Abstract
A method to isolate large quantities of directly accessible plasma membrane from attached cells is presented. The method is based upon the adhesion of cells to an adsorbed layer of polylysine on glass plates, followed by hypotonic lysis with ice-cold distilled water and subsequent washing steps. Optimal conditions for coating glass plates and time for cell attachment were established. No additional chemical or mechanical treatments were used. Contamination of the isolated plasma membrane by cell organelles was less than 5 %. The method uses inexpensive, commercially available, polylysine and re-usable glass plates. Plasma membrane preparations can be made in 15 minutes. Using this method, we determined that methyl-β-cyclodextrin differentially extracts cholesterol from fibroblast cells and their plasma membranes and that these differences are temperature dependent. Determination of the cholesterol:phospholipid ratio from intact cells does not reflect methyl-β-cyclodextrin plasma membrane extraction properties.
Keywords: fibroblast cells, cholesterol/phospholipid ratio, differential extraction, methyl-β-cyclodextrin, polylysine
INTRODUCTION
Techniques currently used for the isolation of plasma membranes include 1) zonal or density-gradient centrifugation [1 – 5] 2) the rip-flip method, developed for the microscopic observation of small pieces of the cytoplasmic side of plasma membranes [6, 7] and 3) methods based on the adhesion of negatively-charged cells to a positively-charged surface such as polylysine coated polyacrylamide or glass beads [8 – 14]. A method to isolate large quantities of directly accessible cytoplasmic surface of the plasma membranes suitable both for microscopy and biochemical analysis was developed. The method is based on the adhesion of cells to an adsorbed layer of polylysine on glass plates, followed by hypotonic lysis with ice-cold distilled water. Optimal conditions were established for all preparation steps including polylysine coating, cell adhesion, and membrane washing, both ensuring high purity and yield of the membrane preparation. This method allows for a) the creation of isolated plasma membranes without chemical (high salt) or mechanical (vortexing or sonication) treatments, b) direct lipid extraction on glass plates, and c) the ability to study both biochemical and structural properties of isolated plasma membranes.
Methyl-β-cyclodextrin (MβCD) is used to alter the cholesterol content of cells, and in particular, the cholesterol content of the plasma membrane [15]. The cholesterol content of cellular membranes and the relationships between cholesterol enriched domains and physiological function is an active area of research. While questions remain unanswered with regard to detailed kinetics, dependence upon composition and temperature and specific and nonspecific effects, exposure of cells to MβCD reduces cellular cholesterol (Table 1 of Ref. [15]). However, reduction in cellular cholesterol following MβCD treatment may not quantitatively reflect the changes in plasma membrane cholesterol content. Having developed a method to isolate plasma membranes in quantities suitable for biochemical examination, MβCD cholesterol depletion evaluated using intact cells and their plasma membranes were compared. MβCD treatment extracted cholesterol from the plasma membrane of HAB2 cells, a hemagglutinin expressing fibroblast cell line, and intact HAB2 cells in a temperature-dependent way and the reduction in plasma membrane cholesterol content was not proportional to the decrease observed using intact cells. Almost complete removal of plasma membrane cholesterol can be achieved by extraction at physiological temperature (37 °C). At 4 °C, MβCD extraction of cholesterol from the plasma membrane is less. These data indicate that one cannot predict the loss of cholesterol from the plasma membrane based on the loss determined from intact cells.
MATERIALS AND METHODS
Chemicals and solutions
Poly-L-lysine solution (Sigma Chemical Co., St. Louis, MO), boric acid (Mallinckrodt Baker Inc., Paris, KY), Tris buffered saline (TBS,10X, Cellgro, Mediatech, Inc. Herndon, VA), ultra pure water (KD Medical, Columbia, MD), and Pyrex dishes, 100×20mm (CORNING, NY) were used. Fluorescent probes for staining mitochondria (Mito Traker Green FM; MTG), lysosomes (Lyso Tracker Red DND-99; LTR), Golgi apparatus (BODIPY FL C5-ceramide), endoplasmic reticulum (Rhodamine B, hexyl ester, perchlorate; R6), and nucleus (Hoechst 33342; HO342) were purchased from Molecular Probes Eugene, OR; cell membrane dye (PKH 26 Red Fluorescent cell linker kit, Sigma Chemical Co., St. Louis, MO); Albumin, Bovine and Methyl-β-cyclodextrin (Sigma Chemical Co., St. Louis, MO), 0.25% Trypsin-EDTA (1x, GIBCO/Invitrogen, Grand Island, NY), chloroform and methanol (Burdick&Jackson, Muskegon, MI) were used.
