Abstract
The mechanistic basis of how cells respond to increased fatty acids (FA) is murky but potentially involves receptor-mediated activation or inhibition by different FA classes. Holzer et al (2011) recently propose in Cell that expansion of intracellular membrane microdomains induced by saturated FA recruit and activate c-Src for JNK activation.
In this era of unprecedented caloric excess, we face increased incidences of obesity, metabolic syndrome, and diabetes mellitus; natural selection has left us ill equipped for unrestricted food. The first adverse sign is insulin resistance – decreased glucose transport into cells which is matched by an increase in serum insulin at the cost of elevated blood insulin, free fatty acids (FA), and inflammatory mediators to maintain blood glucose homeostasis. Although the insulin receptor signaling cascade is redundant, with one insulin receptor substrate compensating for the loss of the other’s function, c-Jun n-terminal kinase family members 1 and 2 (JNK, aka stress-activated protein kinases, a subset of mitogen-activated protein kinases), when activated, acts as an intracellular mediator of insulin resistance by disrupting both arms of this cascade. The Randle hypothesis links increased free FA to insulin resistance, proposing that FA compete with glucose as the major energy substrate for mitochondrial oxidation. But not all FA are alike. They vary by chain length, number of double bonds per chain (degree of unsaturation), and configuration (cis or trans). In the diet, they have very different effects on health; the mechanism underlying their adverse or beneficial effects, and how they are distinguished is unknown. Holzer et al. (2011) now propose in a recent issue of Cell that long chain fatty acids (≥C16) lead to formation of intracellular lipid domains that recruit and activate c-Src kinase, linking the elevated saturated FA of obesity to insulin resistance via downstream activation of c-Jun N-terminal kinase 1.
FA activation of JNK is not stringent -- a class, rather than one molecular species, of FA causes JNK activation, i.e. two long chain saturated FA (palmitic and stearic acids) but not unsaturated FA can induce robust JNK activation. Even the addition of a single double bond to the saturated palmitic acid is sufficient to prevent JNK activation. Following up on the finding that the activation of the upstream JNK activator mixed-lineage kinase-3 (MLK3) is dependent upon saturated fatty acids (Jaeschke and Davis, 2007), Holzer et al (2011) tested other tyrosine kinases known to interact with MAP kinases. Clearly, FA activation of both MLK3 and JNK is dependent upon FA saturation, due to the activation of one of these tyrosine kinase, the membrane associated c-Src. Moreover, they explain the mystery of soluble MLK3 activating membrane-bound JNK: the activation of c-Src promotes the translocation of MLK3 to the same FA-induced internal membrane fractions that c-Src and JNK reside in.
How is this selectivity between saturated and non-unsaturated FA for colocalization and activation of these kinases achieved? In general, we can envision both a purely proteinaceous mechanism and a lipidic mechanism. In a proteinaceous mechanism, a FA binding protein (FABPs) selective for saturated FA can signal the translocation of these molecules through protein-protein interactions. For example, FABPs selective for unsaturated lipids can interact with downstream targets that include nuclear regulators and transcription factors like PPARs (Hostetler et al., 2010). Additionally, recently identified G-protein coupled receptors like GPR40-43 and GPR120 can bind FA, with GPR120 able to selectively interact with omega-fatty acids (Oh et al., 2010). A lipidic mechanism is unprecedented but attractive. Increased substrate for lipidic membrane components could lead to increased synthesis of membranes whose cytoplasmic surface facilitates binding and activation of c-Src. Selectivity can arise on the enzymatic level since serine palmitoyltransferase uses palmitate but not palmitoleate as a substrate (Chavez et al., 2003). Palmitate is a precursor of ceramides that in turn gives rise to sphingomyelins, presumably the building blocks of raft domains (Yeung et al., 2008). Rafts by definition accumulate high densities of saturated chains; palmitoylating c-Src activates it constitutively. However, the very existence of rafts is questionable. While the plasma membrane is undoubtedly highly organized and heterogeneous with respect to proteins, the nature of lipid domains in cell membranes is unclear. The ease that cell membranes are fractionated into domains by simple density gradients with or without detergent is provocative, but there are many possible interpretations of such results (Heerklootz et al., 2002). Similarly, the simple concept of membrane fluidity loses its intuitive predictive value given that the diffusion of individual lipids has now been measured directly, revealing almost all regions of the membrane as similarly “fluid” in living cells at physiological temperature. It is possible that as yet poorly characterized protein fences, which impede large-scale motion of lipids and proteins, could generate lipid domains, but this remains to be tested. Clearly more specific language concerning fluidity and lipid domains, and future studies that directly assess lipid composition near proteins of interest without cell disruption, would be valuable.
