Abstract
BACKGROUND
Packed Red Blood Cell (PRBC) transfusion is associated with Acute Lung Injury (ALI) development after trauma, but this risk may not be constant through time after trauma. We hypothesized the relationship between PRBC delivery and ALI risk varies through time after injury.
METHODS
Data were collected prospectively from 1999–2006. Inclusion criteria: age > 13 years, SICU admission, and injury severity score (ISS) ≥ 16. Exclusion criteria included discharge/death within 24 hours of admission. Patients were followed prospectively for ALI development for 5 days after trauma. Discrete time models were fit to test the association of timing of PRBC delivery with development of ALI while controlling for patient demographics, resuscitation variables, ISS, and APACHE III scores.
RESULTS
At total of 602 patients were included. Median age was 33 years, 77% were male, and 50% were African American. Using a discrete time-survival model, the relation between transfusion and ALI development was found to vary by transfusion time-window (p<0.0001). The major effect of PRBC delivery on ALI risk occurred in the first 24 hours after trauma; this finding persisted in multivariable modeling (adjusted OR = 1.07 per unit; 95%CI 1.02–1.11, p<0.001). Cumulative incidence of ALI approached 50% in patients receiving ≥ 6u PRBC in the first 24 hours.
CONCLUSIONS
The association between PRBC transfusion and ALI development in trauma patients is time-dependent, with PRBC delivery in the first 24 hours after injury driving the overall relation. Each PRBC unit during this time period increases odds of subsequent ALI development by 7%.
Introduction
Injury remains one of the ten most common causes of death worldwide1, with exanguination being responsible for over 50% of all deaths after trauma. Advances in transfusion medicine over the past century have lead to the survival of patients who would have otherwise died, but at the cost of morbidities related to transfusion therapy. Risks of transfusion include hemolytic reactions, transmission of blood-borne diseases, hypothermia, and immunosupression2. In trauma patients, transfusion is associated with increased rates of infection3, organ failure4, and mortality.5
Blood transfusion is recognized as an important risk factor for the development of acute lung injury (ALI) after trauma5–7. The impact of packed red blood cell (PRBC) delivery on ALI risk after a trauma may vary through time; prior studies demonstrating an association between packed red blood cell transfusion and the development of ALI focused on transfusion in the first 24 or 48 hours of hospitalization and were unable to assess whether the association varied during the days following traumatic injury.
We sought to better understand the relationship between the timing of PRBC administration and ALI development. We hypothesized that the relationship between PRBC delivery and risk of ALI after trauma varies by time after traumatic injury and that the majority of the risk of transfusion is incurred early after traumatic injury.
Materials and Methods
A prospective cohort study was conducted at the Hospital of the University of Pennsylvania (a state-designated level I trauma center) from June 28, 1999 to November 20, 2002 (ARDS I study) and again from October 1, 2005 to April 30, 2010 (ARDS II study). All critically ill trauma patients presenting to the hospital’s emergency department (ED) and admitted to a surgical intensive care unit (SICU) were studied to determine clinical and molecular factors associated with the development of ALI 8. The two year hiatus was due to a funding gap. All subjects over the age of 13 admitted to the SICU from the ED with severe trauma as defined by an injury severity score (ISS) ≥ 16 were eligible. Major exclusion criteria included discharge or death within 24 hours of admission, or the presence of an isolated head injury (defined as an Abbreviated Injury Score ≥ 3 for head/neck but ≤ 1 for all other body regions). Patients were also excluded if they had current or prior congestive heart failure, respiratory disease, morbid obesity, were burned over >30% of total body area, or were lung or bone marrow transplant recipients. Funding for this effort was provided a prior National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (NHLBI) Specialized Center of Research (SCOR) in ALI/ARDS (grant number P50 HL60290) and continued with funding by NIH NHLBI P01-HL79063.
The institutional review board for the University of Pennsylvania reviewed and approved this study with waiver of informed consent. All patients were followed prospectively for the first 5 days following ICU admission after acute trauma for the development of ALI. All blood gas, clinical laboratory, and ventilator data from admission through day 5 in the SICU were recorded using specific case report forms designed for the trauma population. To be designated as having ALI, subjects had to meet all American-European Consensus Conference (AECC) definition criteria within a 24-hour period while endotracheally intubated and receiving mechanical ventilation. Determination of ALI was made by two physician investigators who underwent training to standardize chest radiograph interpretation and independently reviewed all arterial blood gas and ventilator data without knowledge of transfusion or other clinical variables9. Time was windowed into 24 hour periods after admission with day ‘0’ being defined as the first 24 hours after ED admission; day of onset of ALI was defined as the 24 hour time period after admission in which criteria for ALI were first met.
Logistic regression models were fit to test the association of recent PRBC delivery with development of ALI while adjusting for potential baseline confounding factors. Potential confounders considered included age, ISS, mechanism of trauma (blunt vs. penetrating), sex, ICU admission APACHE III scores, and amount of crystalloid fluids delivered. The exposure of interest was the transfusion of PRBC coded as a dichotomous factor of any transfusion received. To achieve a parsimonious model, we excluded variables that did not affect the association of PRBC and ALI, as indicated by a change in odds ratio of greater than 15% on adjustment10.
