Abstract
Introduction
Tumor recurrence remains a main limitation to the long-term survival of patients following liver transplantation for hepatocellular carcinoma (HCC). While the majority of patients recur in the first two years after transplantation, late recurrence is not infrequent.
Discussion
Most common sites of recurrence in order of decreasing frequency are liver graft, lung, bone, abdominal lymph nodes, adrenal glands and peritoneum. Reported five-year survival after surgical resection ranges from 27-88%. Few patients, however, are candidates for surgical resection. Other therapeutic options for recurrent HCC include systemic therapy, intra-arterial therapy, or radiation therapy. Although systemic molecular targeted therapy is generally tolerated with very few interactions with immunosuppressive medications, there is only modest success regarding prolongation of survival. Utilization of radiation therapy for extrahepatic recurrences similarly has minimal impact on overall survival, but may effectively in palliate symptoms. While late recurrence is associated with a more favorable prognosis than early recurrences, prognosis is still poor.
Conclusion
Late recurrence of HCC following transplantation should be borne in mind even after many years from transplant. Surgical salvage, when feasible, remains a viable treatment option in select patients with a chance for long-term survival. A multi-disciplinary approach is critical as different therapeutic modalities have a role in treating recurrent HCC following transplant.
Keywords: Hepatocellular carcinoma, Recurrence, Liver transplantation, Salvage resection, Multidisciplinary, Outcomes
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer mortality worldwide, accounting for more than 500,000 deaths annually.1 Major risk factors include chronic liver disease and cirrhosis due to hepatitis B and C infections, alcoholic liver disease and non-alcoholic steatohepatitis. Surgical management remains the only potentially curable treatment option for patients with HCC. While partial liver resection is a reasonable approach for patients with no or well compensated cirrhosis, orthotopic liver transplantation is the preferred treatment for patients with early stage HCC in the background of cirrhosis. In 1996, Mazzaferro at al. proposed a set of macro-morphologic selection criteria for HCC, including a single tumor<5 cm, 2–3 lesions each<3 cm, and the absence of macroscopic vascular invasion.2 Using these now widely adopted Milan criteria, liver transplantation for HCC is associated with an excellent long-term outcome with a 5- and 10-year survival of 73% and 70%, respectively.3 HCC recurrence can, however, occur in a subset of patients and represents one of the major threats to long-term survival after liver transplantation for HCC. We herein review the literature on the pattern and timing of HCC recurrence after transplantation, as well as review management options and outcomes of recurrent HCC disease following transplantation.
Liver transplantation is the preferred treatment of early stage hepatocellular carcinoma in the setting of cirrhosis. Unlike resection, transplantation provides the benefit of complete tumor excision as well as removal of the underlying diseased liver. While long-term survival following liver transplantation now exceeds 70%, disease recurrence remains an issue in a subset of patients and can adversely affect long-term survival. The incidence of recurrent HCC following transplantation has been reported to vary, ranging from 6-56%4-7 – perhaps reflecting differences in patient selection for initial transplantation. While several recipient and tumor-specific factors are prognostically important, primary tumor size, number of lesions, and presence of vascular invasion have been noted to be the most significant clinical risk factors for both recurrence and survival.4, 6, 8-10 Given this, the overall incidence of recurrence has decreased dramatically since the adoption of the so-called Milan criteria, which ushered in a more stringent selection of HCC patients for transplantation.11 In an analysis of the United Network for Organ Sharing (UNOS) database that included 985 patients undergoing liver transplantation between 1987 and 2001, Yoo at al. demonstrated that survival after transplantation dramatically improved following the adoption of the Milan criteria (5-year survival, pre-Milan criteria: 25% versus post-Milan, 61% p=0.001).12
Following hepatic resection of HCC, the majority of recurrences occur within the liver (Fig. 1).13 While removal of the entire diseased liver attenuates the risk of intrahepatic recurrence following transplantation, tumor recurrence in the liver allograft does occur. De-novo tumor development from recurrent hepatitis and cirrhosis in the liver graft can occur, however most intrahepatic recurrences after liver transplant probably are secondary to hematogenous spread. Some data have suggested that the transplanted liver may be particularly susceptible to circulating tumor cells by liver specific adhesion molecules (homing pattern) and a positive microenvironment for cell growth.14, 15 Recurrent disease following liver transplantation for HCC may involve an extrahepatic site in 10-43% of patients (Table 1). The presence of microscopic extrahepatic foci of disease in lymph nodes or distant organs at the time of transplantation, as well as hematogenous or peritoneal tumor dissemination during transplantation, are mechanisms attributed to disease recurrence.16 The most common extrahepatic sites of recurrence include lung, bone, abdominal lymph nodes, adrenal glands and peritoneum, in descending order of frequency (Fig. 1) (Table 1).
Fig. 1.
