Abstract
The dysferlinopathies (e.g. LGMD2b, Myoshi myopathy) are progressive, adult-onset muscle wasting syndromes caused by mutations in the gene coding for dysferlin. Dysferlin is a large (~200 kDa) membrane-anchored protein, required for maintenance of plasmalemmal integrity in muscle fibers. To facilitate analysis of dysferlin function in muscle cells, we have established a dysferlin-deficient myogenic cell line (GREG cells) from the A/J mouse, a genetic model for dysferlinopathy. GREG cells have no detectable dysferlin expression, but proliferate normally in growth medium and fuse into functional myotubes in differentiation medium. GREG myotubes exhibit deficiencies in plasma membrane repair, as measured by laser wounding in the presence of FM1-43 dye. Under the wounding conditions used, the majority (~66%) of GREG myotubes lack membrane repair capacity, while no membrane repair deficiency was observed in dysferlin-normal C2C12 myotubes, assayed under the same conditions. We discuss the possibility that the observed heterogeneity in membrane resealing represents genetic compensation for dysferlin deficiency.
Keywords: dysferlin, myoblast, myotube, resealing
Introduction
Limb girdle muscular dystrophy 2b (LGMD2b) and Myoshi Myopathy (MM) are late-onset muscular dystrophies caused by point mutations and deletions resulting in reduced levels, or absence, of the protein dysferlin [1–4]. In dysferlinopathy patients, the sarcolemma displays characteristic abnormalities, including 0.1–2.0 µm discontinuities, thickened basal lamina, and accumulations of small vesicles at the sarcolemma [5]; these features suggest that dysferlin is required for maintenance of sarcolemmal integrity. Dysferlin is a large (~200 kDa) membrane-anchored protein with six C2 domains, having sequence similarity to synaptotagmins. By analogy to synaptotagmin’s function as a possible calcium sensor in exocytosis, it has been proposed that dysferlin may serve as a calcium sensor for membrane repair [6–8]. Isolated wild-type mouse muscle fibers can reseal sarcolemmal wounds in the presence of ~1 mM [Ca2+]free, but fibers from a dysferlin knock-out mouse are defective in resealing, based on the unimpeded uptake of FM1-43 fluorescent dye following laser wounding [6].
Studies of muscle damage and repair in vivo suggest that dysferlin may have other functions in addition to membrane resealing. The A/J mouse strain has a spontaneous dysferlin mutation, due to a retrotransposon insertion in intron 4 of the dysf gene, and no detectable dysferlin protein expression [9]. A/J mice exhibit a progressive muscular dystrophy, appearing ~2 months in the proximal muscles and spreading to the distal muscles by 5 months. A/J mice exhibit a defect in recovery from muscle injury caused by a single large strain lengthening contraction [10, 11]. Large strain lengthening contractions produce microtears in muscle fibers which spontaneously reseal, based on retention of fluorescent dextran. A/J muscle fibers appear to reseal normally following injury, but become necrotic a few days later, and must be replaced by new myogenesis [10, 11].
Myogenic cell lines derived from mouse models for muscular dystrophies have proven to be of great value in understanding the pathobiology of these diverse diseases. Efforts to define the function(s) of dysferlin in membrane repair have been hampered by a lack of dysferlin-deficient myogenic cell lines. In order to study membrane resealing using a model cell system, we have developed a dysferlin-deficient myogenic cell line (GREG) from the A/J mouse strain. These cells differentiate into morphologically typical myotubes which do not express detectable levels of dysferlin. GREG myotubes exhibit a heterogeneous membrane resealing deficiency which varies in severity between individual myotubes, suggesting they possess both dysferlin-dependent and independent modes of membrane repair. Dysferlin-independent membrane repair could represent a compensatory process operant in the presence of dysferlin deficiency.
Materials and Methods
Isolation of GREG cells
Thigh muscles from a nine day old A/J strain mouse were excised, minced and digested with a mixture of 0.25% trypsin-0.1% collagenase/dispase in phosphate buffered saline (PBS) to obtain a suspension of mononucleated cells [12, 13]. The cells were resuspended in Ham’s F-10 medium (Invitrogen, 11550) with 20% fetal bovine serum (FBS) (Gemini Bio Products, 100–106), 0.5% chick embryo extract (CEE) (Accurate Chemical & Scientific, CE650TL), Penicillin-Streptomycin liquid (Invitrogen, 15070) and L-Glutamine 200mM (Invitrogen, 25030), and incubated in (uncoated) 100 mm tissue culture dishes for 1 hr to deplete contaminating fibroblasts (which selectively adhere to the dishes), and then were transferred to collagen-coated dishes for myoblast attachment.