Subjects who were diagnosed with ALI within 6 hours of PRBC delivery could theoretically have been considered to have transfusion related lung injury (TRALI). To assess the possible contribution of TRALI to the overall incidence of ALI in this cohort, any patient who received PRBC in the 6 hours immediately prior to meeting the criteria for diagnosis of ALI was labeled as possible TRALI (P-TRALI) as per 2004 consensus criteria11. In a sensitivity analysis to assess for the potential impact of these cases, we repeated our analyses after removing these patients.
The development of “damage control resuscitation” paradigms in which PRBC, fresh frozen plasma (FFP), and platelets are transfused in predefined ratios occurred prior to this study. Our own institutional massive transfusion protocol (MTP) was initiated in 1999 and called for PRBC, FFP, and platelets to be delivered in a 10:6:1 ratio in the first cycle of delivery; subsequent cycles of delivery call for units in a 6:4:1 ratio. These cycles of delivery occurred until discontinuation was requested by the attending trauma surgeon. To further assess the impact of the ratio of blood product delivery on ALI, the ratio of FFP to PRBC was calculated and binned into a categorical variable. Logistic regression analysis was used to examine the relationship between product ratio and the development of ALI using patients who received no products as the referent category.
To address the timing (day) of ALI onset and the observed follow-up time (six days total), we implemented a discrete time failure model of the probability that on any day a patient developed ALI, given that the patient was ALI-free on all prior days. This model allowed us to derive an odds ratio assessing the odds of developing ALI on each day, given being “ALI-free” the previous day (it is conceptually similar to Cox’s proportional hazard model12). We implemented this model using the GENMOD procedure in SAS by entering daily PRBC separately in the model. Heterogeneity of PRBC over study days was assessed. To address the potential bias attributable to patients receiving PRBC as a function of other baseline factors that might also be related to ALI in the above modeling framework, we also implemented marginal structural models13. This method estimated inverse probability weights (IPW) with respect to the probability of receipt of PRBC for each patient. These weights were then used in the response model of the association of PRBC (and other factors) and the development of ALI. This method of weighted regression was used to control for the potential confounding among the baseline factors and the receipt of PRBC on estimates of factors leading to the subsequent development of ALI. Parameter estimates for the marginal model were empirical estimates from the generalized estimating equations (GEE) model implemented using data as appropriate from the discrete time failure model.
Due to a funding gap, patients in this study were enrolled from two different time periods (June 28, 1999 to November 20, 2002 and from October 1, 2005 to April 30, 2010). To ensure that changes in clinical care through time did not have an impact on our findings, we repeated the analyses for the overall cohort to patients from each time period and found no significant changes in our point estimates or confidence intervals.
Results
A total of 609 patients met eligibility criteria and were enrolled in the study. Transfusion data were unavailable on 7 patients, leaving 602 patients for analysis. Patients in the cohort tended to be young (median age 33 years), male (77%), and were equally split between African American and Caucasian (Table 1). Compared to patients who did not develop ALI, patients in the ALI group were more likely to be older (median age 38 (IQR 23–50) vs. 33 (IQR 23–47) years), have a blunt mechanism of injury (73% vs. 66%), to be more severely injured (median ISS 25 (IQR 20–29) vs. 22 (IQR 18–27)), and to have a greater degree of physiologic derangement as measured APACHE III scores (median score 65 (IQR 52–77) vs. 58 (IQR 48–71)). When the contribution of the arterial blood gas was removed, APACHE III scores were similar between the two groups (58 (IQR 49–72) in ALI group vs. 56 (IQR 46–67) in non-ALI group)).
Table 1.
Patient demographics, injury severity, physiologic derangement with univariate analysis of ALI risk.