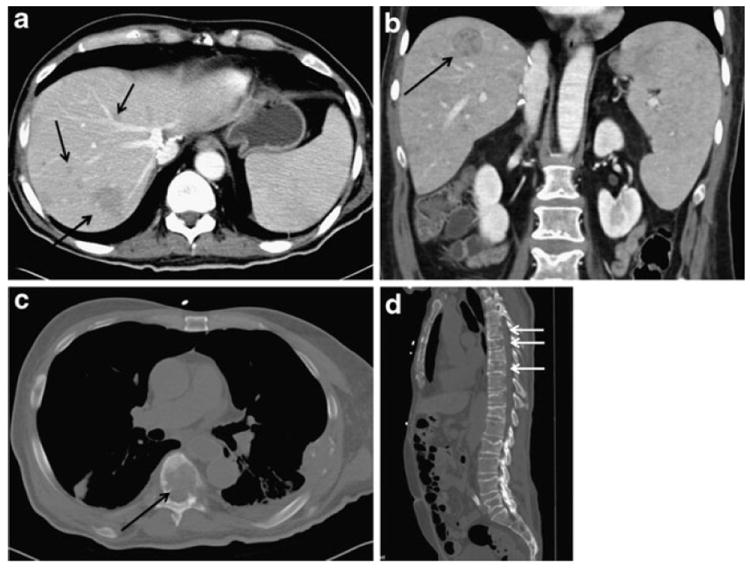
Recurrence following liver transplantation. Axial (A) and coronal images demonstrating intrahepatic recurrence. Axial (C) and sagittal images (D) demonstrating bone metastasis of the spine
Table 1.
Pattern and timing of recurrence of hepatocellular carcinoma after liver transplantation
| Author | Location | Time-frame | No. of patients | HCC recurrence rate | Median time to recurrence (months) | Late recurrence | Multiple site recurrence | Intrahepatic recurrence | Extrahepatic recurrence | Extrahepatic sites |
|---|---|---|---|---|---|---|---|---|---|---|
| Schlitt et al.8 | Hannover, Germany | 1972-1994 | 69 | 39 (56.5%) | 14.5 | 14 (20.5%) ≥3 years | 19 (27.5%) | 24 (34.8%) | 30 (43.5%) | Lung (22), bone (7), adrenal (5), lymph node (4) |
| Regalia et al.9 | Milan/Genova, Italy | 1987-1996 | 132 | 21 (15.9%) | 7.8 | 0≥3 years | 15 (11.4%) | 4 (3.0%) | 17 (12.9%) | Lung (4), bone (3), peritoneum (1), subcutaneous (1) |
| Roayaie et al.22 | New York, USA | 1988-2002 | 311 | 57 (18.3%) | 12.3 | 6 (1.9 %) ≥4 years | 38 (12.2%) | 39 (12.5%) | 48 (15.4%) | Lung (25), bone (19), others (14) |
| Shin et al.19 | Seoul, Korea | 1992-2005 | 138 | 28 (20.3%) | 7.9 | NR | 7 (5.1%) | 14 (10.1%) | 24 (17.4%) | Lung (10), bone (8), lymph node (3), peritoneum (2), adrenal (1) |
| Kornberg et al.18 | Jena, Germany | 1994-2007 | 60 | 16 (26.7%) | 23 | 1 (1.7%) ≥5 years | 5 (8.3%) | 4 (6.7%) | 12 (20.0%) | Lung (5), bone (4), adrenal (1), peritoneum (1), cerebrum (1) |
| Taketomi et al.21 | Fukuoka, Japan | 1996-2007 | 101 | 17 (16.8%) | 12.9 | 1 (%) ≥4 years | NR | NR | NR | NR |
| Chok et al.17 | Hong Kong, China | 1994-2007 | 139 | 24 (17.3%) | NR | 2 (1.4%) ≥5 years | NR | 11 (7.9%) | 15 (10.8%) | Lung (6), bone (3), lymph node (3), adrenal (2), peritoneum (1) |
| Valdivieso et al.20 | Bilbao, Spain | 1996-2008 | 182 | 23 (12.6%) | 23.4 | NR | 5 (2.7%) | 7 (3.8%) | 21 (11.5%) | Lung (12), bone (5), adrenal (2), lymph node (2) |
NR, not recorded
While patients who present with disseminated disease are generally not candidates for local therapy, successful surgical salvage has been reported for intrahepatic and/or confined extrahepatic HCC metastases. In a study involving several Italian centers, 7 out of 21 patients (30%) underwent salvage resection of recurrent HCC of the liver (2), lung (2), bone (1), skin or other sites (2). Surgical resection was associated with a survival of 15.5 months, which was better than the 5.5 months noted among patients treated with a non-surgical approach (Fig. 2).9 A review of studies that have evaluated outcomes of patients with recurrent metastatic HCC following liver transplantation revealed an associated overall 5-year survival of 13% - 35% (Table 2). Albeit in a select group of patients, surgical resection of both intra and/or extrahepatic HCC recurrence may be associated with an improved survival ranging from 27%-88% (Table 2). Therefore, in addition to reduction of immunosuppressive therapy, a thorough assessment of surgical options for a possibly curative intervention should be considered in patients with recurrent HCC.
