Abstract
Varieties of genetic tests are currently available for the domestic cat that support veterinary health care, breed management, species identification, and forensic investigations. Approximately thirty-five genes contain over fifty mutations that cause feline health problems or alterations in the cat’s appearance. Specific genes, such as sweet and drug receptors, have been knocked-out of Felidae during evolution and can be used along with mtDNA markers for species identification. Both STR and SNP panels differentiate cat race, breed, and individual identity, as well as gender-specific markers to determine sex of an individual. Cat genetic tests are common offerings for commercial laboratories, allowing both the veterinary clinician and the private owner to obtain DNA test results. This article will review the genetic tests for the domestic cat, and their various applications in different fields of science. Highlighted are genetic tests specific to the individual cat, which are a part of the cat’s genome.
Keywords: Domestic cat, Feline, Genetic testing, Identification, Mutations, Parentage
1. Introduction
Genetic testing has been available in the domestic cat since the 1960’s, but as like other species, over the past 50 years, the level of resolution has improved from the chromosome level to the sequence level. Knowing the direct causative mutation for a trait or disease assist cat breeders with the breeding programs and can help clinicians determine heritable presentations versus idiopathic versions of a health concern. Genetic tests cover all the various forms of DNA variants, including chromosomal abnormalities, mtDNA variation, gene loss, translocations, large inversions, small insertions and deletions and the simple nucleotide substitutions. Higher throughput technologies have made genetic testing cheaper, simpler and faster, thereby making cat genetic testing affordable to the lay public and small animal practice clinicians. The genetic resources for cats and other animal species have also opened the doors for animal evidence to be supportive in criminal investigations. This review will highlight the various tests available for the domestic cat and their specific capabilities and role’s in cat health and management.
2. Domestic cat genetic testing
2.1. Cytogenetic testing
Some of the earliest genetic testing for any species was the examination of the chromosomes to determine the presence of the normal and complete genomic complement. Early studies of mitotic chromosomes of the domestic cat revealed an easily distinguishable karyotype consisting of 18 autosomal chromosomes and the XY sex chromosome pair, resulting in a 2N complement of 38 chromosomes for the cat genome [1]. Cat chromosomes are clearly defined by size; centromere position; distinctive giemsa banding patterns of the short (p) and long (q) arms of each chromosome; and the presence of only a few small acrocentric chromosomes. Various cytogenetic techniques, such as R-, RBG-banding and fragile site studies, have also helped distinguish and characterize the cat chromosomes [2–5]. Although a sequential numbering of the chromosomes has been suggested [6], the historical classification of chromosomes into morphologic groups has been retained in the cat. Hence cats have three large metacentric chromosomes (A1 to A3), four large subtelomeric chromosomes (B1 to B4), two medium-size metacentrics (C1 to C2), four small subtelomerics (D1 to D4), three small metacentrics (E1 to E3), and two small acrocentrics (F1 and F2). The X chromosome is midsize and subtelomeric, similar to chromosome B4.
Although the cat genome is conserved to humans, certain well-known chromosomal abnormalities are not found. For example, cats do not have a significant fragile X site on the X chromosome that is associated with mental retardation [5]. An analog to Down’s syndrome is not present in the cat since the genes found on human chromosome 21 are represented on the mid-sized metacenteric chromosome C2, which also has genes from human chromosome 3. However, Turner’s Syndrome (XO), Klinefelter’s Syndrome (XXY) and chimerism has been documented in the domestic cat. Sex chromosome aneuploidies and trisomies of small acrocentric chromosomes were typically associated with cases of decreased fertility and syndromes that displayed distinct morphological presentations. Because cat has a highly recognizable X-linked trait [7–10], Orange, and the X-inactivation process was recognized [11], tortoiseshell and calico male cats were the first feline suspects of chromosomal abnormalities, particularly sex chromosome aberrations. Karyotypic and now gene-based assays are common methods to determine if a cat with ambiguous genitalia [12] or a poor reproductive history has a chromosomal abnormality. Karyotypic studies of male tortoiseshell cats have shown that they are often mosaics, or chimeras, being XX/XY in all or some tissues [10,13–20]. The minor chromosomal differences that are cytogenetically detectable between a domestic cat and an Asian leopard cat are likely the cause of fertility problems in the Bengal cat breed, which is a hybrid between these two species [21]. Other significant chromosomal abnormalities causing common “syndromes” are not well documented in the cat. Several research and commercial laboratories can perform cat chromosomal analyses when provided a living tissue, such as a fibroblast biopsy or whole blood for the analysis of white blood cells.
2.2. Inherited disease tests
The candidate gene approach has been fruitful in domestic cat investigations for the identification of many diseases and trait mutations. The first mutations identified were for a gangliosidosis and muscular dystrophy, discovered in the early and mid-1990’s [22,23], as these diseases have well defined phenotypes and known genes with mutations that were as found in humans. Most of the common diseases, coat colors, and coat types were deciphered in the cat following the same candidate gene approach. To date, other than the muscular dystrophy mutation [22], all other mutations in the cat are autosomal.
Most of the identified disease tests in cats that are very specific to breeds and populations are available as commercial genetic tests (Table 1). Typically, diseases are identified in cat breeds, which are a small percentage of the cat population of the world, perhaps at most 10–15% in the USA [24]. However, some mutations that were found in a specific breed, such as mucopolysaccharidosis in the Siamese [25,26], were found in a specific individual and the mutation is not of significant prevalence in the breed (Table 2). These genetic mutations should not be part of routine screening by cat breeders and registries, but clinicians should know that genetic tests are available for diagnostic purposes, especially from research groups with specialized expertise, such as at the University of Pennsylvania (http://research.vet.upenn.edu/penngen). Other diseases, such as polycystic kidney disease (PKD), are prevalent, PKD in Persians is estimated at 30–38% worldwide [27–29]. Because of cross breeding with Persians, many other breeds, such as British Shorthairs, American Shorthairs, Selkirk Rex, and Scottish Folds, also need to be screened for PKD [30–32]. As PKD testing begins to become less common, as breeders remove positive cats, other genetic tests are becoming more popular, such as coat color and other disease traits (Fig. 1).
Table 1.
Common commercialized DNA tests for domestic cats.
