SUMMARY
The apicomplexan parasite Plasmodium falciparum causes malignant malaria. The mechanism of parasite egress from infected erythrocytes that disseminate parasites in the host at the end of each asexual cycle is unknown [1]. Two new stages of the egress program are revealed: 1) swelling of the parasitophorus vacuole accompanied by shrinkage of the erythrocyte compartment and 2) poration of the host cell membrane seconds before erythrocyte rupture due to egress. Egress was inhibited in dehydrated cells from patients with sickle cell disease, in accord with experimental dehydration of normal cells [2], suggesting that vacuole swelling involves intake of water from the erythrocyte compartment. Erythrocyte membrane poration occurs in relaxed cells, thus excluding involvement of osmotic pressure in this process. Poration does not depend on cysteine protease activity because protease inhibition blocks egress [3–5] but not poration, and poration is required for the parasite cycle because the membrane sealant P1107 interferes with egress. We suggest the following egress program: parasites initiate water influx into the vacuole from the erythrocyte cytosol to expand the vacuole for parasite separation and vacuole rupture upon its critical swelling. Separated parasites leave the erythrocyte by breaching its membrane, weakened by putative digestion of erythrocyte cytoskeleton [3–5] and membrane poration.
RESULTS AND DISCUSSION
Swelling of the parasitophorus vacuole and shrinkage of erythrocyte compartment precede malaria parasite egress
Infected erythrocytes undergo a morphological transformation before rupturing convulsively to release parasites. Based on amphiphiles, osmotic stress, and protease inhibitors, [2, 4] we hypothesize that egress is pressure-driven, through folding and fragmentation of enzymatically altered erythrocyte membrane. Osmotic pressure could build up in either the parasitophorus vacuole (PV) or the host cell cytoplasm [6]. To egress, parasites have to breach both the parasitophorous vacuolar membrane (PVM) and erythrocyte membrane, which is fortified by cytoskeleton. The PVM, devoid of cytoskeletal proteins [7], forms a continuous tubovesicular network (TVN) [8] in the space between the vacuolar and erythrocyte membranes [9]. Cytoplasmic swelling could assist in erythrocyte rupture but at the cost of applying pressure on the vacuole, ultimately interfering with egress, an explosive event [2, 10]. Assuming the PV swells and ruptures, expelling parasites into the erythrocyte compartment, how do parasites move through the viscous erythrocyte cytosol and break the erythrocyte membrane? Since 1) infected erythrocytes lose optical density shortly before erythrocyte rupture [2], and 2) cysteine proteases are involved in egress [3–5], we propose and test the idea that parasites easily pass through a hemoglobin-depleted erythrocyte cytoplasm and breach a weakened erythrocyte membrane.
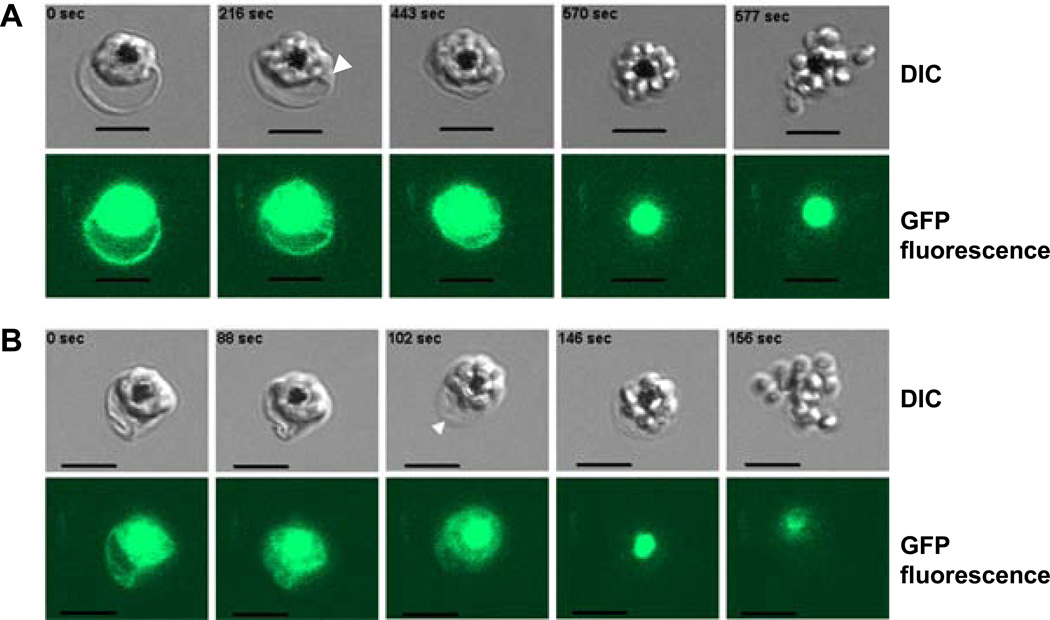
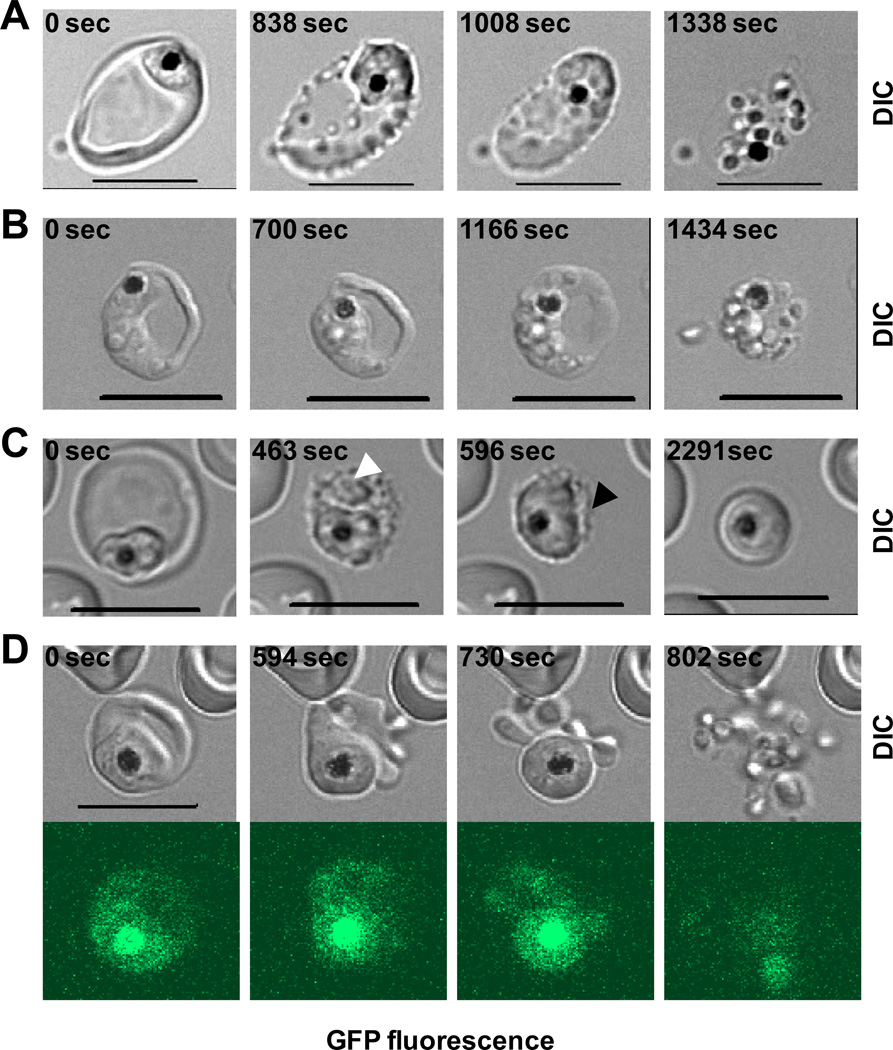