Fig. 2.
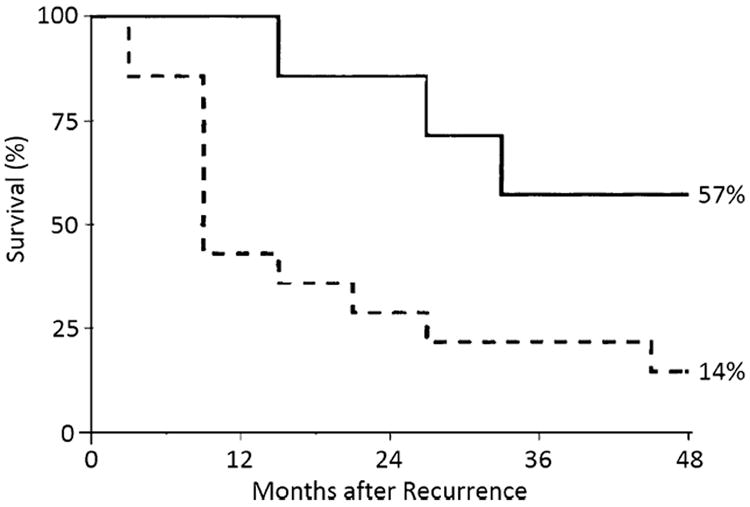
Survival after HCC recurrence following liver transplantation: Comparison of resectable (solid) vs. unresectable (dotted line) patients (57% vs. 14% at 4-years, p=0.02); Used with permission: Regalia E, et al.: Pattern and management of recurrent hepatocellular carcinoma after liver transplantation. J Hepatobiliary Pancreat Surg 1998;5:29–34.
Table 2.
Studies assessing surgical salvage of recurrent hepatocellular carcinoma after liver transplantation
| Author | Location | Time-frame | No. of patients with HCC recurrence | Subset meeting Milan criteria | Patients subjected to surgical salvage | Liver directed therapy (resection, RFA, TACE) | Re-Transplant | Metastasectomy | Radiation | Systemic therapy | Overall 5-year survival after recurrence | 4/5-year survival after surgical salvage |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Schlitt et al.8 | Hannover, Germany | 1972-1994 | 39 | NR | 15 (38%) | 3 (7.7%) | 2 (5.2%) | 13 (33.3%) | 2 (5.2%) | 5 (12.8%) | NR | 47%a |
| Regalia et al.9 | Milan/Genova, Italy | 1987-1996 | 21 | 6 (28.6%) | 7 (32%) | 2 (9.5%) | 0 | 5 (23.8%) | 5 (23.8%) | 9 (42.9%) | 23% | 57%a |
| Roayaie et al.22 | New York, USA | 1988-2002 | 57 | NR | 18 (32%) | 11 (19.3%) | 0 | 10 (17.5%) | 4 (7.0%) | 15 (26.3%) | 22% | 47%b |
| Shin et al.19 | Seoul, Korea | 1992-2005 | 28 | 8 (28.6%) | 4 (14.3%) | 1 (3.6%) | 1 (3.6%) | 3 (10.7%) | NR | NR | NR | 50%a |
| Kornberg et al.18 | Jena, Germany | 1994-2007 | 16 | 1 (6.3%) | 7 (44%) | 4 (25.0%) | 0 | 5 (31.3%) | 3 (18.8%) | 1 (6.3%) | 31% | 71%b |
| Taketomi et al.21 | Fukuoka, Japan | 1996-2007 | 17 | 1 (5.9%) | 9 (52.9%) | 4 (23.5%) | 0 | 8 (47.1%) | 7 (41.2%) | 5 (29.4%) | 35% | 88%b |
| Chok et al.17 | Hong Kong, China | 1994-2007 | 24 | 11 (45.8%) | 17 (70.8%) | 11 (45.8%) | 0 | 12 (50.0%) | 9 (37.5%) | 16 (66.7%) | 25% | NR |
| Valdivieso et al.20 | Bilbao, Spain | 1996-2008 | 23 | 14 (60.9%) | 11 (47.8%) | 2 (8.7%) | 0 | 6 (26.1%) | 0 | 4 (17.4%) | 13% | 27%b |
NR, not recorded; RFA, radiofrequency ablation; TACE, transarterial chemoembolization
4- year survival rate after surgical salvage
5-year survival rate after surgical salvage
When recurrent disease is intrahepatic in location, in some cases, resection and/or ablation of the liver allograft may be feasible (Table 2). As with HCC of the native liver, the utilization of resection versus ablation to treat recurrence in the allograft is dependent on surgical judgment, as well as the size and location of the tumor. While resection may be more applicable to more superficial and larger tumors, ablative techniques may be sufficient and appropriate in the setting of smaller and more deeply situated tumors. Although liver resection for intrahepatic HCC recurrence has been reported by several centers, most series are limited by a small sample size (< 10 cases).17-21 Roayaie et al. published a series that included 5 patients who underwent liver resection and 3 patients who underwent radiofrequency ablations for intrahepatic recurrences after transplant.22 Other patients included in the study had extrahepatic disease and underwent lung resection (7), adrenalectomy (3) or resection of a chest wall recurrence (1). Although 15 of the 19 surgically managed patients developed re-recurrence, 5-year survival was 47% post-transplant. Multivariate analysis revealed that surgical treatment was independently associated with prolonged survival (HR 2.5, p=0.016). The absence of bone metastases (HR 0.54, p=0.021), as well as a time to recurrence of >1 year after transplant (HR 0.96, 0.002) were each independently associated with a better outcome following surgical resection of recurrent disease. In a separate study, Schlitt et al. reported on 39 patients with recurrent disease, of whom 9 patients had intrahepatic recurrence, 15 extrahepatic disease and 15 patients who had both intra- and extrahepatic recurrence.8 Eleven of these patients were able to undergo complete removal of the recurrent disease, including 5 patients with an intrahepatic recurrence; 7 (63%) were alive at 4.3 years of follow-up.