Primary myoblast cells were cultured in F-10 medium (with 20% FBS and 0.5% CEE, as above) for three weeks to suppress fibroblast cell growth. To further select for myoblasts and deplete fibroblasts, cells were lifted in PBS without Ca2+, Mg2+, trypsin or EDTA. Growth medium was aspirated, cells rinsed with PBS and a small amount of PBS was added to the dish. Cells were placed in a 37 °C incubator until they became rounded and could be knocked off the plastic. This procedure was used for the first month of culture expansion until most of the fibroblasts were depleted. During this time the cells grew slowly. The growth medium was changed every other day and cells were lifted and transferred to fresh collagen coated dishes every 5 days regardless of cell density. After approximately 6 weeks of growth, a majority of the initial cell population died but the proliferation rate of the remaining myoblasts accelerated, possibly representing spontaneous transformants, allowing selection for a pure myoblast culture. At this point F10 medium was replaced by Dulbeco’s modified Eagle medium (DMEM) containing GlutaMAX instead of L-Glutamine (Invitrogen, 10569). This medium supported faster myoblast growth; media was changed every two days and a strong precaution was taken to avoid cell overgrowth. Cells now were able to grow routinely on regular tissue culture plastic; collagen coated dishes were reserved for experimental work.
For routine culture, GREG myoblasts are maintained in growth medium: DMEM plus 20% FBS and 0.5% CEE (Accurate Chemical & Scientific, CE650TL), plus antibiotic Primocin 100 mg/ml (InvivoGen, ant-pm-2). For passage, the cells are lifted using 0.25% Trypsin/EDTA (Invitrogen, 25200), at intervals of 3 days (when the cells are 80% confluent), and seeded at a density of 2×104/ml in T-25 flasks. For differentiation into myotubes, myoblasts are plated in growth medium on collagen-coated plastic dishes (e.g. BD Biocoat, 354456) and allowed to reach 80% confluency (1–3 days). The growth medium is replaced with differentiation medium (DMEM plus 5% horse serum) with 4 washings; cells are refed daily. Differentiation into myotubes occurs over a period of 2–5 days; >90% of the myoblasts fuse into myotubes. The cultures appear to be depleted of contaminating fibroblasts. Fibroblasts, when present, will overgrow cultured myotubes in a few days. We have not observed any fibroblast proliferation in myotube cultures maintained for up to 100 days.
C2C12 cells
Parental C2C12 cells and an shRNA-transduced dysferlin “knock down” cell line were generously provided by Dr. Michelle Maxwell, Harvard Medical School [14]. These cells were cultured and differentiated as described for GREG cells (see above).
Genomic Analysis
Genomic DNA was prepared from GREG and C2C12 myoblasts using the Wizard genomic DNA purification kit (Promega, A1120). PCR primers for detection of the retrotransposon (ETn) insertion in the dysferlin gene were as described in [9]: dysf-F, dysf-R, ETn-oR, ETn-R2. 25 µl reactions contained 50 ng DNA, 0.4 µM primers, 20 mM Tris (pH 8.4), 50 mM KCl, 1.5 mM MgSO4, 200 µM each dNTP, and 1U Taq DNA polymerase (Invitrogen). Reactions were denatured for 3 min. at 94 °C; then subjected to 35 cycles of 30 sec at 94 °C, 30 sec at 55 °C, 60 sec at 72 °C; followed by a 10 min. extension at 72 °C. PCR products were analyzed by electrophoresis in a 1.5% agarose gel run in 1X TAE buffer, and visualized by staining with ethidium bromide.
Detection and quantification of muscle fiber proteins
The expression of specific proteins in cultured cells was detected using fluorescent-labeled ligands or antibodies. Cells were washed with serum-free medium, fixed with 3.7% paraformaldehyde in PBS for 20 minutes, permeabilized with 0.1% triton X100 for 2 min. F-actin was detected by staining with 0.88 µM rhodamine-labeled phalloidin (Molecular Probes, R415) for 20 min. at room temperature. Nicotinic acetylcholine receptor (nAChR) was detected by staining live cells with fluorescein-conjugated α-bungarotoxin (1ug/ml in PBS; Molecular Probes, F-1176) for 90 minutes.