The vacuole swells several minutes before parasite egress (Fig. 1A and Movie 1A). Starting adjacent to the parasite’s space (arrowhead in Fig. 1A), vacuole swelling later extends in all directions. At the same time the visible area of the erythrocyte compartment shrinks, suggesting redistribution of water between the erythrocyte cytosol and vacuole. At some point, the erythrocyte membrane becomes relaxed (Fig. 1B, arrowhead; Movie 1B) but still preserves its integrity (note fluorescent signal in cytosol). Membrane relaxation, more or less prominent in each individual erythrocyte, could indicate the cytoskeleton digestion by activated cysteine proteases [3–5]. Eventually dissociated parasites leave the host cell by breaching first the PVM (presumably, when PV critical volume is reached) and then the erythrocyte membrane (Movies 1A-B). Thus, dependence of egress on osmotic pressure [2] can be described in terms of erythrocyte hydration, which affects the swelling and rupture of the vacuole. To test the relationship between erythrocyte hydration and parasite egress, we used dehydrated erythrocytes [11] from donors homozygous for sickle hemoglobin gene (HbSS vs. normal HbAA) (Fig. S1). Sickle erythrocytes do support P. falciparum replication. Live cell microscopy and statistical analysis confirmed that dehydrated erythrocytes have inefficient parasite egress or aborted parasite cycles. Specifically, we chose schizonts (multinucleated parasites) within erythrocytes with the characteristics of irreversible dehydration: flattened appearance and extension in one dimension (9.8±0.3 µm, mean±SEM, n=28 vs. normal erythrocyte diameter 7.9±0.1 µm, mean±SEM, n=29, p<0.001). Parasite egress suffered in dehydrated cells both quantitatively and qualitatively: less than 40% (n=31) of schizonts released parasites while majority could not finish the cycle. Parasites were captured inside the host cell (Fig. 2A, Movie 2A) or only one or two parasites were released (Fig. 2B). The prominent feature of an aborted cycle was a slow vacuole swelling (taking up to 40 minutes), and no membrane rupture in about half of the schizonts (Fig. 2C, Movie 2B). This slow kinetics of preparation to egress gives us a unique opportunity to follow the process, which proceeds faster and less prominently in HbAA-infected erythrocytes. We observed sequential swelling of the remote areas in the erythrocyte cytosol (Fig. 2C, white arrowhead, Movie 2B) that are presumably parasite-derived TVN because erythrocytes are devoid of any membrane-organized compartments. It seems the entire system of continuous membrane structures, PVM and TVN, undergoes volume expansion while the now clearly separated cytosol continues to contract (Fig. 2C, black arrowhead). A decreased erythrocyte cytoplasm volume would increase hemoglobin concentration and the probability of its polymerization [11], which can contribute in the defective parasite egress from sickle cells. In several recordings of egress, the host cell membrane undergoes blebbing/vesiculation, typical for membranes with the defective cytoskeleton [12]. Blebbed membrane preserves its integrity (judged by the GFP signal in blebs, Fig. 2D, Movies 3A). Thus, host cell dehydration diminishes parasite egress from HbAA erythrocytes in hypertonic medium [2] and from the dehydrated HbSS erythrocyte in isotonic medium, likely by preventing the vacuole to reach the threshold of osmotic pressure