Labeling procedures
The following stains were used [16] with concentrations adjusted for HAB2 cells. Nuclei were labeled using 0.5µg/ml Hoechst 33342; HO342 (blue) for 10 min at room temperature; Lysosomes were labeled using 50 nM Lyso Tracker Red DND-99 for 1 hr at 37 °C; Golgi were labeled using 5µM BODYPY FL C5-ceramide (green) for 10 min at 37 C; Endoplasmic Reticulum were labeled using 200 nM Rhodamine B, R6 (red) for 30 min at 37 °C; and Mitochondria were labeled using 250 nM MitoTracker Green FM, MTG for 30 min at 37 °C. Isolated membranes were labeled using 1 µM PKH 26 for 10 min at room temperature.
Cells
HAB2 cells, a generous gift of J. M. White (University of Virginia, Charlottesville, VA) were used. HAB2 cells, a sub-clone of stably transfected NIH-3T3 (mouse embryonic) fibroblasts express hemagglutinin of the A/Japan/305/57 strain influenza virus [17, 18, 19]. Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM, ATCC, Manassas, VA) supplemented with 10% fetal bovine serum (FBS, Gemini Bio-Products, Woodland, CA), 100 U/ml penicillin and 10 µg/ml streptomycin (SIGMA, St. Louis, MO) in 5% CO2.
Membrane isolation procedure
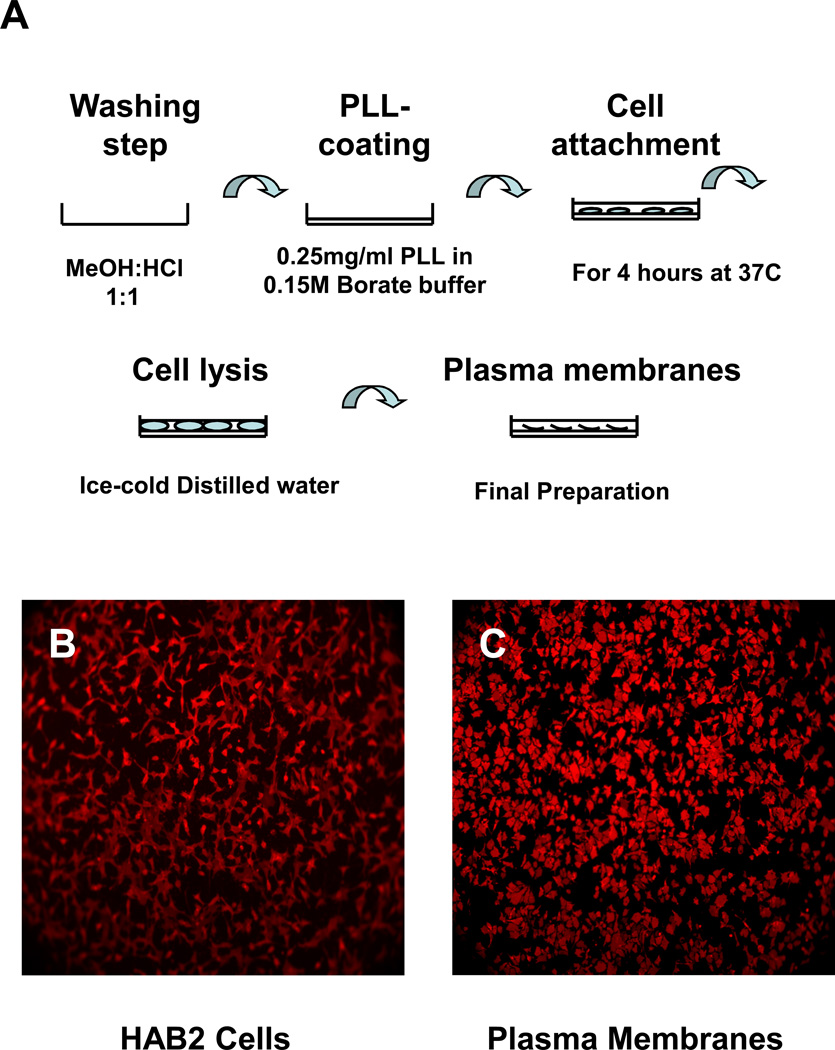
Optimal membrane isolation (Fig. 1) is dependent upon a) cleaning the glass plates, b) coating the glass plates with polylysine, and c) cell attachment. Cleaning the glass plates is a critical step of the procedure. Plates were soaked in hydrochloric acid:methanol (1:1) for at least 30 minutes at room temperature and then washed repeatedly with distilled water, rinsed with ethanol, and allowed to dry. Optimal conditions for coating glass plates with polylysine were established.