| Disease/trait (alleles) | MOIb | Phenotype | Breeds | Gene | Mutationd |
|---|---|---|---|---|---|
| Agouti (A, a) [76] | AR | Banded fur to solid | All breeds | ASIP | c.122_123delCA |
| Amber (E, e) [77] | AR | Brown color variant | Norwegian forest | MC1R | c.250G > A |
| Brown (B, b, bl) [78,79] | AR | Brown, light brown color variants | All breeds | TYRP1 | b = c.8C > G, bl = c.298C > T |
| Color (C, cb, cs, c) [79–81] | AR | Burmese, Siamese color pattern, full albino | All breeds | TYR | Cb = c.715G > T, Cs = c.940G > A, c = c.975delC |
| Dilution (D, d) [40] | AR | Black to grey/blue, Orange to cream | All breeds | MLPH | c.83delT |
| Gloves (G, g) [48] | AR | White feet | Birman | KIT | (Submitted) |
| Hairless (Hr, hr) [52] | AR | Atrichia | Sphynx | KRT71 | c.816 + 1G > A |
| Long fur (L, l) [49,50] | AR | Long fur | All breedsc | FGF5 | c.356_367insT, c.406C > T, c.474delT, c.475A > C |
| Rexing (R, r) | AR | Curly hair coat | Cornish Rex | PYP2R5 | (Submitted) |
| Rexing (Re, re) [52] | AR | Curly hair coat | Devon Rex | KRT71 | c.1108-4_1184del, c.1184_1185ins AGTTGGAG, c.1196insT |
| AB blood type (A, b) [39] | AR | Determines type B | All breeds | CMAH | c.1del-53_70, c.139G > A |
| Gangliosidosis 1 [82] | AR | Lipid storage disorder | Korat, Siamese | GBL1 | c.1457G > C |
| Gangliosidosis 2 [83] | AR | Lipid storage disorder | Burmese | HEXB | c.1356del-1_8, c.1356_1362delGTTCTCA |
| Gangliosidosis 2 [23] | AR | Lipid storage disorder | Korat | HEXB | c.39delC |
| Glycogen storage dis. IV [92] | AR | Glycogen storage disorder | Norwegian forest | GBE1 | IVS11 + 1552_IVS12-1339 del6.2 kb ins334 bp |
| Hypertrophic cardiomyopathy [34] | AD | Cardiac disease | Maine Coon | MYBPC | c.93G > C |
| Hypertrophic cardiomyopathy [85] | AD | Cardiac disease | Ragdoll | MYBPC | c.2460C > T |
| Hypokalemia | AR | Potassium deficiency | Burmese | WNK4 | (Submitted) |
| Progressive retinal atropy [86] | AR | Late onset blindness | Abyssinian | CEP290 | IVS50 + 9T > G |
| Progressive retinal atropy [87] | AD | Early onset blindness | Abyssinian | CRX | c.546delC |
| Polycystic Kidney disease [32] | AD | Kidney cysts | Persian | PKD1 | c.10063C > A |
| Pyruvate kinase def.a | AR | Hemopathy | Several | PKLR | c.693 + 304G > A |
| Spinal muscular atrophy [88] | AR | Muscular atrophy | Maine Coon | LIX1-LNPEP | Gene deletion |
Unpublished test, presented only as abstract, paper submitted.
Mode of inheritance of the non-wildtype variant.
Long fur variants are more or less common depending on the breed.
Not all transcripts for a given gene may have been discovered or well documented in the cat, mutations presented as interpreted from original publication.
Table 2.
Other mutations for Inherited domestic cat diseases.a
| Disease | Gene | Mutationb | Disease | Gene | Mutationb |
|---|---|---|---|---|---|
| Gangliosidosis 2 [89] | HEXB | c.1467_1491inv | Mucopolysaccharidosis VI [26] | ARSB | c.1427T > C |
| Gangliosidosis 2 [90] | HEXB | c.667C > T | Mucopolysaccharidosis VI [25,91] | ARSB | c.1558G > A |
| Gangliosidosis 2 [84] | GM2A | c.390_393GGTC | Mucopolysaccharidosis VII [92] | GUSB | c.1052A > G |
| Hemophilia B [93] | F9 | c.247G > A | Niemann-Pick C [94] | NPC | c.2864G > C |
| Hemophilia B [93] | F9 | c.1014C > T | Polydactyla [95] | SHH | c.479A > G |
| Hyperoxaluria [96] | GRHPR | G > A I4 acceptor site | Polydactyla [95] | SHH | c.257G > C, c.481A > T |
| Lipoprotein lipase def. [97] | LPL | c.1234G > A | Porphyria (congenital erythropoietic) [98] | UROS | c.140C > T, c.331G > A |
| Mannosidosis, alpha [99] | LAMAN | c.1748_1751delCCAG | Porphyria (acute intermittent) [100] | HMBS | c.842_844delGAG, c.189dupT, c.250G > A, c.445C > T |
| Mucolipidosis II [101] | GNPTA | c.2655C > T | Vitamin D resistant rickets [102] | CYP27B1 | c.223G > A, c.731delG |
| Mucopolysaccharidosis I [103] | IDUA | c. 1107_1109delCGA or c. 1108_1110GAC | Vitamin D resistant rickets [104] | CYP27B1 | c.637G > T |
The presented conditions are not prevalent in breeds or populations but may have been established into research colonies.
Not all transcripts for a given gene may have been discovered or well documented in the cat, mutations presented as interpreted from original publication.
Fig. 1.
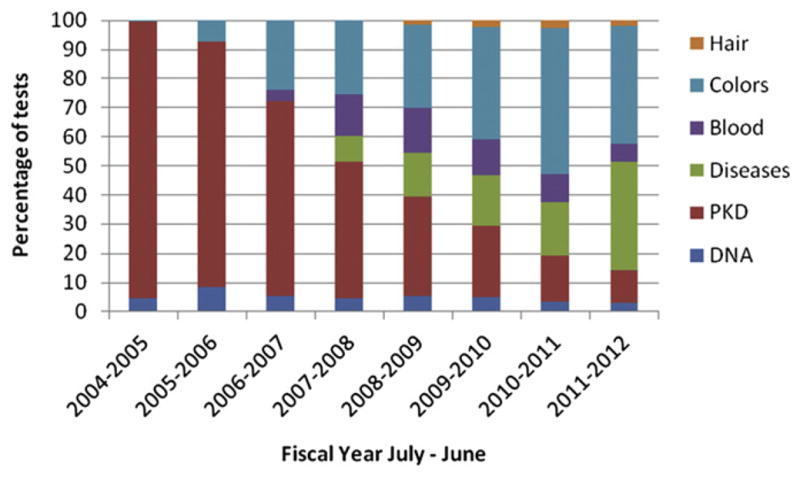
Trends of genetic testing in the domestic cat. DNA-based genetic tests are presented for the cat. Parentage and individual identification (DNA) has not increased as cats do not require testing for registration. One of the most popular tests, PKD, is presented separately to show that the testing requests are decreasing as breeders are eliminating positive cats from breeding programs. Other disease tests and color tests are becoming more popular tests in the cat market. Data from UC Davis Veterinary Genetics Laboratory.
To date, most cat genetic tests have been for traits that have nearly complete penetrance, having little variability in expression, and early onset. However, some recognized mutations in cats might be considered risk factors, predisposing an individual to health problem. Excellent examples of mutations that confer a risk in cats are the DNA variants associated with cardiac disease in cats. Hypertrophic cardiomyopathy (HCM) is a recognized genetic condition [33]. In 2005, Drs. Meurs, Kittleson and colleagues published that a DNA alteration, A31P, in the gene cardiac myosin-binding protein C 3 (MYBPC3) was strongly associated with HCM in a long-term research colony of Maine Coon cats at UC Davis [34]. The data clearly showed not all cats with the mutation had HCM and some cats with HCM did not have the DNA mutation. Age of onset, variable expression, and disease heterogeneity were alluded to in this report. These aspects suggested that the identified DNA variant should be considered more of a “risk factor” than a directly causative mutation. Recent studies have shown that not all Maine Coon cats with the A31P mutation get HCM [35,36] and one of those papers has mistakenly interpreted this lack of penetrance as being evidence that the A31P mutation is not causal [36]. This interpretation is misleading, causing debate as to the validity of the Maine Coon HCM test. As true in humans with cardiac disease, the finding that not all cats with the A31P mutation in MYBPC3 get HCM is actually usual in the field of HCM genetic testing.
Like cat HCM mutations, other disease mutations have shown variation in penetrance and expression. The CEP290 PRA mutation in Abyssinians has a late age of onset and some cats with subclinical disease have been identified [37]. Some cats with the pyruvate kinase deficiency can have very mild and subclinical presentations [38]. Thus, disease or trait causing mutations may not be 100% penetrant, thus, they do not always cause clinically detectable disease.