required to expel parasites. The decreased erythrocyte volume may contribute to malarial protection in individuals with sickle erythrocytes. Notably, decreased erythrocyte volume is a characteristic for individuals with thalassemia and iron deficiency. Alternatively, one may speculate that P. vivax gained an advantage by targeting reticulocytes, the largest circulating erythrocytes in the host. The negative effect of erythrocyte dehydration on parasite egress, demonstrated here, and on invasion [13] emphasizes the general importance of host cell hydration for the asexual cycle of malaria parasites.
Figure 1.
Swelling of parasitophorus vacuole, shrinkage of erythrocyte compartment and erythrocyte membrane relaxation precede malaria parasite egress from erythrocytes infected with P. falciparum (clone 3D7 KAHRP (+His)–GFP). Selected frames from the recordings of parasite egress (see also Movies 1A and 1B; the time stamps of the fist frames in the figures were arbitrary set to “0 sec”). Upper set: differential interference contrast (DIC) images; lower set: corresponding fluorescent images. Scale bar = 5 µm. A. Note the increase of the vacuole/parasite compartment area (by 57% in the third frame in comparison to the first one) and swelling of the conical area adjusted to vacuole (arrowhead) accompanied by a shrinkage of erythrocyte compartment (by 80% in the third frame in comparison to the first one). All these changes observed inside infected erythrocytes that preserve membrane integrity judged by the presence of GFP fluorescent signal in host cell cytoplasm up to the last few seconds before parasite egress. B. Loss of erythrocyte membrane tension (arrowhead) at the moment of the putative digestion of cytoskeleton by cysteine proteases. Membrane relaxation happens during the time of vacuole swelling/erythrocyte compartment shrinkage and does not affect membrane integrity judged by the preservation of GFP fluorescent signal inside infected cell. Note that food vacuoles (black spots in the DIC images) have strong fluorescence originated from the GFP-tagged protein delivered into this organelle by hemoglobin-utilization process.
Figure 2.
Defective end of parasite cycle in dehydrated sickle erythrocytes infected with P. falciparum (clone 3D7 KAHRP (+His)–GFP). Different outcomes illustrated in four sets of timed images (DIC microscopy in panels A-C and DIC/fluorescent microscopy in panel D, see also Movies 2–3). A. Inefficient vacuole swelling/erythrocyte shrinkage resulted in the failure to extrude parasites from infected erythrocyte; Movie 2A. B. Extrusion of individual parasite from infected cell with the preservation of parasite cluster inside erythrocyte. C. Slow vacuole-TVN swelling (white arrowhead) illustrating the simultaneous shrinkage of erythrocyte compartment (black arrowhead). This process was blocked on the stage of unresolved vacuolar swelling during ~ 45 min of observation (Movie 2B). D. Parasite egress from the erythrocyte with a blebbed-vesiculated membrane transformed at the time of the putative cytoskeleton digestion but preserved its integrity, judged by the presence of GFP fluorescent signal inside blebs; Movie 3A. See also Movies 3B. Scale bar = 10 µm.