Reports of repeat liver transplantation as a treatment of recurrent intrahepatic HCC are limited to a few very select case series. Schlitt et al. reported on two patients with intrahepatic recurrence who were managed with re-transplantation at 6 and 10 years following initial transplantation, but long term outcome was note described.8 Shin et al. reported on one patient who experienced an intrahepatic HCC recurrence at one year after initial liver transplant who subsequently underwent a re-transplantation.19 The authors noted that this patient had survived for 45 months without additional recurrences.
Another potential approach to intrahepatic HCC recurrence is the utilization of intra-arterial therapy (IAT) with chemoembolization (e.g. TACE). Data on the use of IAT for recurrent intrahepatic HCC of the allograft is, however, also lacking. Ko et al. reported on 28 patients with recurrent HCC who underwent one or more cycles of TACE after transplantation (mean, 2.5 cycles).23 In this study, the targeted tumor reduced in size by≥25% in 19 of the 28 study patients (68%). However, intrahepatic or extrahepatic metastasis occurred in 21 of the 28 patients (75%) during the 3-month follow-up period and mean survival was only 9 months. While Ko et al. noted no significant morbidity, other investigators have noted an elevated risk of biliary ischemia / necrosis of the liver allograft following IAT.18, 22, 24
Systemic therapeutic options for recurrent HCC are limited (Table 2). While cytotoxic agents have traditionally had marginal effect in the treatment of HCC, systemic therapy with molecular targeted therapy has been shown to prolong survival in recent trials. Sorafenib, a multi-targeted kinase inhibitor, demonstrated a significant overall survival benefit in patients with advanced or metastatic HCC when compared with placebo in two separate Phase 3 trials. In the landmark Sorafenib HCC Assessment Randomized Protocol (SHARP) over 600 patients with advanced HCC, intact liver function (Child-Pugh class A) and good performance status were randomized to sorafenib or placebo.25 The study was stopped at the second planned interim analysis due to a significant difference in overall survival favoring the sorafenib arm, with median survival of 10.7 months and 7.9 months, respectively, for the sorafenib and placebo arms (HR 0.69 (0.55-0.87); p<0.001). A similar overall survival benefit from sorafenib was noted in a second phase 3 trial, carried out in the Asia-Pacific region, with similar entry criteria and treatment plan.26 In this study of over 200 patients, those on the sorafenib arm had a median survival of 6.5 months compared with 4.2 months for those on placebo. The HR of 0.68 (0.50-0.93) was almost identical to the SHARP trial, although the absolute survival was notably lower, likely due a study population with more advanced disease (radiographically and biochemically), and a slightly worse performance status as a whole.