Cats are one of the few species to have their blood type genetic mutation determined [39]. Blood type incompatibilities can lead to transfusion reactions and neonatal isoerythrolysis for the cat, but inherently this characteristic is not necessarily a disease. A point mutation and an 18 base pair deletion have both been implicated in the gene CMAH as indicating the presence of the B blood type, or a B blood type carrier. Because both mutations are on the same allele, a clear indication of the true causative mutation could not be determined. Thus, both mutations should be examined in cats to genetically determine blood type at the current time.
Genome-wide association studies are now feasible for domestic cats and their breeds due to the recent development and release of an Illumina 63K Inflnium feline iSelect DNA array. With this resource, the localization of disease and phenotypic traits can proceed via case – control or cross-breed designs, supporting rapid localization of causative loci, hopefully implicating regional candidate genes. Improvements in the cat genome sequence will also greatly facilitate mutation detection. Therefore, genetic tests for simple disease traits should develop more rapidly for the cat in the future. The genome arrays should also assist with genetic studies on traits that may already have known mutations. As found in many species, specific presentations, such as PKD or HCM, can be caused by different genes and mutations in different and even the same populations, known as disease heterogeneity. The current Maine Coon HCM mutation does not account for all Maine Coons with HCM and many other breeds have HCM and not the Maine Coon or Ragdoll mutations. Likewise, many forms of PKD in humans exist. Thus in cats, there would be no reason why a second mutation in a different gene could cause PKD in another breed or as a different form in Persians. The DNA arrays should help clarify disease heterogeneity problems.
2.3. Phenotypic trait tests
The coat color mutations are common to all cats and are effective for genetic typing in all breeds and populations. Six different genes with nine different mutations affect the coat colors of cats, controlled by the common mammalian loci Agouti (A), Brown (B), Color (C), Dilute (D), and Extension (E) (Table 1). Most of the mutations have been identified by candidate gene approaches, however, the Dilute locus [40], required support from mapping approaches. Several other cat coat color or pattern loci have been localized, such as Tabby (T) [41,42], Ticked (Ti) [41,42], Inhibitor (I) [43,44], Spotting (S) [45], and Orange (O) [46,47], therefore the causative mutations should be forthcoming. The White (W) and Spotting (S) loci are likely to be complex with various alleles, as in other species and as demonstrated in initial studies of white feet, Gloves, in cats [48].
The common locus for long hair in mammals, Long (L), which is controlled by fibroblast growth factor 5, FGF5, is also the major factor for cat hair length [49,50]. However, even though long fur is common in breeds and random bred cats, long fur genetic testing is an exception because four different mutations in FGF5 can cause a cat to have long fur. One mutation, c.475A > C, is common to most all breeds and populations, suggesting this mutation to be the most ancient mutation, but the others are more specific to particular breeds [51]. A second mutation, c.365insT, is highly prevalent in the Ragdoll breed and a third, c.406C > T, is rare among pedigreed breeds but is highly prevalent in Norwegian Forest cats. The additional mutation, c.474delT, was noted in various breeds. Thus, to determine accurately if a cat carries a mutation for long fur, all four mutations must be genotyped.
Additional hair type mutations have also been identified in cats, including nakedness or Hairless (Hr) of the Sphynx [52]. The keratin 71 (KRT71) gene has a complex mutation that causes the rexoid – curly coat of the Devon Rex, which is one of the oldest curly coated breeds [52]. Although listed originally listed as different loci then later considered allelic, hairless and Devon curly are alleles within KRT71, the hairless mutation is recessive but dominant to curly [53]. Several other rexoid-curly coated cats are documented, another historical breed, the Cornish Rex has proven by breeding to be non-allelic to Devon Rex. The first genome-wide association study of the cat has led to the identification of the causative gene and mutation for this rexoid mutation (LA Lyons, personal communication).
2.4. Genetic testing concerns in hybrid cat breeds
Several cat breeds were formed by crossing with different species of cats. The Bengal breed is acknowledged worldwide and has become a highly popular breed. To create Bengals, Asian leopard cats (Felis bengalensis) were and are bred with domestic cat breds like Egyptian Mau, Abyssinian and other cats to form a very unique breed in both color and temperament [54]. The breed termed Chaussie is developing from crosses of domestic cats with Jungle cats (Felis chaus) and the breed termed Savannahs are from crosses with domestic cats and Servals (Felis serval). Bobcat (Lynx rufus) hybrids with domestic cats have not been genetically proven, to date. An Asian leopard cat had a common ancestor with the domestic cat about 6 million years ago, the bobcat about 8 million years ago, the Serval about 9.5 million years ago [55]. The Jungle cat is more closely related to a domestic cat than the leopard cat to the domestic cat. In addition, for some of these wild felid species, different sub-species were incorporated into the breed. The DNA sequence between a domestic cat and one of these wild felid species will have many genetic differences, less for the Jungle cat, more for Serval as compared to a domestic cat. The genetic differences are most likely silent mutations, but, the variation will interplay with genetic assays and may cause more allelic drop-out than what would be normally anticipated. No genetic tests are validated in the hybrid cats breeds, although the tests are typically used very frequently in Bengal cats. Thus, the accuracy for any genetic test is not known for hybrid cat breeds.
2.5. Species identification
Many markers in the genome can delineate species differences due to high mutation rates. However, historically, the mtDNA genome has been a more simple locus to explore and characterize between species, especially since the mutation rates of the control region and the coding genes tend to be higher than the nuclear genes on chromosomes [56]. Restriction fragment length polymorphism analysis initially described species [57], followed by control region length and coding gene variation [58,59]. Recent studies have furthered the development and analysis of universal primers to distinguish common mammalian species [60–62]. Specifically in cats, the 12S rRNA and cytochrome B genes [63–65], as well as the 16S rRNA genes can be amplified with universal PCR primers to distinguished Felidae [66].
The mtDNA control region has also been analyzed in detail to distinguish individual cats. A study considering 1394 cats, including cats from 25 distinct worldwide populations and 26 breeds determined twelve major mitotypes represented 83% of cats [67,68]. An additional 8.0% of cats are clearly derived from the major mitotypes. Unique mitotypes were found in 7.5% of the cats. The overall genetic diversity for this data set was 0.8813 ± 0.0046 with a random match probability of 11.8%. This region of the cat mtDNA has discriminatory power suitable for forensic application worldwide.
Now that full genome sequences are developing for many species, direct comparisons of genes, including their presence or absence, can be analyzed across species. Recent studies have shown cats to be lacking two common genes, a sweet receptor [69,70] and UDP-glucuronosyltransferase (UGT) 1A6, an enzyme important for drug metabolism [71]. Closer cross-species comparisons of genomes will likely unveil many more species-specific differences that can lead to class, order, family, species and sub-species diagnostics.
2.6. Parentage and individual identification
DNA-based parentage and individual identification typing methods have evolved over the years from restriction fragment length polymorphisms (RFLPs) and multi-locus probes, such as variable number tandem repeat (VNTRs), to single locus assays and short tandem repeat (STR) typing. DNA-based genetic testing is used for most domesticated animals to confirm identity and parentage, particularly to validate their registries. The domestic cat is one of the leading household pets, but parentage and identification testing lags for this species since no cat registry requires parentage validation.