Figure 1. Plasma membrane isolation.
A. Glass plates were soaked in Hydrochloric Acid: Methanol (1:1) at room temperature, washed with water, rinsed with ethanol, and dried; glass plates were coated with polylysine; cells were incubated at 37 °C for 4 hours; cells were ruptured with ice-cold distilled water; intracellular debris was removed with ice-cold TBS buffer washes. B. HAB2 cells on a polylysine coated glass plate after incubation for 4 hours at 37 °C in serum-free media. C. Plasma membranes of HAB2 cells on a polylysine coated glass plate. Both samples were stained with 1µM PKH 26 for 10 min at room temperature.
Initially, cells were cultured on collagen and polylysine coated glass plates; HAB2 cells grew well on both substrates. However, in the absence of cells, the background levels of cholesterol and phospholipids were lower in polylysine coated plates. Stronger cell attachment was obtained when polylysine was dissolved in 0.15M borate buffer, pH 8.3, (Grace Bio Labs) instead of distilled water. Equally strong attachment was observed using 1 mg/ml, 0.5 mg/ml, and 0.25 mg/ml concentrations of polylysine; 0.25 mg/ml was used to both conserve material and minimize background. Specifically, polylysine was prepared at a concentration of 0.25 mg/ml in 0.15 M borate buffer (pH 8.3); 10 ml of this solution was applied to the surface and allowed to adsorb over night at room temperature. Prior to cell application, plates were washed 5 times (10 ml each time) with distilled water to remove excess polylysine.
Cells previously grown to 85–90% confluence were lifted, counted, and seeded in the glass plates at a concentration of 2.5×105 cells per ml. Cells were lifted with a 0.25% Trypsin-0.53mM EDTA solution. Growth media was removed first and the flask rinsed with the Trypsin-EDTA solution. The rinse solution was removed and an additional 1 ml of Trypsin-EDTA solution was added to the flask; the flask was maintained in the incubator at 37 °C for 2 – 5 minutes, until the cells detached. 5 ml of fresh complete medium were then added, cells mixed by gently pipetting, cell suspension collected, and cells pelleted by centrifugation (2,000 rpm for 5 min). Cells were resuspended in 5 ml of serum free media and centrifuged with the same settings; this wash step was repeated two times to remove serum from the cell suspension. 10 ml of cell suspension was applied to each glass plate following cell density adjustment.
The optimal incubation time for HAB2 cell attachment to the polylysine coated glass surface was 4 hours at 37 °C, 5% CO2 in serum free media. Under these conditions the number of cells and their quality of attachment were sufficient for our experiments; the cells were strongly attached with maximal cell surface adhesion area. Serum free media was used to avoid cholesterol and phospholipid contamination from the serum. Plates were then washed 3 times with Tris-buffered saline, TBS, to remove unbound cells. For cell disruption, 10 ml of ice-cold distilled water was added for 1 min. The plates were then washed 2 times with 10 ml of TBS buffer to remove intracellular debris. Cell disruption with ice-cold water application was repeated two more times. When required, the plasma membrane preparation was visualized using the membrane indicator, PKH 26 (Fig. 1).
Lipids extraction
Total lipids were extracted from the plasma membranes of HAB2 cells and intact HAB2 cells directly on glass plates by the method of Folch [20]. 5 ml of chloroform:methanol (2:1, v/v) was added to one glass plate with plasma membrane of HAB2 cells or intact HAB2 cells and then shaken gently at room temperature for 1hour. All work with chloroform and methanol was carried out in a chemical fume hood. The mixture was collected and the solvent was washed with 0.2 volume of 0.9% NaCl solution. After vortexing for several seconds, the mixture was centrifuged at low speed to separate the two phases (2000 rpm for 15 min at room temperature, Allegra X-22 Centrifuge, SX4250 rotor, Beckman Coulter). The upper phase was removed by a glass pipette and discarded. The interface was rinsed once with methanol:water (1:1) without mixing the whole preparation. The lower chloroform phase, containing lipids, was carefully transferred to fresh tubes and evaporated under a stream of argon. The lipid residue was dissolved in chloroform and stored at −30 °C.