These studies were carried out in patients who presented initially with advanced disease (mostly liver confined disease), and did not include patients who had previously undergone curative-intent therapy, such as surgical resection or liver transplantation. Since the approval of sorafenib in 2007, there has been interest in assessing its effectiveness in patients with recurrent disease after resection or transplant. A number of retrospective studies have reported acceptable safety data for sorafenib in liver transplant patients, with very few unexpected toxicities or interaction with immunosuppressive medications.27, 28 The numbers in these studies are small, and there is clearly a need for a prospective trial to fully assess the potential survival benefit of sorafenib in this setting. Work is also ongoing to assess the benefit of sorafenib as adjuvant therapy in patients undergoing liver transplantation, after a retrospective analysis suggested a reduced recurrence rate and improved disease-free and overall survival at one year post transplant.29
Radiation therapy is another option for patients with recurrent unresectable HCC.30 Three-dimensional conformal radiation, as well as stereotactic body radiation therapy and radioembolization, have been utilized in the treatment of primary unresectable HCC.31, 32 In addition, radiation therapy is a treatment option for symptomatic palliation of extrahepatic disease. In one study, Seong et al. investigated the effectiveness of palliative radiation therapy for HCC bone metastasis.33 In this study, 51 patients received radiation therapy for 77 bony metastatic lesions, with a median total dose of 30 Gy. There was pain relief in 56 lesions (73%), however, median and 1-year survival were only 5 months and 15%, respectively. In another study, Kaizu et al. reported on the use of radiation and noted that higher doses (48 Gy/2 Gy fxs, 39 Gy/3 Gy fxs) were associated with improved tumor responses when compared with lower standard palliative doses of RT (30 Gy/3 Gy fxs).34 In this study the response rate was 83.8%, and the median survival period from the time of the initial occurrence of a bone metastasis was 6 months. Other studies have similarly suggested that radiation therapy can be associated with palliative relief of symptomatic recurrent metastatic disease.35 While radiation therapy has been utilized mostly in the palliation of bone metastasis, radiation may also be useful in palliating metastatic disease to other sites.36 In one study, intensity modulated radiation therapy (IMRT) was used to treat an orbital metastatic HCC tumor. One month after completion of IMRT (58 Gy/1.8 Gy fxs) the patient had a radiologic (MRI) and symptomatic response and has local control in the orbit 1.7 years after therapy completion.37 Another study demonstrated favorable control of metastatic HCC to the adrenal gland with stereotactic body radiation therapy (SBRT).36 Yamashita et al. reported on 28 patients with metastatic HCC involving the portal, peripancreatic, and/or portal lymph nodes who were treated with radiation therapy.38 Patients received between 46 and 60 Gy in daily 2.0-Gy fractions. A total of 18 (64%) and five (18%) patients achieved partial responses and complete responses, respectively. The 1- and 2-year overall survival rates and the median survival time were 53% and 33%, respectively. In aggregate, these studies suggests that recurrent metastatic HCC may be sensitive to palliative radiation therapy with a recommended dose of ~50-60 Gy in 2–3 Gy fractions. Although, RT has historically been underutilized to palliate metastatic HCC, advancement in radiation technologies (SBRT/IMRT and daily imaging) has resulted in more focal radiation of HCC metastases. In turn, this has allowed for dose escalation of radiation that appears to result in improved tumor control with less normal tissue toxicity. Therefore, radiation therapy should be considered for palliation of metastatic HCC lesions.
Although the vast majority of HCC recurrences are detected in the first 2 years after transplantation, cases of late recurrence have been documented (Table 1). Roayaie et al. reported a median time to recurrence of 12.3 months after transplantation.22 Among 59 patients with a recurrence, only 6 (10%) patients developed a recurrence 4 or more years after transplantation (Fig. 3). Tumor factors associated with late recurrence were small tumor size (<5 cm: 24 vs. ≥ 5 cm: 13 months, p=0.008) and well to moderate differentiation (well: 24 vs. moderate: 16 vs. poor: 11 months, p=0.018). Interestingly, in this study, patients with HCC due to cryptogenic cirrhosis (13 months) or hepatitis B (14 months) were noted to recur earlier than patients with hepatitis C (17 months) or alcoholic cirrhosis (33 months) (p=0.043). In a separate study by Schlitt et al, the authors similarly noted that the overwhelming majority of recurrences occurred within the first few years following transplantation (69% recurrences were within the first two years, 10% in the third year, and only 21% after 3 years).8 These authors also noted that late recurrences were more common among patients transplanted with lower stage disease (p=0.016) and well differentiated tumors (p=0.011). Of note, time to recurrence did not differ significantly between patients with intrahepatic or extrahepatic recurrence. Although the natural history of late HCC recurrences after liver transplant is not well understood, several studies have noted more favorable outcomes – indicating perhaps a more indolent tumor biology. For example, Chok et al. reported that patients who had a late recurrence defined as 2 or more years after transplantation had a more favorable 5-year survival (71%) compared with patient who recurred earlier (7%) (p=0.001).17 Other studies that have assessed survival after diagnosis of post-transplant HCC tumor recurrence have similarly found that a late recurrence was an independent predictor of survival.18, 22
Fig. 3.