| No ALI n=418 | ALI n=184 | OR | 95% CI | p | |
|---|---|---|---|---|---|
| Age in years* | 33 (23–47) | 38 (23–50) | 1.02 | (0.93 –1.12) | 0.68 |
| Male | 323 (77.3%) | 145 (78.8%) | 1.04 | (0.69 –1.57) | 0.86 |
| Race | |||||
| African American | 221 (52.9%) | 86 (46.7%) | 0.78 | (0.55 –1.10) | 0.16 |
| Caucasian | 182 (43.5%) | 92 (50.0%) | 0.77 | (0.54 –1.09) | 0.13 |
| Hispanic | 14 (3.3%) | 4 (2.2%) | 0.64 | (0.21 –1.98) | 0.44 |
| Other | 0 (0.0%) | 1 (0.5)% | 1.18 | (0.71 –1.97) | 0.52 |
| Comorbidities | |||||
| Alcohol Use | 67 (19.0%) | 24 (16.7%) | 1.18 | (0.71 –1.97) | 0.52 |
| Diabetes | 19 (5.2%) | 14 (8.3%) | 0.61 | (0.29 –1.26) | 0.18 |
| Hypertension | 53 (14.6%) | 24 (15.5%) | 0.94 | (0.56 –1.59) | 0.82 |
| Respiratory Disease | 7 (1.9%) | 7 (4.5)% | 0.42 | (0.15 –1.23) | 0.11 |
| Smoking Status | |||||
| Never smoker | 142 (46.0%) | 71 (56.8%) | Reference | ||
| Current Smoker | 150 (48.5%) | 28 (39.2%) | 0.66 | (0.43 –1.02) | 0.06 |
| Former Smoker | 17 (5.5%) | 5 (4.0%) | 0.58 | (0.21 –1.65) | 0.31 |
| Blunt Mechanism | 275 (66%) | 133 (73%) | 1.37 | (0.93 –2.00) | 0.11 |
| Pulmonary Contusion | 89 (21.4%) | 63 (34.2%) | 1.95 | (1.33 –2.85) | <0.001 |
| Rib Fractures | 135 (32.6%) | 63 (34.2%) | 1.05 | (0.73 –1.52) | 0.78 |
| ISS Category† | |||||
| 16 – 27 | 320 (76.6%) | 119 (64.7%) | 1.20 | (0.95 –1.52) | 0.13 |
| 28 – 75 | 98 (23.4%) | 65 (35.3%) | 1.27 | (1.01 –1.60) | 0.04 |
| APACHE III score | 58 (48–71) | 65 (52–77) | 1.19 | (1.09 –1.29) | <0.001 |
| Modified APACHE III score | 56 (46–67) | 58 (49–72) | 1.13 | (1.04 –1.23) | 0.01 |
Definition of abbreviations: OR = Odds Ratio; 95% CI = 95% confidence interval; ISS= Injury Severity Score; APACHE III = Acute Physiologic and Chronic Health Estimate, version III; Modified APACHE III score = APACHE III score without blood gas
Categorical data are expressed as number and percentages, continuous data are expressed as Median with Interquartile Range (IQR). OR for age expressed per 10 years; OR for ISS expressed per 5 score points; OR for APACHE III and modified APACHE III score expressed per 10 score points.
Because the association between ISS and ALI was found to vary by range of ISS, subjects were divided into ISS categories 16–27 and 28–75.
In univariate analysis, APACHE III score, ISS, pulmonary contusion, and blood products delivered at resuscitation were associated with the development of ALI (Table 1). Using logistic regression, we examined the risk of ALI across the range of ISS seen in our subjects and found that there was an inflection point in ALI risk corresponding to an ISS of 28, above and below which ISS scores were linear in the logit for ALI risk. Injury severity scores below 28 were not found to be significantly associated with the development of ALI, whereas an ISS ≥ 28 was found to contribute significantly to the development of ALI (OR 1.2 per 5 ISS points, 95% CI 1.01–1.60).
Age, sex, race, mechanism of injury were not found to be associated with the development of ALI. Patient comorbidities of alcohol use, diabetes, hypertension, respiratory disease, and smoking status were also not found to be associated with ALI development, likely due to small numbers of subjects with these exposures.
Nearly 20% of patients who developed ALI received greater than 6 units of PRBC, compared to 7% of patients who did not develop ALI. Similarly, the number of fresh frozen plasma (FFP) transfusion units were greater than in those who developed ALI than those who did not (50% vs. 33%), while platelet transfusion volume was similar between the two groups. Ratios of FFP:PRBC in patients who developed ALI and those who did not were not significantly different and were not associated with the development of ALI (Table 2). Patients who developed ALI tended to receive greater volume of crystalloid and blood products than those who did not. Median crystalloid volume infused was 2.3L (IQR 0.7–5.0L) in the ALI group vs. 2.7L (IQR 0.4–5.1L) in the non-ALI group, but this was not significantly associated with the development of ALI.
Table 2.
Crystalloids, blood component delivery, and blood component ratios at resuscitation(with univariate analysis of ALI risk. Definition of abbreviations: OR = Odds Ratio; 95% CI = 95% confidence interval; PRBC = Packed Red Blood Cells; FFP = Fresh Frozen Plasma. Categorical data are expressed as number and percentages, while continuous data are expressed as Median with Interquartile Range (IQR).