What is the identity of the membrane domain for the signaling pathway proposed by Holzer et al. (2011)? c-Src targets to endosomes, plasma membrane, and focal adhesions, requiring RhoB endosome-associated actin assembly (Sandilands et al., 2008). Most intracellular membranes, of endoplasmic reticulum and Golgi origin, are excluded, as c-Src has a polybasic domain that binds to anionic lipids, which these membranes are relatively devoid of. The cellular structures whose membranes harboring the putative c-Src signaling domains accumulate intracellularly, like endosomes. Could they include lipid droplets (LDs), organelles composed of neutral lipids and covered with a phospholipid monolayer (Fujimoto, T., 2011)? LD too are induced upon FA uptake by cells with similar time scales. Notably, the LD monolayer also contains the typical raft markers flotilin-1 and caveolin-1, and the LD monolayer may function as a “monolayer domain” and a signaling hub. It is conceivable that LDs enriched in saturated FA may possess biophysical properties positive for c-Src selectivity in a similar way as suggested for rafts. However LD monolayers derived from ER may have insufficient anionic lipid, despite sufficient phosphatidyl inositol (PI) for signaling. Nevertheless, saturated FA may differentially induce lipid droplets from membranes of the endosomal system, rich in anionic phospholipids (C. Jackson and K. Soni, personal communications). Thus the special domains for activation may be on endosomal-derived LD.
Beyond the kinase activation step, toxicity of FA may trigger endoplasmic reticulum (ER) stress and apoptotic pathways. Indeed, palmitate but not palmitoleate is reported to induce ER stress, with mono- and polyunsaturated FA protecting (Diakogiannaki, 2008). Whether ER stress precedes or follows c-Src and JNK activation is not clear, as both processes have timescales of a few hours after palmitate treatment. Clearly more work is needed to understand the effect of dietary lipid on the lipids of our own membranes and on signaling processes that modulate our metabolism. If you are what you don’t metabolize or excrete, we can start to fathom some of the aspects of human nutrition that depend upon FA chain chemistry.