Total cholesterol and phospholipids determination
Total cholesterol was measured using the Amplex Red Cholesterol Assay Kit (A12216), from Molecular Probes St. Louis, MO. Typically, five standards and a blank, in duplicate, covering a range from 0 to ~ 300 ng cholesterol, were used for calibration. 20 independent calibrations were used to evaluate the assay variance for each cholesterol standard; unequal variance, heteroscedasticity, was observed and required the use of weighted curve fitting. Limited replicates argued against direct 1/Variance weighting; multiple weighting models were evaluated according to Almeida et al. 2002 [21]. Weighted (1/[Cholesterol]2 and 1/VarianceBlank), linear curve fitting was used to establish calibration functions for each experiment. Sample unknowns, background subtracted and in triplicate, were evaluated, confidence errors determined, and weighted averages and weighted standard deviations of the pooled data (n = 6 to 11 independent experiments) were determined. Background subtracted unknowns with signal level less than zero were evaluated at the limit of detection, LOD, for the specific calibration line (YIntercept + 3*(Standard Error of Fit)). Evaluation at the LOD is required because the absence of a detectable signal is not synonymous with zero cholesterol concentration; the LOD is an estimate of the maximum amount of cholesterol that could be present but not detected due to the statistical properties of the calibration line.
Total phospholipids were measured using procedures for Determination of Total Phosphorus, from Avanti Polar Lipids. Typically, four standards, covering a range from ~ 0.01 – 0.1 µmoles of lipid, corresponding to an absorbance of ~0.1 – ~1.0 were used for calibration. 11 independent calibrations were used to evaluate the assay variance for each phospholipid standard; equal variance, homoscedasticity, was observed and did not require weighted curve fitting. Calibration functions were determined using un-weighted, linear curve fitting and weighted averages and standard deviations of the pooled data were determined. Weighted averages and standard deviations were used because there was variability in the statistical properties of each calibration line; parameter confidence estimates varied between calibration lines. Weighting the pooled estimates, even when derived from un-weighted fitting, resulted in a more statistically reliable estimate; parameters derived from a “statistically weaker” fit were not given the same weight as those derived from a “statistically stronger” fit. Errors in derived quantities (ratios) were calculated using standard error propagation [22].
Methyl-β-cyclodextrin treatment
Cells attached to glass plates were treated with 10 mM MβCD in serum free media for 30 minutes at the required treatment temperature (37 or 4 °C). MβCD was then removed using a TBS rinse. Two glass plates were used for evaluation of intact cells, while four plates were used to create plasma membrane preparations. Total lipid extraction was done directly on the glass plates by the Folch method. Typically, two preparations, intact cells and plasma membranes, were processed in parallel; cholesterol and total phospholipids content were then determined.
Imaging and Image Processing
Cells and their membranes were imaged using a Zeiss Axiovert-25 with 32× or 10× objectives, using a digital camera (ORCA-ER, HAMAMATSU) controlled by IPLab v 3.61 (SCANALYTICS Inc. Fairfax, VA). For each fluorescent probe evaluated, camera settings were kept constant for imaging both cells and their membranes. A custom macro (available upon request) for Image J v1.34S software (National Institutes of Health, USA) was used for analysis. Briefly, background was subtracted using a Rolling Ball Algorithm, a mask was created using the lowest intensity region of a Multi (4 level) Otsu Thresholding, background pixels were “removed” using the mask, the pixel histogram was created, the total signal (Signal Area) was calculated by summing the product of the pixel frequency (fi)and pixel intensity (Ii) (Signal Area = ), and the signal corrected for “cell coverage” using the number of background and total pixels, NB and NTotal(Signalcorrected = Signal Area/(1-NB/NTotal). The “cell coverage” correction insures that pixel intensity comparisons are not influenced by unequal cell counts in the field of view by scaling the Signal Area by the fraction of contributing (non-background) pixels. Loss of fluorescence, as reflected in the ratio of the SignalMembrane:SignalCell served as a surrogate marker for membrane purity. Nuclei, were counted following background subtraction and binary thresholding. Imaging results are derived from two independent experiments consisting of 1 – 12 images per probe (endoplasmic reticulum, Golgi, mitochondria, nuclei, lysosomal membrane).