| No ALI n=418 | ALI n=184 | OR | 95% CI | p | |
|---|---|---|---|---|---|
| Crystalloid (L) | 2.3 (0.7–5.0) | 2.7 (0.4–5.1) | 1.00 | (0.94 –1.07) | 0.98 |
| PRBC(units) | |||||
| 0 | 280 (67.0%) | 105 (57.1%) | Reference | ||
| 1–3 | 75 (17.9%) | 30 (16.3%) | 1.07 | (0.07 –1.72) | 0.79 |
| 4–5 | 31 (7.7%) | 14 (7.6%) | 1.17 | (0.60 –2.23) | 0.65 |
| ≥ 6 | 21 (7.4%) | 35 (19.0%) | 3.01 | (1.77 –5.13) | <0.001 |
| Fresh Frozen Plasma (units) | |||||
| 0 | 278 (66.5%) | 92 (50.0%) | Reference | ||
| 1–3 | 71 (17.0%) | 38 (20.7%) | 1.62 | (1.02 –2.56) | 0.04 |
| 4–5 | 37 (8.9%) | 26 (14.1%) | 2.12 | (1.22 –3.70) | 0.01 |
| ≥ 6 | 32 (7.7%) | 28 (15.2%) | 2.64 | (1.51 –4.63) | <0.001 |
| Platelets (units) | |||||
| 0 | 383 (91.6%) | 163 (88.6%) | Reference | ||
| 1–3 | 29 (6.9%) | 16 (8.7%) | 1.30 | (0.69 –2.45) | 0.42 |
| 4–5 | 1 (0.2)% | 0 (0.0%) | - | - | - |
| ≥ 6 | 5 (1.2%) | 5 (2.7%) | 2.35 | (0.67 –8.23) | 0.18 |
| FFP: PRBC Ratio | |||||
| No FFP or PRBC | 280 (67.0%) | 105 (57.1%) | Reference | ||
| > 1:1 | 33 (7.9%) | 18 (9.8%) | 1.45 | (0.79–2.70) | .234 |
| 1:1 –1:2 | 36 (8.6%) | 19 (10.3%) | 1.41 | (0.77 –2.56) | .264 |
| 1:2 –1:3 | 11 (2.6%) | 9 (4.9%) | 2.18 | (0.88 –5.42) | .093 |
| < 1:3 | 58 (13.9%) | 33 (17.9%) | 1.52 | (0.94 –2.46) | .090 |
Possible TRALI was identified in 7 out of 184 patients who developed ALI after receiving PRBC within 6 hours prior to diagnosis of ALI. Repeating our analyses with these cases censored did not demonstrate significant changes in univariate analyses.
During the course of the study, individuals who died, were transferred out of the ICU, or developed ALI were censored from the next study day’s analysis. The number of patients at risk per day of the study is shown in Table 3. Focusing on repeated delivery of PRBC over the ICU period using the discrete time failure model, we found that PRBC transfusion beyond the first 24 hours after ED admission was not consistently associated with subsequent development of ALI. Assessment the risk of delivery of FFP on ALI risk was confounded by collinearity with PRBC delivery and therefore could not be included simultaneously in the multivariable model as PRBC delivery. The strength of the association between ALI and transfusion was dependent on the time-window for the transfusion of PRBC.
Table 3.
Subjects at risk at the start of each study day through the study period. Subjects who met endpoints of ALI, death, or transfer out of the ICU were censored for the following day’s analysis. On study day 5, all patients at-risk who did not develop ALI or die were considered to be transferred from the study.
| Study Day | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| At-risk | 602 | 480 | 390 | 314 | 244 |
| ALI | 94 | 26 | 27 | 24 | 13 |
| Died | 0 | 2 | 4 | 0 | 1 |
| Transferred | 28 | 62 | 45 | 46 | 230 |
The overall number of PRBC transfusion per day was not found to have a significant effect on subsequent development of ALI (Chi (1) =0.06, p=0.80). However, including the interaction of the effect of interest (PRBC) and time yielded a significant interaction (Chi (4) =819.70, p<0.0001), indicating that the effect of PRBC on ALI risk was modified by day of transfusion. The degree to which amount of daily PRBC delivery impacted on of subsequent development of ALI was therefore dependent on the study day in the ICU; the effect of PRBC delivery in this model was driven by the amount of PRBC delivered during the first 24 hours. We therefore included the number of units of PRBC transfused during the first 24 hours as a risk factor variable in our discrete time survival model and found a significant effect (Chi (1) =13.37, p=0.0003) and OR=1.07 (95% CI: 1.03 – 1.11). Therefore, with each additional unit of PRBC at resuscitation during the initial 24 hours the odds of subsequent ALI development increased by 7%. Because some patients received uncrossmatched PRBC the first 24 hours, we assessed the relationship between uncrossmatched PRBC delivery and ALI risk. In our final model, uncrossmatched PRBC delivery was not found to be associated with risk of ALI in (Chi (1) =2.81, p=0.094). Adjusting for age, ISS, sex, mechanism of trauma (blunt vs. penetrating), APACHE III score, and total fluids delivered during the resuscitation period, yielded a similar effect (Chi(1)=10.52, p=0.0012, OR = 1.07(95%CI: 1.02–1.11). Similarly, implementing the marginal mode to control for the potential confounding of the delivery of any blood product yielded a similar effect (Z=3.68, p=0.0002, OR=1.08 (95%CI: 1.04–1.13).
In univariate analysis, pulmonary contusion was found to be significantly associated with the development of ALI. To examine the effect of pulmonary contusion on the PRBC-ALI relationship of our above results, we examined the consistency of the relationship for both groups and found consistency of the effect of PBRC in the Pulmonary Contusion group (OR=1.07 (95% CI: 1.01–1.14) and the No Pulmonary Contusion group (OR=1.07 (95%CI: 1.02–1.12).