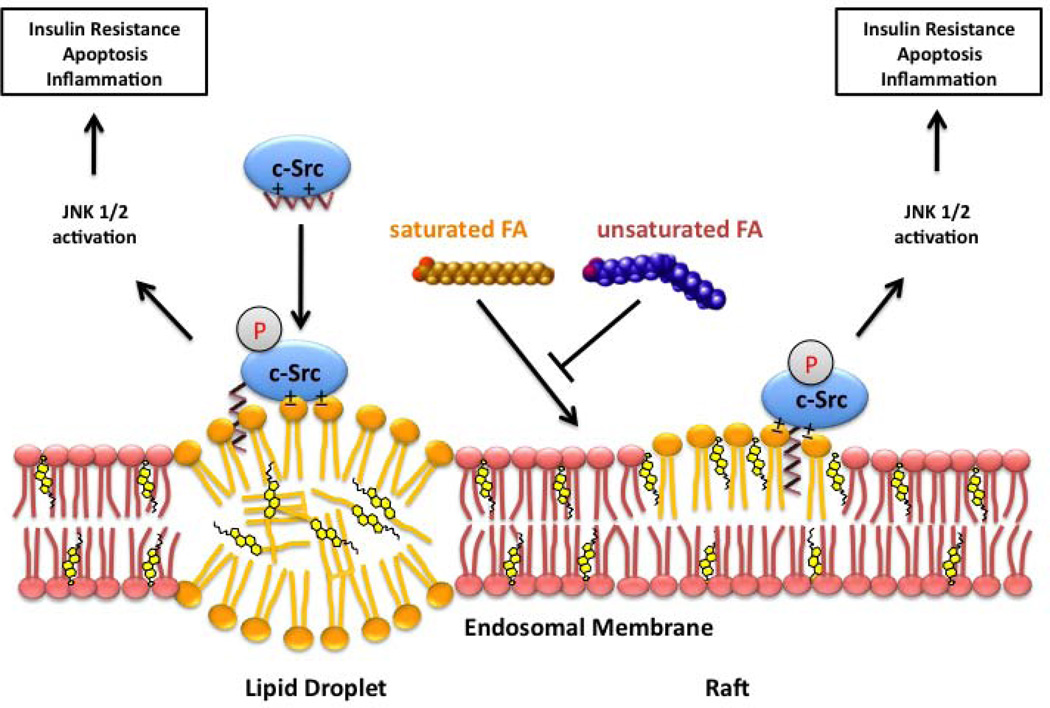
Figure 1. Hypothetical mechanisms linking fatty-acid-induced activation of c-Src and JNK to insulin resistance and inflammatory response.
Holzer et al. (2011) propose that saturated long chain fatty acids (FA) can induce new intracellular membranes to form that bind c-Src, leading to its autophosporylation and activation, and starting a JNK signaling cascade giving rise to insulin resistance. These new membrane domains result from an imbalance in the incorporation of saturated (orange) and unsaturated (blue) fatty acids from diet. Dietary fat can also induce new lipid droplets. Rich in saturated chains, these new surfaces, bilayer or monolayer, could induce the accumulation of dually myristoylated and palmitoylated proteins via interaction of their saturated alkyl chains. c-Src is myristoylated, not palmitoylated, and binds to surfaces with anionic lipids. Long chain saturated fatty acids may also overload sphingomyelin synthesis pathway and lead to the accumulation of ceramides, lipid signaling molecules also capable of induction of insulin resistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chavez JA, Knotts TA, Wang LP, Li G, Dobrowsky RT, Florant GL, Summers SA. J Biol Chem. 2003;278 doi: 10.1074/jbc.M212307200. 10297–102303. [DOI] [PubMed] [Google Scholar]
- Diakogiannaki E, Welters HJ, Morgan NG. J Endocrinol. 2008;197:553–563. doi: 10.1677/JOE-08-0041. [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Parton RG. Cold Spring Harb Perspect Biol. 2011 Mar 1;3(3) doi: 10.1101/cshperspect.a004838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerklotz H. Biophys. J. 2002;83:2693–2701. doi: 10.1016/S0006-3495(02)75278-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer RG, Park E-J, Li N, Tran H, Chen M, Choi C, Solinas G, Karin M. Cell. 2011 doi: 10.1016/j.cell.2011.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetler HA, Balanarasimha M, Huang H, Kelzer MS, Kaliappan A, Kier AB, Schroeder F. J. Lipid Res. 2010;51:3103–3116. doi: 10.1194/jlr.M005041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke A, Davis RJ. Mol. Cell. 2007;27:498–508. doi: 10.1016/j.molcel.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. Cell. 2010;142(5):687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandilands E, Frame MC. Trends Cell Biol. 2008;18:322–329. doi: 10.1016/j.tcb.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Yeung T, Gilbert GE, Shi J, Silvius J, Kapus A, Grinstein S. Science. 2008;319:210–213. doi: 10.1126/science.1152066. [DOI] [PubMed] [Google Scholar]