For detection of dysferlin and myoferlin we used an antigen retrieval procedure as described in Robinson, et al., 2001[15]. Following Triton-X100 treatment (see above), cells were treated with 0.5% SDS for 5 min., washed with PBS and blocked with 5% normal goat serum in PBS for 1 hr. Cells were incubated with Hamlet mAb to dysferlin (NCL-Hamlet, Leica Biosystems) diluted 1:40, or myoferlin (Cov-7D2, Novus Biologicals) diluted 1:50, for 1 hr., washed with PBS, and incubated with Alexa Fluor-labeled goat anti-mouse IgG (Invitrogen A11029) diluted 1:1500 for 1 hr. Cells were imaged using a Zeiss Axiovert 25 with a 10X objective and images were recorded using a digital camera (Hamamatsu Orca ER) controlled by IPLab (Version 3.61, Scanalytics, Fairfax, VA, USA). Fluorescence intensity was quantified using ImageJ as described in [16]; coverage-corrected pixel frequency graphs were used to compare hamlet labeling.
Myoferlin antibody staining of fixed myotubes and myoblasts were imaged using a Zeiss 510 confocal microscope. Samples were imaged using a Zeiss Plan-Apochromat 63×, 1.4 NA, oil immersion objective with 488 nm laser excitation and 500–550 nm bandpass filter. Image stacks were acquired with nominal voxel dimensions of 0.28 um × 0.28 um × 0.50 um and consisted of 20 to 40 image planes. Orthogonal projections of the image stacks, processed using ImageJ (NIH), were used to evaluate the distribution of myoferlin fluorescence.
Immunoprecipitation and western blotting
Immunoprecipitation of dysferlin was performed with VhH heavy chain antibody fragments specific for dysferlin [17] as described previously [18]. Briefly, cells were homogenized in lysis buffer (50mM Tris HCl, pH7.5, 150mM NaCl, 0.2% Triton X100, 1× protease inhibitor cocktail), spun down and precleared with ProtA Sepharose beads. 20 μg of dysferlin VhH H7 or F4 were incubated overnight at 4°C tumbling. As a negative control we used a VhH against amyloid-β [19], which does not recognize dysferlin. As a positive control lysate we used cultured IM2 myotubes. Subsequently, pre-equilibrated ProtA Sepharose beads were added and incubated for 2 hr at 4°C tumbling. Beads were spun down and washed 5× in lysis buffer, and finally boiled in sample buffer. IP samples were loaded onto SDS-PAGE gels and stained with Coomasie Blue, or blotted onto PVDF membrane for western blot detection. Blots were blocked with 4% Marvel in PBS. Hamlet mouse anti-dysferlin (see above) was incubated 2 hr at room temperature in blocking buffer. Secondary detection was performed with goat anti-mouse IRdye680 (Westburg). Blot and gel were imaged with an Odyssey scanner (Licor).
Membrane repair assay
Plasma membrane repair capacity in cultured myotubes was measured by the uptake of FM 1-43 dye following laser wounding [6, 20]. Myotubes grown in 35 mm plastic dishes were washed 3X with Dulbecco’s PBS (DPBS) supplemented with 5.6 mM D-glucose and 0.33 mM sodium pyruvate, and the buffer replaced with DPBS containing 2 µM FM 1-43 (Molecular Probes, T35356). The dish was mounted on a Zeiss 510 (Axioskop 2) confocal microscope and imaged at room temperature (18 – 21 C°) using a 63×, 0.9 NA, water immersion objective (Zeiss) with laser scanning at 488 nm. To laser wound the myotubes, a square 7.8 µm2 region of interest (ROI) overlapping the plasma membrane was scanned using the maximum output (~ 2.5 W) of the Ti-Sapphire infrared laser (Coherent Chameleon) operating at 800 nm. The amount of energy delivered was controlled by the number of scan repetitions within the wounding ROI and varied (1 – 100) depending upon the degree of wounding desired. An image stack was recorded at 10 sec intervals for 200 sec. Uptake of FM1-43 dye into the wounded myotubes was quantified using NIH ImageJ. Background was subtracted using the Rolling Ball algorithm, as implemented in NIH ImageJ [21], an image mask created using the maximum projection of the image stack in time, and the average intensity of the myotube calculated using the Time Series Plugin. The fluorescence intensity was normalized by the pre-wound intensity and the normalized response (Δ F/F) plotted as a function of time.