RESULTS
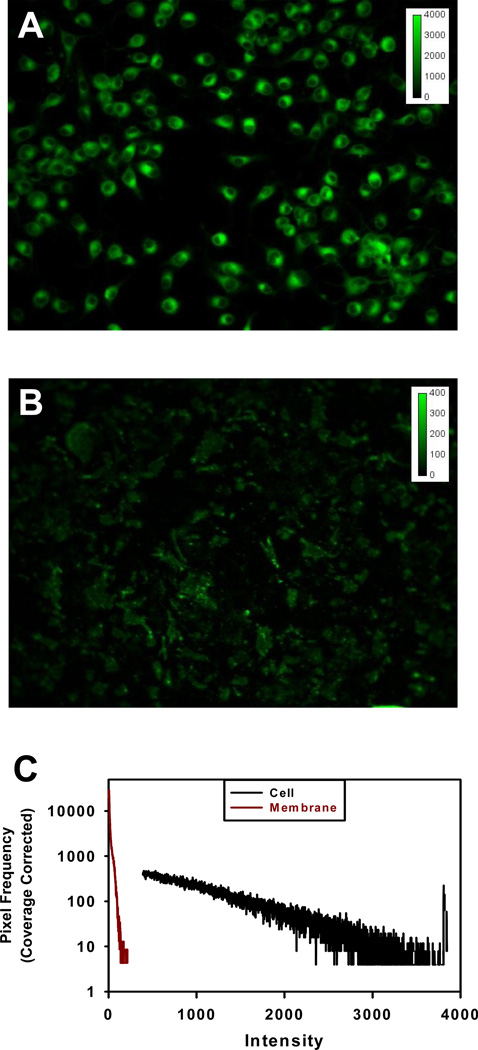
The purity of the plasma membrane preparation was evaluated by labeling with fluorescent, organelle-specific probes. An example of HAB2 cells and plasma membranes of HAB2 cells labeled with BODYPY FL C5-ceramide specific for the Golgi apparatus is shown in Fig. 2. The detection of nuclei, endoplasmic reticulum, lysosomal membrane, Golgi and mitochondria in plasma membrane preparations corresponded to contamination levels, relative to intact cells, of 0.9 %, 2.9 %, 3.4 %, 5.7 % and 3.0 %, for an average of 96.8 +/− 1.7 % purity over all probes tested.
Figure 2. Staining of Golgi apparatus and purity of plasma membrane preparation.
HAB2 cells (A) and plasma membranes of HAB2 cells (B) were stained with 5 µM BODYPY FL C5-ceramide (green) specific for Golgi apparatus for 10 min at 37 °C in serum-free medium (DMEM) and visualized using a 32× objective. (C) Intensity histogram used to calculate membrane purity. In this example, the Signal Area, for the HAB2 cells and plasma membranes were 8.79*107 and 3.59*106 and the number of background pixels, 2.39*105 and 2.50*105respectively. With 3.22*105 total pixels, the coverage correction for cells and membranes is, 0.26 and 0.22. The corresponding Signalcorrectedare 3.41*108 and 1.60*107 representing a purity of 4.7%. For direct comparison, the pixel frequency, fi, was normalized by the coverage correction, fi/0.26 and fi/0.22, respectively, and designated as Coverage Corrected on the axis.