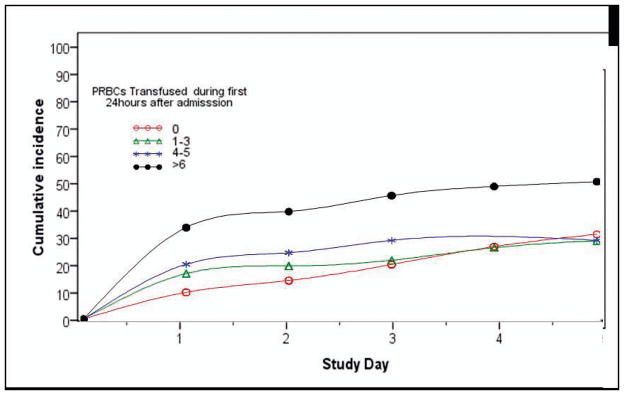
To examine the effect of the dose of PRBC transfused in the first 24 hours on the subsequent cumulative incidence of ALI, PRBC transfusion was binned in to 0, 1–3, 4–5, and >6 units of PRBC in the first 24 hours. The distribution of transfusion of PRBC showed a heavy positive skew, with more than 60% of patients receiving no transfusion in the first 24 hours. Overall, 17% of patients received 1–3 units of PRBC, 8% of patients received 4–5 units of PRBC, and 11% of patients received ≥ 6 units of PRBC in the first 24 hours after presenting to the ED (Table 1). Cumulative incidence rates of ALI during the ICU study period varied as a function of the amount of PRBC delivered within the first 24 hours (Figure 1). Transfusion of any amount of PRBC in the first 24 hour time period was associated with an increased risk of ALI at 24 hours. By 48 hours, the cumulative incidence of ALI was no longer statistically different between patients receiving no PRBC transfusion and those receiving 1–3 units. At 72 hours, the cumulative incidence of ALI was no longer statistically different between patients receiving no units of PRBC and 4–5 units of PRBC. Patients who were transfused >6 units of PRBC in the first 24 hours exhibited a steep rise in the cumulative incidence of ALI between 24–72 hour time period and had a significantly greater cumulative incidence of ALI than all other transfusion groups throughout all days of the study. At day 5, the cumulative incidence of ALI in the > 6 PRBC transfusion group was 53% and was found to be significantly higher than in the 4–5 unit PRBC transfusion group (30%), the 1–3 unit PRBC transfusion group (29%), or the no PRBC transfusion group (27%), (p < 0.001 for each group).
Figure 1.
Cumulative incidence of ALI by PRBC transfusion during resuscitation period according to number of units of PRBCs transfused during the first 24 hours after admission. Transfusion of any amount of PRBCs in the first 24 hour time period lead to an increased incidence of ALI at 24 hours(Day 1). By 48 hours(Day 2), the cumulative incidence of ALI was no longer statistically different between patients receiving no PRBC transfusion and those receiving 1–3 units. At 72 hours(Day 3), the cumulative incidence of ALI was no longer statistically different between patients receiving no units of PRBCs and 4–5 units of PRBCs. Patients who were transfused >6 units of PRBCs in the first 24 hours exhibit a steep rise in the cumulative incidence of ALI between 24–72 hour time period and had a greater cumulative incidence of ALI than patients in the no transfusion, 1–3 unit transfusion, or 4–5 unit transfusion group throughout all days of the study.
Discussion
In this prospective cohort study of patients who sustained severe trauma, transfusion of PRBC was associated with an increase in subsequent risk of development of ALI when transfusion occurred within the first 24 hours after ED presentation but not during other time periods. Furthermore, patients who received any PRBC transfusion during the first 24 hours were nearly twice as likely to develop ALI compared to those who received no PRBC transfusion at all during the first 24 hours after ED admission. While there are other studies that demonstrate an adverse impact of transfusion after trauma, ours is distinguished by a rigorous definition of ALI (prospective collection of data, dual independent physician review of radiographs, blood gasses, and ventilator data). In addition, our study is the first study to use a discreet time failure model methodology to prospectively examine the time-dependent relationship between transfusion and risk of ALI. Therefore, we can more confidently state that the risk of ALI conferred by transfusion occurs most strongly due to transfusion within the first 24 hours after admission and not in the following days.
Our findings regarding the relationship between RBC transfusion and the development of ALI after trauma are consistent with previous studies that did not use time dependent models. Silverboard et al. found that the number of units of PRBC transfused in the first 24 hours was an independent predictor of ALI in severely injured trauma patients (ISS>16). They found that 21% of patients who received 0–5 units, 31% of patients receiving 6–10 units, and 57% of patients receiving >10 units developed ALI. This effect was differential by mechanism of injury, with blunt trauma patients having a lower transfusion threshold to develop ALI5. This study found no association between markers of concomitant shock (as measured by APACHE III scores, base deficit, and use of vasopressor in the first 24 hours) or thoracic trauma and the development of ALI, but the sample size was relatively small (n=102). Chaiwat et al. found that with respect to patients receiving no blood products, patients receiving 1–5, 6–10, or >10 units of PRBC had an OR of 1.88, 2.48, and 2.62 of developing ALI, respectively14.