Analysis of FM 1-43 uptake
Laser wounding assays do not distinguish between the size of the initial wound (wounding potential) and the ability of the cell to repair the wound (repair potential). Therefore, the magnitude and or rate in the uptake of dye may not be optimal parameters for comparing membrane repair. To resolve this problem, membrane repair was defined as the presence of a plateau in the FM 1-43 signal, in the absence of entire cell saturation, while a continuous increase in the FM 1-43 signal was taken as the absence of repair. These behaviors were quantified by formally fitting the dye uptake kinetics with three different kinetic models:
Repair Model: Normalized Signal = (1-exp(-t/τ))*A
Continuous linear uptake Model: Normalized Signal = A*t + B
Super linear uptake Model: Normalized Signal = A*t^B
Results
Proliferation and Differentiation of GREG myoblasts in culture
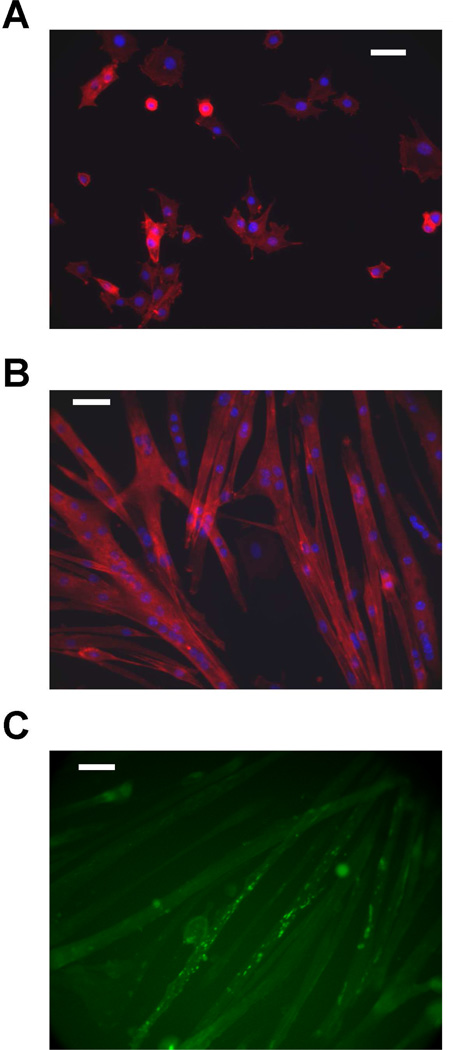
To obtain a dysferlin-deficient myogenic cell line, primary myoblasts were prepared from a 9 day old A/J mouse (see Methods). To suppress the growth of fibroblasts, the myoblasts were cultured under selective conditions for three weeks. Fibroblasts were depleted by selective detachment of myoblasts in PBS, without trypsin or chelators (see Methods). During this period, the growth rate of the myoblasts increased, and a pure myoblast population, free of fibroblast contamination, was obtained. These cells have undergone > 60 population doublings in culture, demonstrating a large proliferation potential. We compared the doubling time of this dysferlin-deficient “GREG” cell line with the well-characterized C2C12 mouse myogenic cell line. The population doubling time decreased with passage number from 34.5 ± 1.2 hrs at passage 20 to 18.6 ± 0.2 hrs at passage 40; this decreased doubling time is similar to C2C12 myoblasts (Table 1). When the growth medium is replaced with differentiation medium (DMEM plus 5% horse serum), the GREG myoblasts fuse and form myotubes over a period of three days (Figure 1A, B). In time, the myotubes develop long, branching structures, and exhibit spontaneous contraction. Myotubes have been maintained in culture up to one month, with no evidence of fibroblast contamination. The GREG myotubes express proteins characteristic of muscle fibers including F-actin (Figure 1A, B) and nicotinic acetylcholine receptors (nAChR) (Figure 1C).
Table 1.
Doubling time comparison between GREG and C2C12 myogenic cell lines; influence of passage number on growth kinetics. TD, doubling time.
| Cell line | Low passage # | TD (hrs) | High passage # | TD (hrs) |
|---|---|---|---|---|
| C2C12 | 22 | 19.6 ± 0.4 | 38 | 18.4 ± 0.2 |
| GREG | 20 | 34.5 ± 1.2 | 40 | 18.6 ± 0.2 |
Figure 1.
Differentiation of myogenic GREG cell line in culture. (A) GREG myoblasts cultured in growth medium; nuclei stained with DAPI (blue), actin stained with rhodamine-labeled phalloidin (red) (B) formation of myotubes after 3 days in differentiation medium; nuclei stained with DAPI (blue), actin stained with rhodamine-labeled phalloidin (red). (C) nicotinic acetylcholine receptors detected by fluorescein-conjugated α-bungarotoxin. Scale bar is 200 µm.