Standardized genetic tests are important for sharing information, combining datasets and assisting with population management. Peer-review, research collaborations, and forums and comparison tests hosted by the International Society of Animal Genetics (ISAG) allow both formal and informal oversight of parentage test development in domesticated species. Under the auspices of ISAG, a parentage comparison test was performed among 17 worldwide commercial and research laboratories to identify and to validate a microsatellite-based profiling panel for parentage and identification in the domestic cat [72]. Nine of the 19 markers, plus two additional gender markers [107], were found to be sufficient for parentage, gender determination, and identification testing of random bred, purebred and several wild felid species (Table 3). However, as more breeds are evaluated and as the tests become more popular, additional panels of markers are being developed for the cat under the auspice of ISAG.
Table 3.
Genetic markers selected as a “core” panel for ISAG cat parentage and identification testing.
| Marker | Chr. | Repeat | Forward primer 5′–3′
|
Label | uM | PE (min–max) (breeds) | PE (min–max) (random) |
|---|---|---|---|---|---|---|---|
| Reverse primer 5′–3′ | |||||||
| FCA069 | B4 | AC | AATCACTCATGCACGAATGC | VIC | 0.20 | 0.1324–0.5336 | 0.3958–0.5948 |
| AATTTAACGTTAGGCTTTTTGCC | |||||||
| FCA075 | E2 | TG | ATGCTAATCAGTGGCATTTGG | NED | 0.10 | 0.1442–0.5771 | 0.4240–0.5992 |
| GAACAAAAATTCCAGACGTGC | |||||||
| FCA105 | A2 | TG | TTGACCCTCATACCTTCTTTGG | PET | 0.20 | 0.2221–0.5585 | 0.6110–0.7101 |
| TGGGAGAATAAATTTGCAAAGC | |||||||
| FCA149a | B1 | TG | CCTATCAAAGTTCTCACCAAATCA | PET | 0.18 | 0.1783–0.5995 | 0.3586–0.5767 |
| GTCTCACCATGTGTGGGATG | |||||||
| FCA220 | F2 | CA | CGATGGAAATTGTATCCATGG | FAM | 0.30 | 0.0000–0.3383 | 0.1851–0.4221 |
| GAATGAAGGCAGTCACAAACTG | |||||||
| FCA229 | A1 | GT | CAAACTGACAAGCTTAGAGGGC | NED | 0.25 | 0.0452–0.5131 | 0.3927–0.5813 |
| GCAGAAGTCCAATCTCAAAGTC | |||||||
| FCA310a | C2 | (CA)5TA(CA)7
TA(CA)8 |
TTAATTGTATCCCAAGTGGTCA | FAM | 0.30 | 0.1196–0.5256 | 0.3417–0.5611 |
| TAATGCTGCAATGTAGGGCA | |||||||
| FCA441b | D3 | TAGA | ATCGGTAGGTAGGTAGATATAG | VIC | 0.15 | 0.2061–0.5774 | 0.3388–0.5505 |
| GCTTGCTTCAAAATTTTCAC | |||||||
| FCA678d | A1 | AC | TCCCTCAGCAATCTCCAGAA | NED | 0.25 | 0.0415–0.4908 | 0.3016–0.5715 |
| GAGGGAGCTAGCTGAAATTGTT | |||||||
| AMELc | XY | – | CGAGGTAATTTTTCTGTTTACT | N/A | N/A | ||
| GAAACTGAGTCAGAGAGGC | |||||||
| ZFXYc | XY | – | AAGTTTACACAACCACCTGG | PET | 0.20 | N/A | N/A |
| CACAGAATTTACACTTGTGCA | |||||||
| Total PE | 0.9008–0.9979 | 0.9947–0.9987 |
Markers that are of the first ten published feline microsatellites [106].
A marker that is currently included in the feline forensic panel [107].
The two markers on the X and Y chromosomes were added to the panel after the comparison test [108].
Newly designed primers presented herein for FCA678 generate a product 30 bp less than originally published primers.
A second panel of STR markers was developed for forensics applications, although a large database has not been developed [105,106]. The system simultaneously amplifies 11 polymorphic tetranucleotide STR loci and one gender identifying sequence tagged site on the Y chromosome sex-determining region Y gene (SRY gene). This panel was tested following the standard 8.1.2.2 of the quality assurance standards for DNA analysis recommended by the DNA Advisory Board (DAB) [73] and recommendations made for animal DNA forensic and identity testing [74]. The overall ability for detection of mutation rate was 0.3%, heterozygosity values ranged from 0.60 to 0.82, while the average cat breed heterozygosity was 0.71, ranging from 0.57 to 0.83. Mutations were observed in seven loci (FCA733, FCA742, FCA749, F124, F53, F85, and FCA441). Null alleles were observed in three loci (FCA736, F53, and F85). Once the null alleles are addressed with potentially new primer designs, this tetra-STR-based panel could have powerful applications in forensics.
2.7. Race and breed identification
A newly developing test for the domestic cat is a race and breed identification panel. Based on the studies by Lipinski et al. (2008) [75], and Kurushima et al. (2012, submitted), STRs have been tested in a variety of random bred cats from around the world and a majority of the major cat breeds of the USA and other regions. The genetic studies have been able to differentiate eight worldwide populations of cats – races – and can distinguish the major breeds. Analyses of the present day random bred cat populations suggest that the regional populations are highly genetically distinct, hence analogous to humans, different races of cats. The regional genetic differentiation is capture and displayed within the breeds that developed later from those populations. The foundation population (race) of the Asian breeds, such as Burmese and Siamese, are the street cats of Southeast Asia, whereas the foundation population (race) of the Maine Coon and Norwegian Forest cat are Western European cats. Phenotypic markers help to delineate breeds within specific breed families, such as the Persian, Burmese, and Siamese families. The cat race and breed identification tests are similar to tests that have been developed for the dog, such as the Mars, Inc. Wisdom Panel (http://www.wisdompanel.com/). Although similar, domestic cats are random bred cats and not a concoction of pedigreed breed cats. Cat breeds developed from the random bred populations that have existed in different regions of the world for thousands of years. Therefore, the claims of the cat race and breed identification tests are different than the dog tests, not claiming that most household cats are recent offspring of pedigreed cats.
3. Conclusion
Genetic testing is an important diagnostic tool for the veterinarian, breeder, and owner. Genetic tests are not 100% foolproof and the accuracy of the test procedure and the reputation and customer service of the genetic testing laboratory needs to be considered. Some traits are highly desired and genetic testing can help breeders to more accurately determine appropriate breedings, potentially becoming more efficient breeders, thus lowering costs and excess cat production. Other traits or diseases are undesired, thus genetic testing can be used to prevent disease and potentially eradicating the concern from the population. Genetic tests for simple genetic traits are more consistent with predicting the trait or disease presentation, but, as genomics progress for the cat, more tests that confer risk will become more common. Veterinarians will have to weigh the relative risk of having a mutation versus having disease as part of their differentials and breeders will have to consider risk factors along with the other important attributes of a cat for their breeding decisions.
Acknowledgments
This project was supported by the National Institute of Health – National Center for Research Resources (NCRR) R24 RR016094, now the Office of Research Infrastructure Programs (ORIP) R24 OD010928, the Winn Feline Foundation, and the Center for Companion Animal Health, School of Veterinary Medicine, University of California, Davis. The work described in your article must have been carried out in accordance with Uniform Requirements for manuscripts submitted to Biomedical journals http://www.icmje.org.