Host cell membrane poration precedes membrane rupture
The second main observation of this paper, that erythrocyte membrane becomes porous seconds before rupture, can support the conclusion that the cytosolic osmotic pressure does not build up sufficiently to drive parasite egress. The same observation, however, can explain the loss in optical density observed prior to parasite egress [2] as a loss of hemoglobin across new erythrocyte membrane pores. We designed a fluorescent assay for the detection of membrane integrity in living erythrocytes (the standard propidium iodide method does not work in enucleated cells). This method utilizes a fluorescent phalloidin (Alexa Fluor 488 Phalloidin, Invitrogen, USA, MW ~ 1.32 kDa), a binding partner of F-actin (a filamentous cytoskeletal protein, the major component of erythrocyte cytoskeleton) that is membrane-impermeant. Upon binding to actin, phalloidin creates a high local concentration of fluorophore underneath the erythrocyte membrane. The signal/noise ratio was very high, reflecting a low level of nonspecific binding of phalloidin, making it a good marker of membrane permeability. Using this method we first showed that labeled phalloidin does not permeate membranes of either normal erythrocytes or immature schizonts (Fig. 3A), and that sufficient filamentous actin resides in residual membrane [2, 14] (Fig. 3B). This method allowed us to follow erythrocyte membrane integrity at the end of the parasite cycle using simultaneous fluorescence and differential interference contrast (DIC) microscopy. Permeability to phalloidin was detected in 31 recordings ~ 13.3±2.5 seconds (mean ± SEM) prior to parasite egress (Fig. 3C and movie 4). In the seven other recordings, there with no imaged ring of cortical fluorescence but rather a diffuse increase in cortical or intracellular fluorescence (data not presented). Thus, although the detection of fluorescent phalloidin may be sensitive to the state of actin polymerization and limited by pore radius, poration most likely always precedes parasite egress. We also tested for cytosolic protein efflux with parasite-derived (clone 3D7 KAHRP (+His)–GFP) 41.6 kDa GFP-tagged soluble protein [15]. A significant drop of fluorescence in erythrocyte cytoplasm was observed before membrane rupture (Fig. 1A-B, frames number four, Movies 1A-B). We confirmed the release of GFP-tagged protein from cells by measuring the GFP-specific fluorescence in the medium using a fluorometric assay (Fig. S2A-C). Since both influx of phalloidin and efflux of fluorescent protein occurred at approximately at the same time, it is likely that a new aqueous permeability pathway develops in the erythrocyte membrane before membrane rupture and parasite egress.
Figure 3.
Detection of erythrocyte membrane permeability using influx of fluorescent phalloidin, which labels erythrocyte cytoskeleton, into the infected erythrocyte from the medium. A. Normal erythrocytes (left cell on DIC image) and immature schizont (right cell on DIC image) have membranes that are not permeable for phalloidin (judged by the absence of fluorescent signal in the fluorescent image). B. Residual membranes of erythrocytes, following parasite egress, preserve filamentous actin. Two sites of parasite egress: DIC and corresponding fluorescent images with the intensely labeled erythrocyte membrane fragments. C. Demonstration that erythrocyte membrane poration precedes membrane rupture and malaria parasite (P. falciparum, clone 3D7) egress using fluorescent phalloidin (green color images) and DIC (black-white color images) microscopy of live infected cells approaching the end of the erythrocyte cycle. Sets of four pictures for one schizont demonstrate morphological transformation of infected erythrocyte during egress (the time stamp of the fist frame was arbitrary set to “0 sec”). Top row images show schizont before membrane poration in the medium with the fluorescent phalloidin (green background). Note that erythrocyte compartment is visible only on DIC images (white arrowheads). Images in the two middle rows show schizonts with a porated erythrocyte membrane that is not swelled. Note that fluorescent contours of erythrocyte membrane are smaller that those in DIC images (white arrowheads) due to the specifics of the light accumulation from the rounded living cells using confocal microscopy. The bottom row captures the erythrocyte membrane rupture and parasite egress. Bar = 5 µm.
Membrane poration occurs with cysteine protease (and egress) inhibitor E-64 [4]. All E-64-induced parasite clusters had a phalloidin-labeled (porous) erythrocyte membrane (Fig. 4 A, black arrowhead) and lacked a GFP signal when 3D7 KAHRP (+His)–GFP-infected cells were analyzed (Fig. 4B, black arrowhead; Fig. S3A). Despite the strong inhibition of parasite egress by E-64 [4], GFP fluorescence in the medium was equal to that of the control culture (Fig. S3B). Thus, this poration prior to parasite egress is independent of cysteine protease activity.
Figure 4.