In our cohort ISS was not found to be consistently associated with ALI development across the entire range of values; for ISS from 16–27, ISS was not significantly associated with the development of ALI. Because the physiology associated with severe injury may be more directly associated with the development of ALI than the injury per se, we also attempted to control for deranged physiology using APACHE using APACHE III scores. Even after controlling for ISS and APACHE III scores, transfusion of PRBC in the first 24 hours was found to be significantly associated with ALI development. Because the abnormal results of arterial blood gas (ABG) measurements contribute to both ALI and APACHE III this analysis was then repeated with using the APACHE III score without ABG with similar findings. These results were similar to those observed by other authors15.
To assess the association between PRBC delivery and ALI risk in a timeframe more remote from acute hemorrhage and injury, Croce et al. found that delayed transfusion was still associated with an increased risk of ALI in a cohort of blunt trauma patients with an ISS of less than 25. In this study, patients who received transfusion within 48 hours of admission were excluded, those patients with delayed transfusion were still found to have increased risk of developing ALI compared to those who did not (OR 3.42; 95% CI 4.02–34.12, p<0.0001).16 A large sample size (n=1040) allowed for ample statistical power to detect small differences; the overall incidence of ARDS in this delayed transfusion cohort was 1%, with an incidence of 0.2% in the non-transfused vs. 2.8% in the transfused group. Our study failed to confirm the effect of transfusion outside of the first 24 hours on subsequent development of ALI, possibly due to our smaller sample size or shorter duration of follow-up. While the short length of follow up may have limited our ability to study longer-term associations between transfusion and ALI, it did allow us to assume that the ALI cases we studied were temporally related to traumatic injury.
There are several putative mechanics through which transfusion may contribute to the subsequent development of ALI. With storage, the plasma membrane of PRBC become less deformable and may “sludge” or lodge in tissue capillary beds after transfusion, directly contributing to local tissue hypoperfusion17. In addition, lipids that accumulate during storage of PRBC may directly activate neutrophils that may become sequestered in the lungs early after trauma leading to neutrophil mediated injury to pulmonary parenchyma18. When ALI occurs within 6 hours of transfusion in the absence of other identified risk factors for ALI, the diagnostic criteria for TRALI have been met11. If other putative risk factors are present such that the development of ALI cannot be definitively ascribed to transfusion, P-TRALI is said to occur. TRALI may result from neutrophil-mediated damage to the pulmonary vascular endothelium. The ‘two-hit hypothesis’ holds that a primary stimulus (such as trauma or surgery) causes pulmonary sequestration of neutrophils followed by a pro-inflammatory stimulus as a result of blood transfusion; this in turn leads to neutrophil activation and local tissue damage, causing fluid and protein to leak into the alveolar space19. There are inherent difficulties with the diagnosis of TRALI and P-TRALI secondary to timing of the diagnosis of ALI. Timing of transfusion was relatively straightforward to capture, but the time at which a patient met criteria for ALI was in part determined by the timing of laboratory values and available chest radiographs. Within these constraints, to the best of our estimation P-TRALI consisted of only 3.8% of cases of ALI after transfusion and repetition of our analysis with these removed did not significantly affect the association between PRBC delivery and ALI.
Evidence of a dose effect for PRBC transfusion on subsequent development of ALI has been suggested by previous studies5,14. Using our continuous risk model we were able to examine the cumulative risk of ALI through the first 5 days of admission by number of units of PRBC transfused. In all categories of PRBC transfusion, the majority of patients who will develop ALI have done so at the end of the first 24 hours, with cumulative incidence rising and additional by 3–5% per day thereafter. In contradistinction, patients who were never transfused exhibit a more linear rise in ALI incidence across the study period. This suggests a synergistic effect between risk of transfusion and other risk factors at play in the first 24 hours. An obvious approach to mitigate ALI risk in this patient population would be to minimize PRBC delivery in the early ICU course. Previous studies of attempts to restrict transfusion in critically ill patients have shown benefit. In 1999, Hebert et al reported the results of a randomized controlled trial which demonstrated a reduction in in-hospital mortality for ICU patients receiving a restrictive (target hemoglobin 7.0 to 9.0 g per deciliter) vs. liberal (target hemoglobin 10.0 to 12.0 g per deciliter) transfusion policy. Although there were decreased ARDS rates in the restrictive vs. liberal transfusion group, this did not reach statistical significance (7.7 vs. 11.4%, p=0.06)20. A post-hoc analysis of trauma patients in Hebert’s original trial demonstrated no difference in mortality or organ dysfunction scores between the two transfusion groups, but ALI rates were not specifically examined in this study21. No studies exist in on restrictive transfusion policies early after trauma; it is obvious that this approach must viewed with extreme caution secondary to risk of ongoing bleeding early after injury.