References
- 1.Wurster-Hill DH, Gray CW. Giemsa banding patterns in the chromosomes of twelve species of cats (Felidae) Cytogenet Cell Genet. 1973;12:388–97. [PubMed] [Google Scholar]
- 2.Shibasaki Y, Flou S, Ronne M. The R-banded karyotype of Felis catus. Cytobios. 1987;51:35–47. [PubMed] [Google Scholar]
- 3.Ronne M, Storm CO. The high resolution RBG-banded karyotype of Felis catus. In Vivo. 1992;6:517–22. [PubMed] [Google Scholar]
- 4.Ronne M, Storm CO. Localization of landmarks and bands in the karyotype of Felis catus. Cytobios. 1995;81:213–22. [PubMed] [Google Scholar]
- 5.Ronne M. Localization of fragile sites in the karyotype of Felis catus. Hereditas. 1995;122:279–83. doi: 10.1111/j.1601-5223.1995.00279.x. [DOI] [PubMed] [Google Scholar]
- 6.Cho KW, Youn HY, Watari T, Tsujimoto H, Hasegawa A, Satoh H. A proposed nomenclature of the domestic cat karyotype. Cytogenet Cell Genet. 1997;79:71–8. doi: 10.1159/000134686. [DOI] [PubMed] [Google Scholar]
- 7.Bamber RC, Herdman EC. The inheritance of black, yellow and tortoiseshell coat colour in cats. J Genet. 1927;18:87–97. [Google Scholar]
- 8.Little CC. Colour inheritance in cats, with special reference to colours, black, yellow and tortoiseshell. J Genet. 1919;8:279–90. [Google Scholar]
- 9.Ibsen HL. Tricolor inheritance. Iii. Tortoiseshell cats. Genetics. 1916:1. doi: 10.1093/genetics/1.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doncaster L. On the inheritance of tortoiseshell and related colours in cats. Proc Camb Philol Soc. 1904;13:35–8. [Google Scholar]
- 11.Lyon MF. Sex chromatin and gene action in mammalian X-chromosome. Am J Hum Genet. 1962;14:135–48. [PMC free article] [PubMed] [Google Scholar]
- 12.Schlafer DH, Valentine B, Fahnestock G, Froenicke L, Grahn RA, Lyons LA, et al. A case of SRY-Positive 38, XY true hermaphroditism (XY sex reversal) in a cat. Vet Pathol. 2011;48:817–22. doi: 10.1177/0300985810382093. Epub 2010 Sep 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishihara T. Cytological studies on tortoiseshell male cats. Cytologia. 1956;21:391–8. [Google Scholar]
- 14.Chu EHY, Thuline HC, Norby DE. Triploid-diploid chimerism in a male tortoiseshell cat. Cytogenetics. 1964;3:1–18. doi: 10.1159/000129794. [DOI] [PubMed] [Google Scholar]
- 15.Thuline HC. Male tortoiseshell, chimerism and true hermaphroditism. J Cat Genet. 1964;4:2–3. [Google Scholar]
- 16.Pyle RL, Patterson DF, Hare WC, Kelly DF, Digiulio T. XXY sex chromosome constitution in a Himalayan cat with tortoise-shell points. J Hered. 1971;62:220–2. doi: 10.1093/oxfordjournals.jhered.a108154. [DOI] [PubMed] [Google Scholar]
- 17.Gregson NM, Ishmael J. Diploid triploid chimerism in three tortoiseshell cats. Res Vet Sci. 1971;12:275–9. [PubMed] [Google Scholar]
- 18.Centerwall WR, Benirschke K. Animal model for the XXY Klinefelter’s syndrome in man: tortoiseshell and calico male cats. Am J Vet Res. 1975;36:1275–80. [PubMed] [Google Scholar]
- 19.Kosowska B, Januszewski A, Tokarska M, Jach H, Zdrojewicz Z. Cytogenetic and histologic studies of tortoiseshell cats. Medycyna Weterynaryjna. 2001;57:475–9. [Google Scholar]
- 20.Kuiper H, Hewicker-Trautwein M, Distl O. Cytogenetic and histologic examination of four tortoiseshell cats. Dtsch Tierarztl Wochenschr. 2003;110:457–61. [PubMed] [Google Scholar]
- 21.Modi WS, Fanning TG, Wayne RK, O’Brien SJ. Chromosomal localization of satellite DNA sequences among 22 species of felids and canids (carnivaora) Cytogenet Cell Genet. 1988;48:208–13. doi: 10.1159/000132630. [DOI] [PubMed] [Google Scholar]
- 22.Winand NJ, Edwards M, Pradhan D, Berian CA, Cooper BJ. Deletion of the dystrophin muscle promoter in feline muscular dystrophy. Neuromuscul Disord. 1994;4:433–45. doi: 10.1016/0960-8966(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 23.Muldoon LL, Neuwelt EA, Pagel MA, Weiss DL. Characterization of the molecular defect in a feline model for type II GM2-gangliosidosis (Sandhoff disease) Am J Pathol. 1994;144:1109–18. [PMC free article] [PubMed] [Google Scholar]
- 24.Louwerens M, London CA, Pedersen NC, Lyons LA. Feline lymphoma in the post-feline leukemia virus era. J Vet Intern Med. 2005;19:329–35. doi: 10.1892/0891-6640(2005)19[329:flitpl]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 25.Yogalingam G, Hopwood JJ, Crawley A, Anson DS. Mild feline mucopoly-saccharidosis type VI. Identification of an N-acetylgalactosamine-4-sulfatase mutation causing instability and increased specific activity. J Biol Chem. 1998;273:13421–9. doi: 10.1074/jbc.273.22.13421. [DOI] [PubMed] [Google Scholar]
- 26.Yogalingam G, Litjens T, Bielicki J, Crawley AC, Muller V, Anson DS, et al. Feline mucopolysaccharidosis type VI. Characterization of recombinant N-acetylgalactosamine 4-sulfatase and identification of a mutation causing the disease. J Biol Chem. 1996;271:27259–65. doi: 10.1074/jbc.271.44.27259. [DOI] [PubMed] [Google Scholar]
- 27.Cannon MJ, MacKay AD, Barr FJ, Rudorf H, Bradley KJ, Gruffydd-Jones TJ. Prevalence of polycystic kidney disease in Persian cats in the United Kingdom. Vet Rec. 2001;149:409–11. doi: 10.1136/vr.149.14.409. [DOI] [PubMed] [Google Scholar]
- 28.Barrs VR, Gunew M, Foster SF, Beatty JA, Malik R. Prevalence of autosomal dominant polycystic kidney disease in Persian cats and related-breeds in Sydney and Brisbane. Aust Vet J. 2001;79:257–9. doi: 10.1111/j.1751-0813.2001.tb11977.x. [DOI] [PubMed] [Google Scholar]
- 29.Barthez PY, Rivier P, Begon D. Prevalence of polycystic kidney disease in Persian and Persian related cats in France. J Feline Med Surg. 2003;5:345–7. doi: 10.1016/S1098-612X(03)00052-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biller DS, Chew DJ, DiBartola SP. Polycystic kidney disease in a family of Persian cats. J Am Vet Med Assoc. 1990;196:1288–90. [PubMed] [Google Scholar]
- 31.Eaton KA, Biller DS, DiBartola SP, Radin MJ, Wellman ML. Autosomal dominant polycystic kidney disease in Persian and Persian-cross cats. Vet Pathol. 1997;34:117–26. doi: 10.1177/030098589703400204. [DOI] [PubMed] [Google Scholar]
- 32.Lyons LA, Biller DS, Erdman CA, Lipinski MJ, Young AE, Roe BA, et al. Feline polycystic kidney disease mutation identified in PKD1. J Am Soc Nephrol. 2004;15:2548–55. doi: 10.1097/01.ASN.0000141776.38527.BB. [DOI] [PubMed] [Google Scholar]