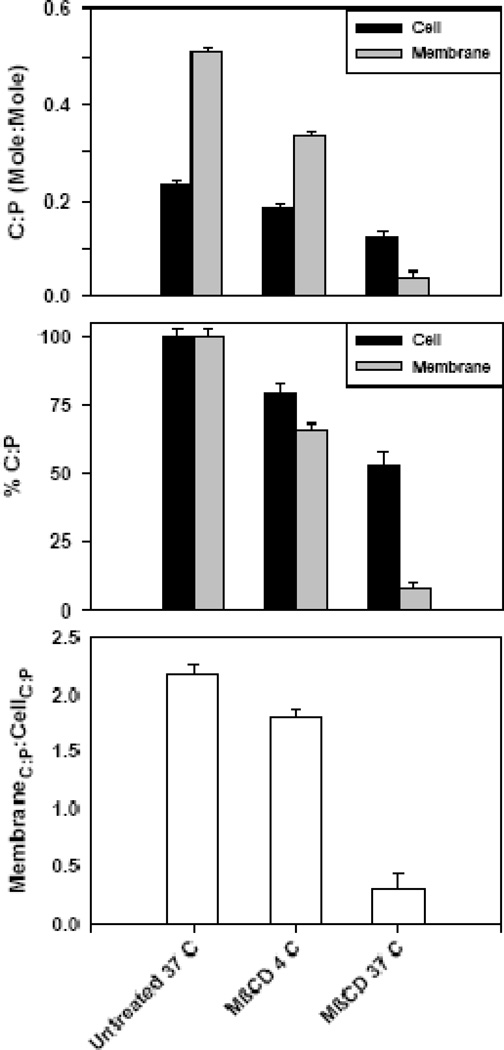
The cholesterol:phospholipid, C:P, ratio (mole:mole) for untreated cells and their plasma membranes was 0.23 +/− 0.01 (n = 7) and 0.51 +/− 0.01 (n = 9) corresponding to a ratio of membrane to cell C:P of 2.18 +/− 0.08. When the C:P ratio was determined using intact cells treated with MβCD, the ratio decreased to 0.19 +/− 0.01 (n = 6) and 0.13 +/− 0.01 (n = 5) for 4 and 37 °C treatment, respectively. MβCD treatment decreases the cellular C:P ratio by ~20% and ~50% from untreated control for 4 and 37 °C treatment, respectively. However, when the isolated membranes are used to evaluate the C:P ratio, larger changes are observed as a function of treatment temperature with the ratio decreasing to 0.34 +/− 0.01 (n = 11) and 0.04 +/− 0.01 (n = 6) for 4 and 37 °C treatment, respectively. MβCD treatment decreases the plasma membrane C:P ratio by ~ 35% and greater than 90% from untreated control for 4 and 37 °C treatment, respectively. The ratio of membrane to cell C:P decreased to 1.80 +/− 0.07 and 0.32 +/− 0.11 for 4 and 37 °C treatment, respectively. The loss of plasma membrane cholesterol is not reflected in the C:P ratios derived from processing intact cells. These results are summarized in Fig. 3.
Figure 3. Cholesterol:phospholipid (C:P) at different extraction temperatures.
(A) C:P ratio for untreated and MβCD extracted HAB2 cells and their plasma membranes. Data are averages of 6 – 11 experiments.
(B) Normalized data from Fig. 4-A, where 100% is the ratio of cholesterol to phospholipids in untreated (control) samples.
C) Membrane C:P to Cell C:P, a measure of the relative concentration of cholesterol in the plasma membranes compared to intact cells.
DISCUSSION
Polylysine-coated glass plates can be used to obtain quantities of plasma membrane suitable for both biochemical and microscopy studies. We used our method of plasma membrane preparation to study how MβCD treatment extracts cholesterol from both plasma membranes and intact HAB2 cells. MβCD treatment extracts cholesterol from intact cells and their membranes in a temperature dependent way. Almost compete removal of plasma membrane cholesterol can be achieved by extraction at physiological temperature, 37 °C. At 4 °C extraction from intact cells and their membranes is limited (20% and 34% decrease), relative to untreated control. Determination of the cholesterol:phospholipid ratio from methyl-β-cyclodextrin treated intact cells did not reflect the plasma membrane extraction properties of methyl-β-cyclodextrin. This isolation procedure is useful for obtaining plasma membrane from other cell types; we have succeeded in obtaining plasma membranes from muscle myoblast cells (C2C12) and Madin-Darby canine kidney epithelial cells (MDCK).
The C:P ratio for intact fibroblasts in our experiments was ~ 0.3, which is consistent with published data (0.29+/−0.03 [23]; <0.3 [24]; 0.38 +/−0.04 [25]; 0.37 and 0.23 +/− lipoprotein [26]). The C:P ratio of untreated cells (0.23 +/− 0.01), in the absence of serum, is in agreement with the value of Davis and Poznansky obtained from microsomal preparations of normal human skin fibroblasts cultured in the absence of lipoprotein [26]. There appears to be a relatively narrow range of C:P ratio over a wide variety of cell types and tissues when averaged over the intact cell or tissue. However, the C:P ratio from isolated plasma membrane preparations is consistently larger in magnitude and range in all cells and tissues measured. C:P ratios range from 0.69 +/− 0.11 to 1.11 depending upon cell and tissue type [25, 27] but cluster around ~0.8 for fibroblasts (0.764 and 0.846 3T3 and SV3T3 cells [24], 0.69 +/− 0.11 [25]). Using 0.3 and 0.8 for intact cells and their membranes, the ratio of membrane to cell C:P is ~ 2.7 which is comparable to the value of 2.18 determined in this study using cells grown in the absence of serum. Having confirmed an increased C:P ratio in plasma membrane relative to the intact cell, the differential extraction of MβCD treatment as a function of temperature and sample (intact cell vs. plasma membrane) becomes an important consideration when evaluating the role of cholesterol in membrane dependent processes.