While in univariate analysis both PRBC and FFP delivery within the 24 hours of injury were both associated with the development of ALI, we did not find any significant association between in ALI risk and ratios of FFP to PRBC; that is, ‘damage control resuscitation’ did not appear to change the risk of ALI. The current literature examining the role of blood product ratios in trauma patients does not specifically address how transfusion ratios are associated with ALI risk, but our finding of that high FFP:PRBC ratios is not associated with subsequent development of ALI must be interpreted with caution. Use of blood product ratios as a predictor of ALI risk is limited as the ratio itself does not account for the overall volume of blood products delivered, which is a known predictor of ALI. Due to significant collinearity between PRBC and FFP delivery during the study period, only one variable could be included in the construction of a parsimonious multivariable model. Because PRBC delivery was found to be a more strongly associated with ALI development than FFP delivery, only PRBC delivery was included in the final multivariable model.
This study has several limitations. While other authors have reported FFP delivery may contribute to the development of ALI22, we cannot specifically comment on this relationship because of collinearity between PRBC and FFP delivery. For the same reason, the ratio of FFP:PRBC did not influence the development of ALI. As this study is observational in nature, we cannot report a causal relationship between PRBC transfusion and ALI. It is possible that the association we describe is related to predictor variables not captured by our study. Transfusion is intertwined with patient physiology, and it is possible that hemorrhagic shock and not the blood transfused in response contributes to the development of ALI (confounding by indication). We have attempted to control for this by using the ISS and APACHE III scores, but ultimately these may not adequately risk-adjust this cohort of patients.
The way in which blood is processed and stored may have implications for the development of transfusion related complications. Specifically, older blood products and non-leukoreduced blood products have in some series been shown to be associated with the development of ALI. While we do not have data on the average age of blood products, there were no systemic changes in the collection or storage of blood that would predispose to changes in the average age of PRBC units across the study period. The use of leukoreduction has gradually increased over the past 10 years and now approaches 90% at our institution, but we do not have specific data on whether or not individual units of PRBCs were leukoreduced. However, since the literature on the impact of leukoreduction on outcomes after transfusion is mixed 23–25 and no study has specifically focused on the impact of leukoreduction on ALI after transfusion in trauma patients, there is no overwhelming argument that the amount of leukoreduced blood a subject received must be controlled for when examining ALI as an outcome. Finally, because this is a single-center study, our findings may not be generalizable to other institutions.
Conclusions
Risk of transfusion of PRBC on the subsequent development of ALI varied within the period after injury. PRBC transfusion in the first 24 hours after injury but not thereafter independently predicted subsequent risk of development of ALI in a dose-dependent fashion. Transfusion of >6 units of blood in the first 24 hours after injury was associated with a cumulative incidence of ALI of >40% after 24 hours; this rate increased to >50% by post-injury day 5.
Acknowledgments
This research was supported in part by National Institutes of Health National Heart, Lung, and Blood Institute grants P50- 60290 and P01-HL79063.
Footnotes
This work was presented as a poster presentation at the 2011 Society for Critical Care Medicine meeting in San Diego, CA.
Contributor Information
Daniel N Holena, Email: daniel.holena@uphs.upenn.edu.
Giora Netzer, Email: gnetzer@medicine.umaryland.edu.
Russell Localio, Email: rlocalio@mail.med.upenn.edu.
Robert J Gallop, Email: Rgallop@wcupa.edu.
Scarlett L Bellamy, Email: bellamys@mail.med.upenn.edu.
Nuala J Meyer, Email: Nuala.Meyer@uphs.upenn.edu.
Michael GS Shashaty, Email: Michael.Shashaty@uphs.upenn.edu.
Paul N Lanken, Email: Paul.Lanken@uphs.upenn.edu.
Sandra Kaplan, Email: Sandra.Kaplan@uphs.upenn.edu.
Patrick M Reilly, Email: Patrick.Reilly@uphs.upenn.edu.
Jason D Christie, Email: Jason.Christie2@uphs.upenn.edu.