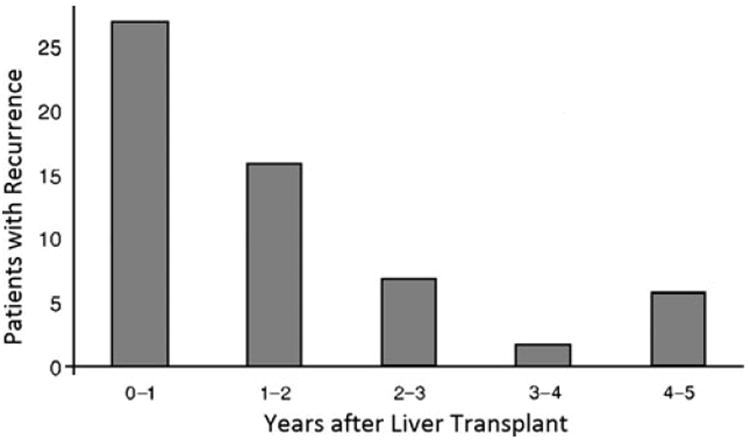
Time Spectrum of HCC recurrence of patients after liver transplantation; Used with permission: Roayaie S, et al.: Recurrence of hepatocellular carcinoma after liver transplant: patterns and prognosis. Liver Transpl 2004;10:534–540
In conclusion, management of patients with recurrent HCC following transplantation is challenging. Although data are scarce, intrahepatic disease can be managed with repeat resection, ablation, transplantation, or IAT therapy. Patients with extrahepatic disease are less likely to benefit from surgical resection. Therapeutic options are limited and included administration of sorafenib or use of radiation therapy. While most recurrences occur early after transplant, late recurrences do occur. Many patients with recurrent metastatic HCC face a difficult clinical course. As such, management of patients with recurrent HCC following transplantation are best served being treated by a multi-disciplinary team that includes surgery, transplantation, hepatology, interventional and diagnostic radiology, as well as medical oncology.
Acknowledgments
Funding None.
Footnotes
Conflict of Interest None
Contributor Information
Peter J. Kneuertz, Department of Surgery, The Sidney Kimmel Comprehensive Cancer Center, The Johns Hopkins University School of Medicine, Baltimore, MD, USA
David P. Cosgrove, Department of Medical Oncology, The Sidney Kimmel Comprehensive Cancer Center, The Johns Hopkins University School of Medicine, Baltimore, MD, USA
Andrew M. Cameron, Department of Surgery, The Sidney Kimmel Comprehensive Cancer Center, The Johns Hopkins University School of Medicine, Baltimore, MD, USA
Ihab R. Kamel, Department of Radiology, The Sidney Kimmel Comprehensive Cancer Center, The Johns Hopkins University School of Medicine, Baltimore, MD, USA
Jean-Francois H. Geschwind, Department of Interventional Radiolog, The Sidney Kimmel Comprehensive Cancer Center, The Johns Hopkins University School of Medicine, Baltimore, MD, USA
Joseph M. Herman, Department of Radiation Oncology, The Sidney Kimmel Comprehensive Cancer Center, The Johns Hopkins University School of Medicine, Baltimore, MD, USA
Timothy M. Pawlik, Department of Surgery, The Sidney Kimmel Comprehensive Cancer Center, The Johns Hopkins University School of Medicine, Baltimore, MD, USA Department of Medical Oncology, The Sidney Kimmel Comprehensive Cancer Center, The Johns Hopkins University School of Medicine, Baltimore, MD, USA; Johns Hopkins Medicine Liver Tumor Center Multi-Disciplinary Clinic, Center for Surgical Trials and Outcomes Research, Johns Hopkins Hospital, 600 N. Wolfe Street, Harvey 611, Baltimore, MD 21287, USA, tpawlik1@jhmi.edu.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 3.Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL, Adam R, Neuhaus P, Salizzoni M, Bruix J, Forner A, De Carlis L, Cillo U, Burroughs AK, Troisi R, Rossi M, Gerunda GE, Lerut J, Belghiti J, Boin I, Gugenheim J, Rochling F, Van Hoek B, Majno P. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35–43. doi: 10.1016/S1470-2045(08)70284-5. [DOI] [PubMed] [Google Scholar]
- 4.Zavaglia C, De Carlis L, Alberti AB, Minola E, Belli LS, Slim AO, Airoldi A, Giacomoni A, Rondinara G, Tinelli C, Forti D, Pinzello G. Predictors of long-term survival after liver transplantation for hepatocellular carcinoma. Am J Gastroenterol. 2005;100:2708–2716. doi: 10.1111/j.1572-0241.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- 5.Marsh JW, Dvorchik I, Subotin M, Balan V, Rakela J, Popechitelev EP, Subbotin V, Casavilla A, Carr BI, Fung JJ, Iwatsuki S. The prediction of risk of recurrence and time to recurrence of hepatocellular carcinoma after orthotopic liver transplantation: a pilot study. Hepatology. 1997;26:444–450. doi: 10.1002/hep.510260227. [DOI] [PubMed] [Google Scholar]
- 6.Hemming AW, Cattral MS, Reed AI, Van Der Werf WJ, Greig PD, Howard RJ. Liver transplantation for hepatocellular carcinoma. Ann Surg. 2001;233:652–659. doi: 10.1097/00000658-200105000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimoda M, Ghobrial RM, Carmody IC, Anselmo DM, Farmer DG, Yersiz H, Chen P, Dawson S, Durazo F, Han S, Goldstein LI, Saab S, Hiatt J, Busuttil RW. Predictors of survival after liver transplantation for hepatocellular carcinoma associated with Hepatitis C. Liver Transpl. 2004;10:1478–1486. doi: 10.1002/lt.20303. [DOI] [PubMed] [Google Scholar]