Treatment with 10 mM MβCD for 30 minutes at 37 °C did not deplete cholesterol from all membrane fractions but essentially depleted all the cholesterol in the plasma membrane. The roles of cholesterol in membrane heterogeneity/domains (“rafts vs. non-rafts”) and the association and function of proteins to specialized domains are of considerable interest. MβCD is often used to selectively deplete cholesterol from low-density and high-density membrane fractions [Table 2, of Ref. 15]. Not only is MβCD concentration and exposure time important parameters in perturbing the cholesterol content of the membrane, but extraction temperature is critical. Having a method to isolate and evaluate biochemical quantities of pure plasma membrane will benefit those studies where cholesterol perturbation needs to be minimized; the combined effects of MβCD concentration, exposure time and temperature extraction now can be evaluated easily.
Acknowledgments
The authors thank Drs. K. Melikov, E. Zaitseva and J. Mazar for fruitful discussions and suggestions. This study was supported by the Intramural Research Program of the NIH, Eunice Kennedy Shriver National Institute of Health and Human Development.
Abbreviations used
- MβCD
methyl-β-cyclodextrin
- TBS
tris-buffered saline
- FBS
fetal bovine serum
- C:P
cholesterol:phospholipid ratio
- NaCl
sodium chloride
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Depierre JW, Karnovsky ML. Plasma membranes of mammalian cells. J. Cell. Biol. 1973;56:275–303. doi: 10.1083/jcb.56.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atkinson PH, Summers DF. Purification and properties of HeLa cell plasma membranes. J. Biol. Chem. 1971;246:5162–5175. [PubMed] [Google Scholar]
- 3.Boone CW, Ford LE, Stuart DC, Lorenz D D. Isolation of plasma membrane fragments from Hela cells. J. Cell. Biol. 1969;41:378–392. doi: 10.1083/jcb.41.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosmann HB, Hagopian A, Eylar EH. Cellular membranes: the isolation and characterization of the plasma and smooth membranes of HeLa cells. Arch. Biochem. Biophys. 1968;128:51–59. doi: 10.1016/0003-9861(68)90008-8. [DOI] [PubMed] [Google Scholar]
- 5.Johnsen S, Stokke T, Prydz H. HeLa cell plasma membranes. I.5-nucleotidase and ouabain-sensetive ATPase as markers for plasma membranes. J. Cell. Biol. 1974;63:357–363. doi: 10.1083/jcb.63.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanan DA, Anderson RG. Simultaneous visualization of LDL receptor distribution and Clathrin Lattices on membranes torn from the upper surface of cultured cells. J. Histochem. Cytochem. 1991;39:1017. doi: 10.1177/39.8.1906908. [DOI] [PubMed] [Google Scholar]
- 7.Wilson BS, Pfeiffer JR, Oliver JM. Observing FceRI signaling from the inside of the Mast cell membrane. J. Cell. Biol. 2000;149:1131–1142. doi: 10.1083/jcb.149.5.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobson BS, Branton D. Plasma membrane: rapid isolation and exposure of the cytoplasmic surface by use of positively charged beads. Science. 1977;195:302–304. doi: 10.1126/science.831278. [DOI] [PubMed] [Google Scholar]
- 9.Cohen CM, Kalish DI, Jacobson BS, Branton D. Membrane isolation on polylysine-coated beads. Plasma membrane from HeLa cells. J. Cell. Biol. 1977;75(1) doi: 10.1083/jcb.75.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalish DI, Cohen CM, Jacobson BS, Branton D. Membrane isolation on polylysine-coated glass beads. Asymmetry of bound membrane. Biochim. Biophys. Acta. 1978;506(1) doi: 10.1016/0005-2736(78)90437-6. [DOI] [PubMed] [Google Scholar]