References
- 1.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006 May 27;367(9524):1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 2.Raghavan M, Marik PE. Anemia, allogenic blood transfusion, and immunomodulation in the critically ill. Chest. 2005 Jan;127(1):295–307. doi: 10.1378/chest.127.1.295. [DOI] [PubMed] [Google Scholar]
- 3.Dunne JR, Riddle MS, Danko J, Hayden R, Petersen K. Blood transfusion is associated with infection and increased resource utilization in combat casualties. The American surgeon. 2006 Jul;72(7):619–625. discussion 625–616. [PubMed] [Google Scholar]
- 4.Moore FA, Moore EE, Sauaia A. Blood transfusion: An independent risk factor for postinjury multiple organ failure. Archives of Surgery. 1997;132(6):620–625. [PubMed] [Google Scholar]
- 5.Silverboard H, Aisiku I, Martin GS, Adams M, Rozycki G, Moss M. The role of acute blood transfusion in the development of acute respiratory distress syndrome in patients with severe trauma. Journal of Trauma - Injury, Infection and Critical Care. 2005;59(3):717–723. [PubMed] [Google Scholar]
- 6.Croce MA, Tolley EA, Claridge JA, Fabian TC. Transfusions result in pulmonary morbidity and death after a moderate degree of injury. The Journal of trauma. 2005 Jul;59(1):19–23. doi: 10.1097/01.ta.0000171459.21450.dc. discussion 23–14. [DOI] [PubMed] [Google Scholar]
- 7.Chaiwat O, Lang JD, Vavilala MS, et al. Early packed red blood cell transfusion and acute respiratory distress syndrome after trauma. Anesthesiology. 2009 Feb;110(2):351–360. doi: 10.1097/ALN.0b013e3181948a97. [DOI] [PubMed] [Google Scholar]
- 8.Christie JD, Gaughan C, Gallop R. Clinical risk factors of development of ARDS in a cohort study of patients with major trauma. Am J Respir Crit Care Med. 2003;167:A740. [Google Scholar]
- 9.Shah CV, Localio AR, Lanken PN, et al. The impact of development of acute lung injury on hospital mortality in critically ill trauma patients. Critical Care Medicine. 2008;36(8):2309–2315. doi: 10.1097/CCM.0b013e318180dc74. [DOI] [PubMed] [Google Scholar]
- 10.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. American Journal of Epidemiology. 1993;138(11):923–936. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 11.Kleinman S, Caulfield T, Chan P, et al. Toward an understanding of transfusion-related acute lung injury: Statement of a consensus panel. Transfusion. 2004;44(12):1774–1789. doi: 10.1111/j.0041-1132.2004.04347.x. [DOI] [PubMed] [Google Scholar]
- 12.Willett JDSaJB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- 13.Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. American Journal of Epidemiology. 2008;168(6):656–664. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaiwat O, Lang JD, Vavilala MS, et al. Early packed red blood cell transfusion and acute respiratory distress syndrome after trauma. Anesthesiology. 2009;110(2):351–360. doi: 10.1097/ALN.0b013e3181948a97. [DOI] [PubMed] [Google Scholar]
- 15.Navarrete-Navarro P, Rivera-Fernández R, Rincón-Ferrari MD, et al. Early markers of acute respiratory distress syndrome development in severe trauma patients. Journal of Critical Care. 2006;21(3):253–258. doi: 10.1016/j.jcrc.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Croce MA, Tolley EA, Claridge JA, et al. Transfusions result in pulmonary morbidity and death after a moderate degree of injury. Journal of Trauma -Injury, Infection and Critical Care. 2005;59(1):19–24. doi: 10.1097/01.ta.0000171459.21450.dc. [DOI] [PubMed] [Google Scholar]
- 17.Frenzel T, Westphal-Varghese B, Westphal M. Role of storage time of red blood cells on microcirculation and tissue oxygenation in critically ill patients. Current Opinion in Anaesthesiology. 2009;22(2):275–280. doi: 10.1097/ACO.0b013e328323f7c4. [DOI] [PubMed] [Google Scholar]
- 18.Silliman CC, Voelkel NF, Allard JD. Plasma and lipids from stored packed red blood cells cause acute lung injury in an animal model. J Clin Invest. 1998;101:1458–1467. doi: 10.1172/JCI1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bux J, Sachs UJH. The pathogenesis of transfusion-related acute lung injury (TRALI) British Journal of Haematology. 2007;136(6):788–799. doi: 10.1111/j.1365-2141.2007.06492.x. [DOI] [PubMed] [Google Scholar]
- 20.Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. New England Journal of Medicine. 1999;340(6):409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 21.McIntyre L, Hebert PC, Wells G, et al. Is a restrictive transfusion strategy safe for resuscitated and critically ill trauma patients? Journal of Trauma - Injury, Infection and Critical Care. 2004;57(3):563–568. doi: 10.1097/01.ta.0000136158.93864.54. [DOI] [PubMed] [Google Scholar]
- 22.Inaba K, Branco BC, Rhee P, et al. Impact of Plasma Transfusion in Trauma Patients Who Do Not Require Massive Transfusion. Journal of the American College of Surgeons. 2010;210(6):957–965. doi: 10.1016/j.jamcollsurg.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 23.Hébert PC, Fergusson D, Blajchman MA, et al. Clinical Outcomes Following Institution of the Canadian Universal Leukoreduction Program for Red Blood Cell Transfusions. Journal of the American Medical Association. 2003;289(15):1941–1949. doi: 10.1001/jama.289.15.1941. [DOI] [PubMed] [Google Scholar]
- 24.Nathens AB, Nester TA, Rubenfeld GD, Nirula R, Gernsheimer TB. The effects of leukoreduced blood transfusion on infection risk following injury: A randomized controlled trial. Shock. 2006;26(4):342–347. doi: 10.1097/01.shk.0000228171.32587.a1. [DOI] [PubMed] [Google Scholar]
- 25.Phelan HA, Sperry JL, Friese RS. Leukoreduction Before Red Blood Cell Transfusion Has No Impact on Mortality in Trauma Patients. Journal of Surgical Research. 2007;138(1):32–36. doi: 10.1016/j.jss.2006.07.048. [DOI] [PubMed] [Google Scholar]