- 8.Schlitt HJ, Neipp M, Weimann A, Oldhafer KJ, Schmoll E, Boeker K, Nashan B, Kubicka S, Maschek H, Tusch G, Raab R, Ringe B, Manns MP, Pichlmayr R. Recurrence patterns of hepatocellular and fibrolamellar carcinoma after liver transplantation. J Clin Oncol. 1999;17:324–331. doi: 10.1200/JCO.1999.17.1.324. [DOI] [PubMed] [Google Scholar]
- 9.Regalia E, Fassati LR, Valente U, Pulvirenti A, Damilano I, Dardano G, Montalto F, Coppa J, Mazzaferro V. Pattern and management of recurrent hepatocellular carcinoma after liver transplantation. J Hepatobiliary Pancreat Surg. 1998;5:29–34. doi: 10.1007/pl00009947. [DOI] [PubMed] [Google Scholar]
- 10.Margarit C, Charco R, Hidalgo E, Allende H, Castells L, Bilbao I. Liver transplantation for malignant diseases: selection and pattern of recurrence. World J Surg. 2002;26:257–263. doi: 10.1007/s00268-001-0214-1. [DOI] [PubMed] [Google Scholar]
- 11.Kooby DA, Egnatashvili V, Graiser M, Delman KA, Kauh J, Wood WC, Staley CA., Iii Changing management and outcome of hepatocellular carcinoma: evaluation of 501 patients treated at a single comprehensive center. J Surg Oncol. 2008;98:81–88. doi: 10.1002/jso.21049. [DOI] [PubMed] [Google Scholar]
- 12.Yoo HY, Patt CH, Geschwind JF, Thuluvath PJ. The outcome of liver transplantation in patients with hepatocellular carcinoma in the United States between 1988 and 2001: 5-year survival has improved significantly with time. J Clin Oncol. 2003;21:4329–4335. doi: 10.1200/JCO.2003.11.137. [DOI] [PubMed] [Google Scholar]
- 13.Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: long-term results of treatment and prognostic factors. Ann Surg. 1999;229:216–222. doi: 10.1097/00000658-199902000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeatman TJ, Nicolson GL. Molecular basis of tumor progression: mechanisms of organ-specific tumor metastasis. Semin Surg Oncol. 1993;9:256–263. [PubMed] [Google Scholar]
- 15.Osada T, Sakamoto M, Ino Y, Iwamatsu A, Matsuno Y, Muto T, Hirohashi S. E-cadherin is involved in the intrahepatic metastasis of hepatocellular carcinoma. Hepatology. 1996;24:1460–1467. doi: 10.1053/jhep.1996.v24.pm0008938181. [DOI] [PubMed] [Google Scholar]
- 16.Zimmerman MA, Ghobrial RM, Tong MJ, Hiatt JR, Cameron AM, Hong J, Busuttil RW. Recurrence of hepatocellular carcinoma following liver transplantation: a review of preoperative and postoperative prognostic indicators. Arch Surg. 2008;143:182–188. doi: 10.1001/archsurg.2007.39. discussion 188. [DOI] [PubMed] [Google Scholar]
- 17.Chok KS, Chan SC, Cheung TT, Chan AC, Fan ST, Lo CM. Late Recurrence of Hepatocellular Carcinoma after Liver Transplantation. World J Surg. 2011 doi: 10.1007/s00268-011-1146-z. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kornberg A, Kupper B, Tannapfel A, Katenkamp K, Thrum K, Habrecht O, Wilberg J. Long-term survival after recurrent hepatocellular carcinoma in liver transplant patients: clinical patterns and outcome variables. Eur J Surg Oncol. 2010;36:275–280. doi: 10.1016/j.ejso.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Shin WY, Suh KS, Lee HW, Kim J, Kim T, Yi NJ, Lee KU. Prognostic factors affecting survival after recurrence in adult living donor liver transplantation for hepatocellular carcinoma. Liver Transpl. 2010;16:678–684. doi: 10.1002/lt.22047. [DOI] [PubMed] [Google Scholar]
- 20.Valdivieso A, Bustamante J, Gastaca M, Uriarte JG, Ventoso A, Ruiz P, Fernandez JR, Pijoan I, Testillano M, Suarez MJ, Montejo M, Ortiz de Urbina J. Management of hepatocellular carcinoma recurrence after liver transplantation. Transplant Proc. 2010;42:660–662. doi: 10.1016/j.transproceed.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 21.Taketomi A, Fukuhara T, Morita K, Kayashima H, Ninomiya M, Yamashita Y, Ikegami T, Uchiyama H, Yoshizumi T, Soejima Y, Shirabe K, Maehara Y. Improved results of a surgical resection for the recurrence of hepatocellular carcinoma after living donor liver transplantation. Ann Surg Oncol. 2010;17:2283–2289. doi: 10.1245/s10434-010-0999-y. [DOI] [PubMed] [Google Scholar]