- 33.Kittleson MD, Meurs KM, Munro MJ, Kittleson JA, Liu SK, Pion PD, et al. Familial hypertrophic cardiomyopathy in maine coon cats: an animal model of human disease. Circulation. 1999;99:3172–80. doi: 10.1161/01.cir.99.24.3172. [DOI] [PubMed] [Google Scholar]
- 34.Meurs KM, Sanchez X, David RM, Bowles NE, Towbin JA, Reiser PJ, et al. A cardiac myosin binding protein C mutation in the maine coon cat with familial hypertrophic cardiomyopathy. Hum Mol Genet. 2005;14:3587–93. doi: 10.1093/hmg/ddi386. [DOI] [PubMed] [Google Scholar]
- 35.Sampedrano C, Chetboul V, Mary J, Tissier R, Abitbol M, Serres F, et al. Prospective echocardiographic and tissue Doppler Imaging screening of a population of maine coon cats tested for the A31P mutation in the myosin-binding protein C gene: a specific analysis of the heterozygous status. J Vet Intern Med. 2009;23:91–9. doi: 10.1111/j.1939-1676.2008.0218.x. [DOI] [PubMed] [Google Scholar]
- 36.Wess G, Schinner C, Weber K, Kuchenhoff H, Hartmann K. Association of A31P and A74T polymorphisms in the myosin binding protein C3 gene and hypertrophic cardiomyopathy in maine coon and other breed cats. J Vet Intern Med. 2010;24:527–32. doi: 10.1111/j.1939-1676.2010.0514.x. [DOI] [PubMed] [Google Scholar]
- 37.Menotti-Raymond M, David VA, Pflueger S, Roelke ME, Kehler J, O’Brien SJ, et al. Widespread retinal degenerative disease mutation (rdAc) discovered among a large number of popular cat breeds. Vet J. 2010;186:32–8. doi: 10.1016/j.tvjl.2009.08.010. Epub 2009 Sep 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kohn B, Fumi C. Clinical course of pyruvate kinase deficiency in Abyssinian and Somali cats. J Feline Med Surg. 2008:10. doi: 10.1016/j.jfms.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bighignoli B, Niini T, Grahn RA, Pedersen NC, Millon LV, Polli M, et al. Cytidine monophospho-N-acetylneuraminic acid hydroxylase (CMAH) mutations associated with the domestic cat AB blood group. BMC Genet. 2007;8:27. doi: 10.1186/1471-2156-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishida Y, David VA, Eizirik E, Schaffer AA, Neelam BA, Roelke ME, et al. A homozygous single-base deletion in MLPH causes the dilute coat color phenotype in the domestic cat. Genomics. 2006;88:698–705. doi: 10.1016/j.ygeno.2006.06.006. Epub 2006 Jul 24. [DOI] [PubMed] [Google Scholar]
- 41.Lyons LA, Bailey SJ, Baysac KC, Byrns G, Erdman CA, Fretwell N, et al. The Tabby cat locus maps to feline chromosome B1. Anim Genet. 2006;37:383–6. doi: 10.1111/j.1365-2052.2006.01458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eizirik E, David VA, Buckley-Beason V, Roelke ME, Schaffer AA, Hannah SS, et al. Defining and mapping mammalian coat pattern genes: multiple genomic regions implicated in domestic cat stripes and spots. Genetics. 2010;184:267–75. doi: 10.1534/genetics.109.109629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Menotti-Raymond M, David VA, Eizirik E, Roelke ME, Ghaffari H, O’Brien SJ. Mapping of the domestic cat “SILVER” coat color locus identifies a unique genomic location for silver in mammals. J Hered. 2009;100(Suppl 1):S8–13. doi: 10.1093/jhered/esp018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turner P, Robinson R. Melanin inhibitor: a dominant gene in the domestic cat. J Hered. 1980;71:427–8. doi: 10.1093/oxfordjournals.jhered.a109401. [DOI] [PubMed] [Google Scholar]
- 45.Cooper MP, Fretwell N, Bailey SJ, Lyons LA. White spotting in the domestic cat (Felis catus) maps near KIT on feline chromosome B1. Anim Genet. 2006;37:163–5. doi: 10.1111/j.1365-2052.2005.01389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmidt-Kuntzel A, Nelson G, David VA, Schaffer AA, Eizirik E, Roelke ME, et al. Linkage map and the sex-linked orange locus-mapping of orange, multiple origins, and epistasis over non-agouti. Genetics. 2009;181:1415–25. doi: 10.1534/genetics.108.095240. Epub 2009 Feb 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grahn RA, Lemesch BM, Millon LV, Matise T, Rogers QR, Morris JG, et al. Localizing the X-linked orange colour phenotype using feline resource families. Anim Genet. 2005;36:67–70. doi: 10.1111/j.1365-2052.2005.01239.x. [DOI] [PubMed] [Google Scholar]
- 48.Gandolfi B, Bach L, Beresford L, Fretwell N, Bailey S, Longeri M, et al. Off with the gloves: Mutation in KIT implicated for the unique white spotting phenotype of Birman cats. submitted. [Google Scholar]
- 49.Drogemuller C, Rufenacht S, Wichert B, Leeb T. Mutations within the FGF5 gene are associated with hair length in cats. Anim Genet. 2007;38:218–21. doi: 10.1111/j.1365-2052.2007.01590.x. [DOI] [PubMed] [Google Scholar]
- 50.Kehler JS, David VA, Schaffer AA, Bajema K, Eizirik E, Ryugo DK, et al. Four independent mutations in the feline fibroblast growth factor 5 gene determine the long-haired phenotype in domestic cats. J Hered. 2007;98:555–66. doi: 10.1093/jhered/esm072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bach L, Gandolfi B, Grahn R, Grahn J, Kurushima J, Froenicke L, et al. The distribution and possible origins of FGF5 mutations affecting fur length in cats. submitted. [Google Scholar]
- 52.Gandolfi B, Outerbridge C, Beresford L, Myers J, Pimentel M, Alhaddad H, et al. The naked truth: Sphynx and Devon rex cat breed mutations in KRT71. Mamm Genome. 2010:509–15. doi: 10.1007/s00335-010-9290-6. Epub 2010 Oct 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Robinson R. The rex mutants of the domestic cat. Genetica. 1971;42:466–8. doi: 10.1007/BF00122078. [DOI] [PubMed] [Google Scholar]
- 54.Johnson G. The Bengal cat. Greenwell Springs, LA: Gogees Cattery; 1991. [Google Scholar]
- 55.Johnson WE, Eizirik E, Pecon-Slattery J, Murphy WJ, Antunes A, Teeling E, et al. The late Miocene radiation of modern Felidae: a genetic assessment. Science. 2006;311:73–7. doi: 10.1126/science.1122277. [DOI] [PubMed] [Google Scholar]
- 56.Kocher TD, Thomas WK, Meyer A, Edwards SV, Paabo S, Villablanca FX, et al. Dynamics of mitochondrial DNA evolution in animals: amplification and sequencing with conserved primers. Proc Natl Acad Sci U S A. 1989;86:6196–200. doi: 10.1073/pnas.86.16.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown WM, George M, Jr, Wilson AC. Rapid evolution of animal mitochondrial DNA. Proc Natl Acad Sci U S A. 1979;76:1967–71. doi: 10.1073/pnas.76.4.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cann RL, Wilson AC. Length mutations in human mitochondrial DNA. Genetics. 1983;104:699–711. doi: 10.1093/genetics/104.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cann RL, Brown WM, Wilson AC. Polymorphic sites and the mechanism of evolution in human mitochondrial DNA. Genetics. 1984;106:479–99. doi: 10.1093/genetics/106.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pun KM, Albrecht C, Castella V, Fumagalli L. Species identification in mammals from mixed biological samples based on mitochondrial DNA control region length polymorphism. Electrophoresis. 2009;30:1008–14. doi: 10.1002/elps.200800365. [DOI] [PubMed] [Google Scholar]