- 11.Kramer RM, Branton D. Retention of lipid asymmetry in membranes on polylysine-coated polyacrylamide beads. Biochim. Biophys. Acta. 1979;556(2) doi: 10.1016/0005-2736(79)90044-0. [DOI] [PubMed] [Google Scholar]
- 12.Jacobson BS, Cronin J, Branton D. Coupling polylysine to glass beads for plasma membrane isolation. Biochim. Biopys. Acta. 1978;506(1) doi: 10.1016/0005-2736(78)90436-4. [DOI] [PubMed] [Google Scholar]
- 13.Cohen CM, Kramer RM, Branton D. Transbilayer mapping of membrane proteins using membranes isolated on polylysine-coated polyacrylamide beads. Biochim. Biophys. Acta. 1980;597(1) doi: 10.1016/0005-2736(80)90147-9. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson BS. Isolation of plasma membrane from eukaryotic cell on polylysine-coated polyacrylamide beads. Biochim. Biophys. Acta. 1977;471(1) doi: 10.1016/0005-2736(77)90260-7. [DOI] [PubMed] [Google Scholar]
- 15.Zidovetzki R, Levitan I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: Evidence, misconceptions and control strategies. Biochim Biophys Asta. 2007;1768(6) doi: 10.1016/j.bbamem.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woodburn KW. Intracellular localization of the radiation enhancer Motexafin Gadolinium using interferometric fourier fluorescence microscopy. Pharmacology. 2001;297(3) [PubMed] [Google Scholar]
- 17.White JM, Helenius A, Gething MJ. Haemagglutinin of influenza virus expressed from a cloned gene promotes membrane fusion. Nature. 1982;300:658–659. doi: 10.1038/300658a0. [DOI] [PubMed] [Google Scholar]
- 18.Sambrook J, Rodgers L, White JM, Gething MJ. Lines of BPV – transformed murine cells that constitutively express influenza virus hemagglutinin. EMBO Journal. 1985;4:91–103. doi: 10.1002/j.1460-2075.1985.tb02322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doxsey SJ, Sambrook J, Helenius A, White JM. An efficient method for introducing macromolecules into living cell. J. Cell Biol. 1985:101, 19–27. doi: 10.1083/jcb.101.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Folch J, Lees M, Stanley GHS. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 21.Almeida AM, Castel-Blanco MM, Falcao AC. Linear regression for calibration lines revisited: weighting schemes for bioanalytical methods. J. Chromatography B. 2002:215–222. doi: 10.1016/s1570-0232(02)00244-1. [DOI] [PubMed] [Google Scholar]
- 22.Bevington P, Robinson DK. Data reduction and error analysis for the physical sciences. second ed. McGraw-Hill Book Inc.; 1992. [Google Scholar]
- 23.Murphy EJ, Zhang H, Sorbi S, Rapoport SI, Gibson GE. Phospholipid composition and levels are not altered in fibroblasts bearing presenilin-1 mutations. Brain Research Bulletin. 2000;52(3) doi: 10.1016/s0361-9230(00)00257-4. [DOI] [PubMed] [Google Scholar]
- 24.Scott RE, Perkins RG, Zschunke MA, Hoerl BJ, Maercklein PB. Plasma membrane vesiculation in 3T3 and SV3T3 cells. J.Cell Sci. 1979;35:229–243. doi: 10.1242/jcs.35.1.229. [DOI] [PubMed] [Google Scholar]
- 25.Lange Y, Swaisgood MH, Ramos BV, Steck TL. Plasma membrane contain half the phospholipids and 90% of the cholesterol and sphingomyelin in cultured human fibroblasts. J. Biol. Chem. 1989;264:3786–3793. [PubMed] [Google Scholar]
- 26.Davis PJ, Poznansky MJ. Modulation of 3-hydroxy-3-methylglutaryl-CoA reductase by changes in microsomal cholesterol content or phospholipid composition. Proc. Natl. Acad. Sci. U. S. A. 1987;84:118–121. doi: 10.1073/pnas.84.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finean JB, Coleman R, Green WA. Studies of isolated plasma membrane preparations. Ann. N. Y. Acad. Sci. 1966;137:414–420. doi: 10.1111/j.1749-6632.1966.tb50173.x. [DOI] [PubMed] [Google Scholar]