- 22.Roayaie S, Schwartz JD, Sung MW, Emre SH, Miller CM, Gondolesi GE, Krieger NR, Schwartz ME. Recurrence of hepatocellular carcinoma after liver transplant: patterns and prognosis. Liver Transpl. 2004;10:534–540. doi: 10.1002/lt.20128. [DOI] [PubMed] [Google Scholar]
- 23.Ko HK, Ko GY, Yoon HK, Sung KB. Tumor response to transcatheter arterial chemoembolization in recurrent hepatocellular carcinoma after living donor liver transplantation. Korean J Radiol. 2007;8:320–327. doi: 10.3348/kjr.2007.8.4.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Llovet JM, Real MI, Montana X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Sola R, Rodes J, Bruix J. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 25.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Haussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 26.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 27.Kim R, El-Gazzaz G, Tan A, Elson P, Byrne M, Chang YD, Aucejo F. Safety and feasibility of using sorafenib in recurrent hepatocellular carcinoma after orthotopic liver transplantation. Oncology. 2010;79:62–66. doi: 10.1159/000319548. [DOI] [PubMed] [Google Scholar]
- 28.Yoon DH, Ryoo BY, Ryu MH, Lee SG, Hwang S, Suh DJ, Lee HC, Kim TW, Ahn CS, Kim KH, Moon DB, Kang YK. Sorafenib for recurrent hepatocellular carcinoma after liver transplantation. Jpn J Clin Oncol. 2010;40:768–773. doi: 10.1093/jjco/hyq055. [DOI] [PubMed] [Google Scholar]
- 29.Saab S, McTigue M, Finn RS, Busuttil RW. Sorafenib as adjuvant therapy for high-risk hepatocellular carcinoma in liver transplant recipients: feasibility and efficacy. Exp Clin Transplant. 2010;8:307–313. [PubMed] [Google Scholar]
- 30.Robertson JM, Lawrence TS, Dworzanin LM, Andrews JC, Walker S, Kessler ML, DuRoss DJ, Ensminger WD. Treatment of primary hepatobiliary cancers with conformal radiation therapy and regional chemotherapy. J Clin Oncol. 1993;11:1286–1293. doi: 10.1200/JCO.1993.11.7.1286. [DOI] [PubMed] [Google Scholar]
- 31.Tse RV, Hawkins M, Lockwood G, Kim JJ, Cummings B, Knox J, Sherman M, Dawson LA. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. 2008;26:657–664. doi: 10.1200/JCO.2007.14.3529. [DOI] [PubMed] [Google Scholar]
- 32.Liapi E, Geschwind JF. Intra-arterial therapies for hepatocellular carcinoma: where do we stand? Ann Surg Oncol. 2010;17:1234–1246. doi: 10.1245/s10434-010-0977-4. [DOI] [PubMed] [Google Scholar]
- 33.Seong J, Koom WS, Park HC. Radiotherapy for painful bone metastases from hepatocellular carcinoma. Liver Int. 2005;25:261–265. doi: 10.1111/j.1478-3231.2005.01094.x. [DOI] [PubMed] [Google Scholar]
- 34.Kaizu T, Karasawa K, Tanaka Y, Matuda T, Kurosaki H, Tanaka S, Kumazaki T. Radiotherapy for osseous metastases from hepatocellular carcinoma: a retrospective study of 57 patients. Am J Gastroenterol. 1998;93:2167–2171. doi: 10.1111/j.1572-0241.1998.00614.x. [DOI] [PubMed] [Google Scholar]
- 35.Kim S, Chun M, Wang H, Cho S, Oh YT, Kang SH, Yang J. Bone metastasis from primary hepatocellular carcinoma: characteristics of soft tissue formation. Cancer Res Treat. 2007;39:104–108. doi: 10.4143/crt.2007.39.3.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Torok J, Wegner RE, Burton SA, Heron DE. Stereotactic body radiation therapy for adrenal metastases: a retrospective review of a noninvasive therapeutic strategy. Future Oncol. 2011;7:145–151. doi: 10.2217/fon.10.165. [DOI] [PubMed] [Google Scholar]
- 37.Quick AM, Bloomston M, Kim EY, Hall NC, Mayr NA. Complete response to radiation therapy of orbital metastasis from hepatocellular carcinoma. World J Gastroenterol. 2009;15:6000–6003. doi: 10.3748/wjg.15.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamashita H, Nakagawa K, Shiraishi K, Tago M, Igaki H, Nakamura N, Sasano N, Siina S, Omata M, Ohtomo K. Radiotherapy for lymph node metastases in patients with hepatocellular carcinoma: retrospective study. J Gastroenterol Hepatol. 2007;22:523–527. doi: 10.1111/j.1440-1746.2006.04450.x. [DOI] [PubMed] [Google Scholar]