Evidence that cysteine proteases are not involved in erythrocyte membrane poration (A-B) and that membrane sealant P1107 interferes with parasite egress (C). A. Parasite clusters (black arrowhead on DIC image) produced in medium supplemented with cysteine protease inhibitor E-64 and fluorescent phalloidin enclosed in porated erythrocyte membrane detected by phalloidin (green color). Note the unlabeled membrane of immature schizont (white arrowheads) and normal erythrocytes (two cells in the upper left corner of the DIC image). Image of 3D7-infected culture. B. E-64-induced cluster (black arrowhead) in 3D7 KAHRP (+His)–GFP-infected culture as well as uninfected cells are lacking fluorescent signal while immature schizont (white arrowhead) demonstrates strong fluorescence. Bar = 5 µm. C. Inhibition of parasite egress by poloxamine P1107 (n = 5, mean ± s.e.). Cultures were treated with poloxamine for 1–2 hours.
Host cell membrane poration is likely an essential step in parasite egress
If erythrocyte membrane poration is required for egress then membrane-sealing agents should inhibit egress. Poloxamine (P1107, BASF, average molecular weight 15 kDa) is a non-ionic surfactant that can seal radiopermeabilized cell membranes [16]. Inhibition of parasite egress in the presence of poloxamine was dose-dependent (Fig. 4C) suggesting that poration is likely essential for parasite egress. Importantly, schizonts did not lose their optical density and were not permeable for fluorescent phalloidin. We observed even less phalloidin-positive schizonts in treated cultures, with 47% inhibition of parasite egress, than in control cultures. Occasionally we observed schizonts weakly labeled with phalloidin. They could represent cells only partially sealed by P1107 (data not illustrated). Fluorescence of the medium in treated cultures with 53% inhibition of parasite egress was 34% less than in controls. It is doubtful that poloxamine acts intracellularly since it is charged and unlikely to enter infected erythrocytes until they are permeabilized. We conclude that P1107 acts from outside the erythrocyte by interfering with the initial events of membrane permeation. Despite the obscurity of the molecular mechanism of membrane sealing by P1107 and its lower molecular weight analog Poloxamer 188, and unknown “side-effects” of reagents on cells, this type of compound has promising medical applications for treating diseases which pathogenicity involves membrane destabilization [17].
The role for poration in parasite egress is not clear. The efflux of hemoglobin could be necessary to minimize protein viscosity in host cytoplasm exacerbated by its shrinkage. Conversely, pores would facilitate the influx of ions into the host cells [18] that could be involved in egress program.
Search for the parasite-specific “egress agents”
The genome of Plasmodium species has five genes coding for perforin-like proteins (PLPs). The role of these proteins in tissue transmigration [19], liver infection [20], and malarial transmission to mosquito [21–22] is documented. TgPLP1 of Toxoplasma gondii, another Apicomplexan parasite, is implicated in egress from the host cells [23]. Expression of PLPs in the erythrocyte stage of P. falciparum is uncertain: a single PfPLP2 peptide (PFL0805w) is detected in merozoites [24]. Because perforins may cause the membrane permeation described here, we tested and detected PfPLP2 messenger RNA in immature schizonts ([25], Fig.S4). Loss of function studies are needed to test what role, if any, perforin has in P. falciparum life cycle.
In summary, we report two new essential steps in the program of Plasmodium falciparum egress from erythrocytes. First, the parasitophorous vacuole swells as the erythrocyte shrinks, suggesting ion/water redistribution between these two compartments of infected cells. At the end of the cycle, vacuole swelling apparently provides the space for parasites dissociation prior to egress, leading to vacuole rupture. In the midst of erythrocyte shrinkage, the tension of erythrocyte membrane decreases without loss of integrity. Second, parasite egress requires host cell membrane poration prior to host cell membrane rupture. Membrane poration is observed in erythrocytes that are not swollen, thus it does result from critical membrane stretching. Perhaps either release of protein from erythrocytes or an influx of ions into the host cells, or both, are needed for the asexual parasite cycle to complete. Alternatively, host cell membrane poration could serve to weaken a barrier, which parasites must breach to egress.
Similarities in parasite egress mechanisms between two families of the phylum Apicomplexa, Plasmodium and Toxoplasma, are emerging: both type of parasites make pores in host cells membrane [26] and activate host cell calpain [5] prior to egress. Since P. falciparum has multiple experimental limitations, Toxoplasma, a more conventional organism, emerges as a model for Apicomplexan biology [27]. Regardless, egress of parasites is a vital step of diseases devastating humanity. Our appreciation of a more complex egress program provides more targets for novel antimalarials, just as it may help explain the selective advantage the sickle trait confers upon its carriers.