- 61.Mitani T, Akane A, Tokiyasu T, Yoshimura S, Okii Y, Yoshida M. Identification of animal species using the partial sequences in the mitochondrial 16S rRNA gene [Tokyo] Leg Med. 2009;11(Suppl 1):S449–50. doi: 10.1016/j.legalmed.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 62.Chaves PB, Graeff VG, Lion MB, Oliveira LR, Eizirik E. DNA barcoding meets molecular scatology: short mtDNA sequences for standardized species assignment of carnivore noninvasive samples. Mol Ecol Resour. 2012;12:18–35. doi: 10.1111/j.1755-0998.2011.03056.x. Epub 2011 Aug 31. [DOI] [PubMed] [Google Scholar]
- 63.Masuda R, Lopez JV, Slattery JP, Yuhki N, O’Brien SJ. Molecular phylogeny of mitochondrial cytochrome b and 12S rRNA sequences in the Felidae: ocelot and domestic cat lineages. Mol Phylogenet Evol. 1996;6:351–65. doi: 10.1006/mpev.1996.0085. [DOI] [PubMed] [Google Scholar]
- 64.Janczewski DN, Yuhki N, Gilbert DA, Jefferson GT, O’Brien SJ. Molecular phylogenetic inference from saber-toothed cat fossils of Rancho La Brea. Proc Natl Acad Sci U S A. 1992;89:9769–73. doi: 10.1073/pnas.89.20.9769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Janczewski DN, Modi WS, Stephens JC, O’Brien SJ. Molecular evolution of mitochondrial 12S RNA and cytochrome b sequences in the pantherine lineage of Felidae. Mol Biol Evol. 1995;12:690–707. doi: 10.1093/oxfordjournals.molbev.a040232. [DOI] [PubMed] [Google Scholar]
- 66.Johnson W, O’Brien SJ. Phylogenetic reconstruction of the Felidae using 16S rRNA and NADH-5 mitochondrial genes. J Mol Evol. 1997;44:s98–116. doi: 10.1007/pl00000060. [DOI] [PubMed] [Google Scholar]
- 67.Grahn RA, Kurushima JD, Billings NC, Grahn JC, Halverson JL, Hammer E, et al. Feline non-repetitive mitochondrial DNA control region database for forensic evidence. Forensic Sci Int Genet. 2011;5:33–42. doi: 10.1016/j.fsigen.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tarditi CR, Grahn RA, Evans JJ, Kurushima JD, Lyons LA. Mitochondrial DNA sequencing of cat hair: an informative forensic tool*. J Forensic Sci. 2011;56(Suppl 1):S36–46. doi: 10.1111/j.1556-4029.2010.01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li X, Li W, Wang H, Bayley DL, Cao J, Reed DR, et al. Cats lack a sweet taste receptor. J Nutr. 2006;136:1932S–4S. doi: 10.1093/jn/136.7.1932S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li X, Li W, Wang H, Cao J, Maehashi K, Huang L, et al. Pseudogenization of a sweet-receptor gene accounts for cats’ indifference toward sugar. PLoS Genet. 2005;1:27–35. doi: 10.1371/journal.pgen.0010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shrestha B, Reed JM, Starks PT, Kaufman GE, Goldstone JV, Roelke ME, et al. Evolution of a major drug metabolizing enzyme defect in the domestic cat and other Felidae: phylogenetic timing and the role of hypercarnivory. PLoS One. 2011;6:e18046. doi: 10.1371/journal.pone.0018046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lipinski MJ, Amigues Y, Blasi M, Broad TE, Cherbonnel C, Cho GJ, et al. An international parentage and identification panel for the domestic cat (Felis catus) Anim Genet. 2007;38:371–7. doi: 10.1111/j.1365-2052.2007.01632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.(DAB), DNA Advisory Board, U. S. Department of Justice, Investigation. FBo. Quality assurance standards for forensic DNA testing laboratories. 1998. Forensic Science Communications. 2000 Oct 01:2.
- 74.Budowle B, Garofano P, Hellman A, Ketchum M, Kanthaswamy S, Parson W, et al. Recommendations for animal DNA forensic and identity testing. Int J Leg Med. 2005;119:295–302. doi: 10.1007/s00414-005-0545-9. [DOI] [PubMed] [Google Scholar]
- 75.Lipinski MJ, Froenicke L, Baysac KC, Billings NC, Leutenegger CM, Levy AM, et al. The ascent of cat breeds: genetic evaluations of breeds and worldwide random-bred populations. Genomics. 2008;91:12–21. doi: 10.1016/j.ygeno.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eizirik E, Yuhki N, Johnson WE, Menotti-Raymond M, Hannah SS, O’Brien SJ. Molecular genetics and evolution of melanism in the cat family. Curr Biol. 2003;13:448–53. doi: 10.1016/s0960-9822(03)00128-3. [DOI] [PubMed] [Google Scholar]
- 77.Peterschmitt M, Grain F, Arnaud B, Deleage G, Lambert V. Mutation in the melanocortin 1 receptor is associated with amber colour in the Norwegian forest cat. Anim Genet. 2009;40:547–52. doi: 10.1111/j.1365-2052.2009.01864.x. [DOI] [PubMed] [Google Scholar]
- 78.Lyons LA, Foe IT, Rah HC, Grahn RA. Chocolate coated cats: TYRP1 mutations for brown color in domestic cats. Mamm Genome. 2005;16:356–66. doi: 10.1007/s00335-004-2455-4. [DOI] [PubMed] [Google Scholar]
- 79.Schmidt-Kuntzel A, Eizirik E, O’Brien SJ, Menotti-Raymond M. Tyrosinase and tyrosinase related protein 1 alleles specify domestic cat coat color phenotypes of the albino and brown loci. J Hered. 2005;96:289–301. doi: 10.1093/jhered/esi066. [DOI] [PubMed] [Google Scholar]
- 80.Imes DL, Geary LA, Grahn RA, Lyons LA. Albinism in the domestic cat (Felis catus) is associated with a tyrosinase (TYR) mutation. Anim Genet. 2006;37:175–8. doi: 10.1111/j.1365-2052.2005.01409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lyons LA, Imes DL, Rah HC, Grahn RA. Tyrosinase mutations associated with Siamese and Burmese patterns in the domestic cat (Felis catus) Anim Genet. 2005;36:119–26. doi: 10.1111/j.1365-2052.2005.01253.x. [DOI] [PubMed] [Google Scholar]
- 82.De Maria R, Divari S, Bo S, Sonnio S, Lotti D, Capucchio M, et al. Beta-galactosidase deficiency in a Korat cat: a new form of feline GM1-gangliosidosis. Acta Neuropathol (Berl) 1998;96:307–14. doi: 10.1007/s004010050899. [DOI] [PubMed] [Google Scholar]
