholar]
- 25.Isbrucker RA, Edwards JA, Wolz E, Davidovich A, Bausch J. Safety studies on epigallocatechin gallate (EGCG) preparations. Part 2: dermal, acute and shortterm toxicity studies. Food Chem. Toxicol. 2006;44:636–650. doi: 10.1016/j.fct.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Yang CS, Lambert JD, Ju J, Lu G, Sang S. Tea and cancer prevention: molecular mechanisms and human relevance. Toxicol. Appl. Pharmacol. 2007;224:265–273. doi: 10.1016/j.taap.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Champoux JJ. DNA topisomerases: structure, function, and mechanism. Annu. Rev. Biochem. 2001;70:369–413. doi: 10.1146/annurev.biochem.70.1.369. [DOI] [PubMed] [Google Scholar]
- 28.Nitiss JL. DNA topoisomerase II and its growing repertoire of biological functions. Nat. Rev. Cancer. 2009;9:327–337. doi: 10.1038/nrc2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pommier Y, Leo E, Zhang H, Marchand C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol. 2010;17:421–433. doi: 10.1016/j.chembiol.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vos SM, Tretter EM, Schmidt BH, Berger JM. All tangled up: how cells direct, manage and exploit topoisomerase function. Nat. Rev. Mol. Cell Biol. 2011;12:827–841. doi: 10.1038/nrm3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nitiss JL. Targeting DNA topoisomerase II in cancer chemotherapy. Nat. Rev. Cancer. 2009;9:338–350. doi: 10.1038/nrc2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez-Lazaro M, Willmore E, Jobson A, Gilroy KL, Curtis H, Padget K, Austin CA. Curcumin induces high levels of topoisomerase I- and II-DNA complexes in K562 leukemia cells. J. Nat. Prod. 2007;70:1884–1888. doi: 10.1021/np070332i. [DOI] [PubMed] [Google Scholar]
- 33.Martin-Cordero C, Lopez-Lazaro M, Galvez M, Ayuso MJ. Curcumin as a DNA topoisomerase II poison. J. Enzyme Inhib. Med. Chem. 2003;18:505–509. doi: 10.1080/14756360310001613085. [DOI] [PubMed] [Google Scholar]
- 34.Worland ST, Wang JC. Inducible overexpression, purification, and active site mapping of DNA topoisomerase II from the yeast Saccharomyces cerevisiae. J. Biol. Chem. 1989;264:4412–4416. [PubMed] [Google Scholar]
- 35.Kingma PS, Greider CA, Osheroff N. Spontaneous DNA lesions poison human topoisomerase IIα and stimulate cleavage proximal to leukemic 11q23 chromosomal breakpoints. Biochemistry. 1997;36:5934–5939. doi: 10.1021/bi970507v. [DOI] [PubMed] [Google Scholar]
- 36.Bender RP, Ham AJ, Osheroff N. Quinone-induced enhancement of DNA cleavage by human topoisomerase IIα: adduction of cysteine residues 392 and 405. Biochemistry. 2007;46:2856–2864. doi: 10.1021/bi062017l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fortune JM, Osheroff N. Merbarone inhibits the catalytic activity of human topoisomerase IIα by blocking DNA cleavage. J. Biol. Chem. 1998;273:17643–17650. doi: 10.1074/jbc.273.28.17643. [DOI] [PubMed] [Google Scholar]
- 38.Lindsey RH, Jr., Bromberg KD, Felix CA, Osheroff N. 1,4-Benzoquinone is a topoisomerase II poison. Biochemistry. 2004;43:7563–7574. doi: 10.1021/bi049756r. [DOI] [PubMed] [Google Scholar]
- 39.Bender RP, Lindsey RH, Jr., Burden DA, Osheroff N. N-acetyl-p-benzoquinone imine, the toxic metabolite of acetaminophen, is a topoisomerase II poison. Biochemistry. 2004;43:3731–3739. doi: 10.1021/bi036107r. [DOI] [PubMed] [Google Scholar]
- 40.Lindsey RH, Bender RP, Osheroff N. Stimulation of topoisomerase II-mediated DNA cleavage by benzene metabolites. Chem. Biol. Interact. 2005;153-154:197–205. doi: 10.1016/j.cbi.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 41.Bender RP, Lehmler HJ, Robertson LW, Ludewig G, Osheroff N. Polychlorinated biphenyl quinone metabolites poison human topoisomerase IIα: altering enzyme function by blocking the N-terminal protein gate. Biochemistry. 2006;45:10140–10152. doi: 10.1021/bi0524666. [DOI] [PubMed] [Google Scholar]
- 42.Pommier Y, Marchand C. Interfacial inhibitors: targeting macromolecular complexes. Nat. Rev. Drug Discov. 2012;11:25–36. doi: 10.1038/nrd3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bandele OJ, Clawson SJ, Osheroff N. Dietary polyphenols as topoisomerase II poisons: B ring and C ring substituents determine the mechanism of enzyme-mediated DNA cleavage enhancement. Chem. Res. Toxicol. 2008;21:1253–1260. doi: 10.1021/tx8000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang H, Mao Y, Chen AY, Zhou N, LaVoie EJ, Liu LF. Stimulation of topoisomerase II-mediated DNA damage via a mechanism involving protein thiolation. Biochemistry. 2001;40:3316–3323. doi: 10.1021/bi002786j. [DOI] [PubMed] [Google Scholar]
- 45.Bender RP, Osheroff N. Mutation of cysteine residue 455 to alanine in human topoisomerase IIα confers hypersensitivity to quinones: enhancing DNA scission by closing the N-terminal protein gate. Chem. Res. Toxicol. 2007;20:975–981. doi: 10.1021/tx700062t. [DOI] [PMC free article] [PubMed] [Google Scholar]