EXPERIMENTAL PROCEDURES
Culture of P. falciparum
Plasmodium falciparum strains 3D7 (ATCC, Manassas, VA) and 3D7 KAHRP(+His) – GFP, (MRA-576, contributed by A.F. Cowman, MR4, ATCC, Manassas, VA) were cultured according to the Trager–Jensen method [28] in human erythrocytes in RPMI 1640 medium (Invitrogen) supplemented with 25 mM Hepes (Invitrogen), 4.5 mg ml−1 glucose (Sigma), 0.1 mM hypoxanthine (Invitrogen), 25 µg ml−1 gentamicin (Invitrogen) and 0.5% AlbuMax (Invitrogen). Erythrocytes infected with late stage malaria parasites were isolated from cultures and a new synchronized infection was initiated in HbAA or HbSS erythrocytes as described in [29]. Blood from four patients with Hb SS mutation was collected after National Institutes of Health Institutional Review Board approval and informed consent obtained in accordance with the Declaration of Helsinki.
Live cell microscopy
A laser scanning confocal microscope (LSM 510, Zeiss) was used with 100× or 63× 1.4 NA oil objectives and laser excitation at 488 nm. A low intensity of cell illumination was used to avoid photodamage of late-infected cells. Fluorescent phalloidin (Alexa Fluor 488 Phalloidin, Invitrogen, USA) was used at the final concentration 66 – 132 nM. Both fluorescent phalloidin and GFP were excited at 488 nm.
Parasite egress assay
To asses the effect of drugs on parasite egress, we employed methods described in detail in [29]. The reagent was added to the culture medium and infected cells were treated for different time intervals. Treatment was carried out at 37°C in chambers for microscopy and then parasite egress was quantified using light microscopy. Parasite egress in drug treated cultures was compared with egress in control cultures. We used the cysteine protease inhibitor E-64 (Sigma, St Louis, MO) at 10 µM concentration as described in [4].
Detection of fluorescence in medium
A fluorescent signal from the GFP-fusion protein released into the medium from infected cells (as the result of parasite egress) was assessed using a FluoroMax-4 spectrofluorimetor (Horiba Jobin Yvon, Israel). Signal-to-noise ratio was measured at the following detection setting: excitation at 480 nm with 5 nm bandpass, emission at 495–550 nm with 5 nm bandpass, interval 1 nm, integration time 1 sec. Signal at 509 nm (maximum of GFP fluorescence) was used to compare fluorescence at the different experimental conditions after intensity of buffer fluorescence was subtracted from the sample fluorescence.
Detection of mRNA expression
Total RNA was prepared from P. falciparum-infected erythrocytes, uninfected erythrocytes and PBMCs using the PureLink Micro-to-Midi Total RNA Purification System (Invitrogen, 12183-018). The RNA yield was measured using the Quant-iT RNA Assay kit (Invitrogen, Q10213). The yields from a 50 µl packed volume of cells were: infected erythrocytes, 34 µg; uninfected erythrocytes, <1 µg; PBMCs, 10 µg. Primers for gene-specific cDNA synthesis (GSPs) and PCR amplification of the PfPL2 (PFL0805w) sequence were designed using Emboss eprimer3 to search GenBank accession no. XM_001350534.1. The primer sequences are:
GSP: P3177R, GGGATCGACATAAGGCAATG
PP1: P1281F, TTTCAGGGGACCTGTATTGC, and P1689R, ATTCGGCAGATGCAGAAAAG
PP2: P1491F, TCATGCTGATGGTGATAAACG, and P1648R, GGGAGTACTAACCTTTACGTCTGA
PP3: P1692F, TTCTGCATCTGCCGAATTTA, and P2112R, AAGCGCTTGATAATCCCATC
PP4: P1786F, ACAGGTATACCAATAACAACAACAAG, and P1935R, ACATCCACTTGTTTACATTTTCTTCA
For RT-PCR analysis [30] of PfPLP2 expression, 1 µg of RNA was treated with 1U amplification grade DNase I (Invitrogen 18068-015) to remove genomic DNA following the manufacturer’s protocol. The ThermoScript RT-PCR System (Invitrogen 11146-024) was used for cDNA synthesis, in a 20 µl reaction with 10 pmole GSP. Reactions were incubated at 45°C for 2 hrs, heat inactivated at 85°C, and treated with RNase H. For detection of the PfPLP2 sequence in cDNA by PCR, 1 µl RT-PCR reaction was added to a 25 µl volume containing 20 mM Tris-HCl (pH 8.4), 50 mM KCl, 3.0 mM MgSO4, 0.2 mM dNTPs, 5 pmole forward and reverse primers, 1U Taq polymerase (Invitrogen 10342-053). The reactions were denatured for 3 min at 94°C and amplified for 30 cycles of 30 sec at 94°C, 30 sec at 56°C, 60 sec at 72°C, followed by a 10 min. extension at 72°C. PCR products were analyzed by electrophoresis in a 1.5% agarose gel in 1X TAE, and visualized by staining with ethidium bromide.