- 83.Bradbury AM, Morrison NE, Hwang M, Cox NR, Baker HJ, Martin DR. Neurodegenerative lysosomal storage disease in European Burmese cats with hexosaminidase beta-subunit deficiency. Mol Genet Metab. 2009;97:53–9. doi: 10.1016/j.ymgme.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 84.Martin DR, Cox NR, Morrison NE, Kennamer DM, Peck SL, Dodson AN, et al. Mutation of the GM2 activator protein in a feline model of GM2 gangliosidosis. Acta Neuropathol. 2005;110:443–50. doi: 10.1007/s00401-005-1040-6. [DOI] [PubMed] [Google Scholar]
- 85.Meurs KM, Norgard MM, Ederer MM, Hendrix KP, Kittleson MD. A substitution mutation in the myosin binding protein C gene in ragdoll hypertrophic cardiomyopathy. Genomics. 2007;90:261–4. doi: 10.1016/j.ygeno.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 86.Menotti-Raymond M, David VA, Schaffer AA, Stephens R, Wells D, Kumar-Singh R, et al. Mutation in CEP290 discovered for cat model of human retinal degeneration. J Hered. 2007;98:211–20. doi: 10.1093/jhered/esm019. [DOI] [PubMed] [Google Scholar]
- 87.Menotti-Raymond M, Deckman K, David V, Myrkalo J, O’Brien S, Narfström K. Mutation discovered in a feline model of human congenital retinal blinding disease. Invest Ophthalmol Vis Sci. 2010;51:2852–9. doi: 10.1167/iovs.09-4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fyfe JC, Menotti-Raymond M, David VA, Brichta L, Schaffer AA, Agarwala R, et al. An approximately 140-kb deletion associated with feline spinal muscular atrophy implies an essential LIX1 function for motor neuron survival. Genome Res. 2006;16:1084–90. doi: 10.1101/gr.5268806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Martin DR, Krum BK, Varadarajan GS, Hathcock TL, Smith BF, Baker HJ. An inversion of 25 base pairs causes feline GM2 gangliosidosis variant. Exp Neurol. 2004;187:30–7. doi: 10.1016/j.expneurol.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 90.Kanae Y, Endoh D, Yamato O, Hayashi D, Matsunaga S, Ogawa H, et al. Nonsense mutation of feline beta-hexosaminidase beta-subunit (HEXB) gene causing Sandhoff disease in a family of Japanese domestic cats. Res Vet Sci. 2007;82:54–60. doi: 10.1016/j.rvsc.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 91.Crawley AC, Yogalingam G, Muller VJ, Hopwood JJ. Two mutations within a feline mucopolysaccharidosis type VI colony cause three different clinical phenotypes. J Clin Invest. 1998;101:109–19. doi: 10.1172/JCI935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fyfe JC, Kurzhals RL, Lassaline ME, Henthorn PS, Alur PR, Wang P, et al. Molecular basis of feline beta-glucuronidase deficiency: an animal model of mucopolysaccharidosis VII. Genomics. 1999;58:121–8. doi: 10.1006/geno.1999.5825. [DOI] [PubMed] [Google Scholar]
- 93.Goree M, Catalfamo JL, Aber S, Boudreaux MK. Characterization of the mutations causing hemophilia B in 2 domestic cats. J Vet Intern Med. 2005;19:200–4. doi: 10.1892/0891-6640(2005)19<200:cotmch>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 94.Somers K, Royals M, Carstea E, Rafi M, Wenger D, Thrall M. Mutation analysis of feline Niemann-Pick C1 disease. Mol Genet Metab. 2003;79:99–103. doi: 10.1016/s1096-7192(03)00074-x. [DOI] [PubMed] [Google Scholar]
- 95.Lettice LA, Hill AE, Devenney PS, Hill RE. Point mutations in a distant sonic hedgehog cis-regulator generate a variable regulatory output responsible for preaxial polydactyly. Hum Mol Genet. 2008;17:978–85. doi: 10.1093/hmg/ddm370. [DOI] [PubMed] [Google Scholar]
- 96.Goldstein R, Narala S, Sabet N, Goldstein O, McDonough S. Primary hyper-oxaluria in cats caused by a mutation in the feline GRHPR gene. J Hered. 2009;100:S2–7. [Google Scholar]
- 97.Ginzinger DG, Lewis ME, Ma Y, Jones BR, Liu G, Jones SD. A mutation in the lipoprotein lipase gene is the molecular basis of chylomicronemia in a colony of domestic cats. J Clin Invest. 1996;97:1257–66. doi: 10.1172/JCI118541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Clavero S, Bishop DF, Giger U, Haskins ME, Desnick RJ. Feline congenital erythropoietic porphyria: two homozygous UROS missense mutations cause the enzyme deficiency and porphyrin accumulation. Mol Med. 2010;16:381–8. doi: 10.2119/molmed.2010.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Berg T, Tollersrud OK, Walkley SU, Siegel D, Nilssen O. Purification of feline lysosomal alpha-mannosidase, determination of its cDNA sequence and identification of a mutation causing alpha-mannosidosis in Persian cats. Biochem J. 1997;328(Pt 3):863–70. doi: 10.1042/bj3280863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Clavero S, Bishop DF, Haskins ME, Giger U, Kauppinen R, Desnick RJ. Feline acute intermittent porphyria: a phenocopy masquerading as an erythro-poietic porphyria due to dominant and recessive hydroxymethylbilane synthase mutations. Hum Mol Genet. 2010;19:584–96. doi: 10.1093/hmg/ddp525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mazrier H, Van Hoeven M, Wang P, Knox V, Aguirre G, Holt E, et al. Inheritance, biochemical abnormalities, and clinical features of feline mucolipidosis II: the first animal model of human I-cell disease. J Hered. 2003;94:363–73. doi: 10.1093/jhered/esg080. [DOI] [PubMed] [Google Scholar]
- 102.Geisen V, Weber K, Hartmann K. Vitamin D-dependent hereditary rickets type I in a cat. J Vet Intern Med. 2009;23:196–9. doi: 10.1111/j.1939-1676.2008.00220.x. [DOI] [PubMed] [Google Scholar]
- 103.He X, Li CM, Simonaro CM, Wan Q, Haskins ME, Desnick RJ, et al. Identification and characterization of the molecular lesion causing mucopoly-saccharidosis type I in cats. Mol Genet Metab. 1999;67:106–12. doi: 10.1006/mgme.1999.2860. [DOI] [PubMed] [Google Scholar]
- 104.Grahn R, Ellis M, Grahn J, Lyons L. No bones about it! A novel CYP27B1 mutation results in feline vitamin D-dependent rickets type I (VDDR-1) J Feline Med Surg. doi: 10.1177/1098612X12446637. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Menotti-Raymond MA, O’Brien SJ. Evolutionary conservation of ten micro-satellite loci in four species of Felidae. J Hered. 1995;86:319–22. doi: 10.1093/oxfordjournals.jhered.a111594. [DOI] [PubMed] [Google Scholar]
- 106.Menotti-Raymond MA, David VA, Wachter LL, Butler JM, O’Brien SJ. An STR forensic typing system for genetic individualization of domestic cat (Felis catus) samples. J Forensic Sci. 2005;50:1061–70. [PubMed] [Google Scholar]
- 107.Pilgrim KL, McKelvey KS, Riddle AE, Schwartz MK. Felid sex identification based on noninvasive genetic samples. Mol Biol Notes. 2005;5:60–1. [Google Scholar]