Supplementary Material
HIGHLIGHTS.
Swelling of the parasitophorous vacuole and erythrocyte shrinkage precede parasite egress; these are slowed and inhibited in sickle cells.
Host cell membrane poration precedes membrane rupture.
Host cell membrane poration is likely to be an essential step in parasite egress.
Candidates for the parasite-specific “egress agents”.
ACKNOWLEDGMENTS
We thank Drs. K. Melikov, E. Pugacheva, V. Lizunov, P. Blank, E. Zaitseva and H. Ginsburg for stimulating discussions. We thank Dr. Shu-Rong Yin for helpful advice on the PCR protocols and also Ms. Erinn Hama and Ms. Dan Yin for their valuable contribution to this project in its early stages. Nurse Practitioner Antoinett Rabel and the patient volunteers are thanked for the collection of clinical samples used in this study. This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Cowman AF, Crabb BS. Invasion of red blood cells by malaria parasites. Cell. 2006;124:755–766. doi: 10.1016/j.cell.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Glushakova S, Yin D, Li T, Zimmerberg J. Membrane transformation during malaria parasite release from human red blood cells. Curr. Biol. 2005;15:1645–1650. doi: 10.1016/j.cub.2005.07.067. [DOI] [PubMed] [Google Scholar]
- 3.Gelhaus C, Vicik R, Schirmeister T, Leippe M. Blocking effect of a biotinylated protease inhibitor on the egress of plasmodium falciparum merozoites from infected red blood cells. Biol. Chem. 2005;386:499–502. doi: 10.1515/BC.2005.059. [DOI] [PubMed] [Google Scholar]
- 4.Glushakova S, Mazar J, Hohmann-Marriott MF, Hama E, Zimmerberg J. Irreversible effect of cysteine protease inhibitors on the release of malaria parasites from infected erythrocytes. Cell. Microbiol. 2009;11:95–105. doi: 10.1111/j.1462-5822.2008.01242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chandramohanadas R, Davis PH, Beiting DP, Harbut MB, Darling C, Velmourougane G, Yeh Lee M, Creer PA, Roos DS, Greenbaum DC. Apicomplexan parasites co-opt host calpains to facilitate their escape from infected cells. Science. 2009;324:794–797. doi: 10.1126/science.1171085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lew VL, Macdonald L, Ginsburg H, Krugliak M, Tiffert T. Excess haemoglobin digestion by malaria parasites: A strategy to prevent premature host cell lysis. Blood Cells, Molecules, and Diseases. 2004;32:353–359. doi: 10.1016/j.bcmd.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Atkinson CT, Aikawa M, Perry G, Fujino T, Bennett V, et al. Ultrastructural localization of erythrocyte cytoskeletal and integral membrane proteins in Plasmodium falciparum-infected erythrocytes. EurJCell Biol. 1988;45:192–199. [PubMed] [Google Scholar]
- 8.Wickert H, Gottler W, Krohne G, Lanzer M. Maurer's cleft organization in the cytoplasm of Plasmodium faliparum-infected erythrocytes: new insights from three-dimensional reconstruction of serial ultrathin sections. Eur J Cell Biol. 2004;83:567–582. doi: 10.1078/0171-9335-00432. [DOI] [PubMed] [Google Scholar]
- 9.Hohmann-Marriott MF, Sousa AA, Azari AA, Glushakova S, Zhang G, Zimmerberg J, Leapman RD. Nanoscale 3D cellular imaging by axial scanning transmission electron tomography. Nature Methods. 2009;6:729–731. doi: 10.1038/nmeth.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trager W. On the release of malaria parasites. Trends Parasitol. 2002;18:60–61. doi: 10.1016/s1471-4922(01)02169-9. [DOI] [PubMed] [Google Scholar]
- 11.Lew VL, Bookchin RM. Ion transport pathology in the mechanism of sickle cell dehydration. Physiological Reviews. 2005;85:179–200. doi: 10.1152/physrev.00052.2003. [DOI] [PubMed] [Google Scholar]
- 12.Agre P, Casella JF, Zinkham WH, McMillan C, Bennett V. Partial deficiency of erythrocyte spectrin in hereditary spherocytosis. Nature. 1985;314:380–383. doi: 10.1038/314380a0. [DOI] [PubMed] [Google Scholar]
- 13.Tiffert T, Lew VL, Ginsburg H, Krugliak M, Croisille L, Mohandas N. The hydration state of human red blood cells and their susceptibility to invasion by Plasmodium falciparum. Blood. 2005;105:4853–4860. doi: 10.1182/blood-2004-12-4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glushakova S, Yin D, Gartner N, Zimmerberg J. Quantification of malaria parasite release from infected erythrocytes: Inhibition by protein-free media. Malaria J. 2007;6:61. doi: 10.1186/1475-2875-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wickham ME, Rug M, Ralph SA, Klonis N, Mcfadden GI, Tilley L, et al. Trafficking and assembly of the cytoadherence complex in plasmodium falciparum-infected human erythrocytes. EMBO J. 2001;20:5636–5649. doi: 10.1093/emboj/20.20.5636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hannig J, Yu J, Beckett M, Weichselbaum R, Lee RC. Poloxamine 1107 sealing of radiopermeabilized erythrocyte membranes. Int. J. Radiat. Biol. 1999;75:379–385. doi: 10.1080/095530099140555. [DOI] [PubMed] [Google Scholar]
- 17.Yasuda S, Townsend D, Michele DE, Favre EG, Day SM, Metzger JM. Dystrophic heart failure blocked by membrane sealant poloxamer. Nature. 2005;436:1025–1029. doi: 10.1038/nature03844. [DOI] [PubMed] [Google Scholar]
- 18.Mauritz JM, Esposito A, Ginsburg H, Kaminski CF, Tiffert T, Lew VL. The homeostasis of Plasmodium falciparum-infected red blood cells. PLoS Comput. Biol. 2009;5:e1000339. doi: 10.1371/journal.pcbi.1000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaiser K, Camargo N, Coppens I, Morrisey JM, Vaidya AB, Kappe SH. A member of a conserved Plasmodium protein family with membrane-attack complex/perforin (MACPF)-like domains localizes to the micronemes of sporozoites. Mol. Biochem. Parasit. 2004;133:15–26. doi: 10.1016/j.molbiopara.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Ishino T, Chinzei Y, Yuda M. A plasmodium sporozoite protein with a membrane attack complex domain is required for breaching the liver sinusoidal cell layer prior to hepatocyte infection. Cell. Microbiol. 2005;7:199–208. doi: 10.1111/j.1462-5822.2004.00447.x. [DOI] [PubMed] [Google Scholar]
- 21.Kadota K, Ishino T, Matsuyama T, Chinzei Y, Yuda M. Essential role of membrane-attack protein in malarial transmission to mosquito host. P. Natl. Acad. Sci. USA. 2004;101:16310–16315. doi: 10.1073/pnas.0406187101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ecker A, Pinto SB, Baker KW, Kafatos FC, Sinden RE. Plasmodium berghei: Plasmodium perforin-like protein 5 is required for mosquito midgut invasion in anopheles stephensi. Exp. Parasitol. 2007;116:504–508. doi: 10.1016/j.exppara.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kafsack BFC, Pena JDO, Coppens I, Ravindran S, Boothroyd JC, Carruthers VB. Rapid membrane disruption by a perforin-like protein facilitates parasite exit from host cells. Science. 2009;323:530–533. doi: 10.1126/science.1165740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Florens L, Washburn MP, Raine JD, Anthony RM, Grainger M, Haynes JD, et al. A proteomic view of the plasmodium falciparum life cycle. Nature. 2002;419:520–526. doi: 10.1038/nature01107. [DOI] [PubMed] [Google Scholar]
- 25.Le Roch KG, Zhou Y, Blair PL, Grainger M, Moch JK, Haynes JD, De La Vega P, Holder AA, Batalov S, Carucci DJ, Winzeler EA. Discovery of gene function by expression profiling of the malaria parasite life cycle. Science. 2003;301:1503–1508. doi: 10.1126/science.1087025. [DOI] [PubMed] [Google Scholar]
- 26.Black MW, Arrizabalaga G, Boothroyd C. Ionophore-resistant mutants of Toxoplasma gondii reveal host cell permeabilization as an early event in egress. Mol. Cell. Biol. 2000;20:9399–9408. doi: 10.1128/mcb.20.24.9399-9408.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim K, Weiss LM. Toxoplasma gondii: the model apicomplexan. Int. J. Parasitol. 2004;34:423–432. doi: 10.1016/j.ijpara.2003.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 29.Glushakova S, Yin D, Gartner N, Zimmerberg J. Quantification of malaria parasite release from infected erythrocytes: Inhibition by protein-free media. Malaria J. 2007;6:61. doi: 10.1186/1475-2875-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prediger EA. Quantitating mRNAs with relative and competitive RT-PCR. In: Schein CH, editor. Methods in Molecular Biology, vol. 160, Nuclease Methods and Protocols. Totowa, NJ: Humana Press; 2001. pp. 49–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.