Myoblast cell lines can lose the capacity to differentiate into myotubes during passaging. GREG myoblasts have retained the capacity for differentiation for up to 40 passages; we routinely differentiate these cells between 10 and 30 passages.
Dysferlin expression not detected in GREG cells
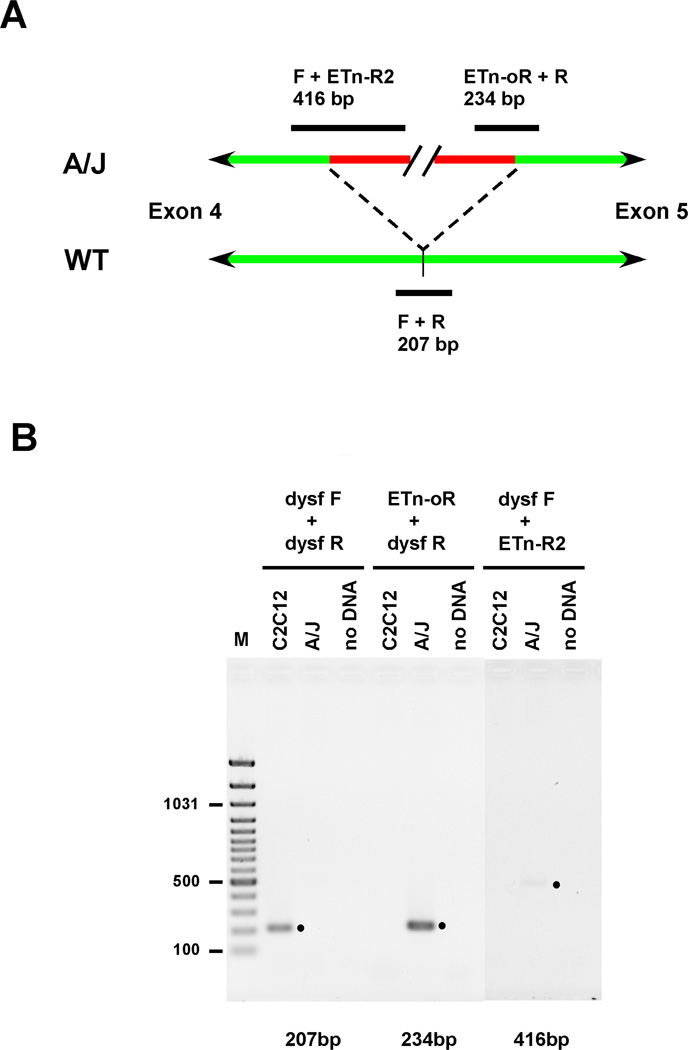
The A/J mouse strain has, in addition to other characterized genetic defects, a ~6 kbp ETn retrotransposon insertion in intron 4 of the dysf gene, and no detectable expression of dysferlin protein [9]. PCR analysis of genomic DNA prepared from GREG myoblasts was used to confirm the presence of the retrotransposon insertion in this cell line. PCR primers which are diagnostic for the presence or absence of the retroposon insertion in the mouse dysferlin gene were used. Primers dysf-F and dysf-R flank the insertion site and yield a 207 bp product from wild-type (no insert) DNA. Primers ETn-oR and ETn-R2 hybridize near the 3’ and 5’ ends of the insertion, and yield 234 bp (ETn-oR + dysf-R) and 416 bp (dysf-F + ETn-R2) products from A/J DNA (Figure 2B). The 207 bp (dysf-F + dysf-R) product was only obtained from C2C12 myoblast DNA, while the A/J-specific products were only obtained from GREG myoblast DNA (Figure 2A), demonstrating that the retroposon insertion is retained in GREG cells.
Figure 2.
Genomic PCR to detect the ETn retrotransposon insertion in the mouse dysf gene. Reactions contained genomic DNA prepared from either C2C12 or GREG myoblasts, or no DNA. Primer pairs for DNA amplification were either (dysf-F + dysf-R) for detection of wild-type sequence, or (ETn-oR + dysf-R) and (dysf-F + ETn-R2) for detection of the retroposon insertion. (A) schematic of the 5-6 kbp ETn retrotransposon (red) insertion site in intron 4 (green) between exons 4 and 5 in mouse dysferlin gene, showing PCR products (black) which derive from either wild type (WT) or A/J strain (A/J) mouse genomic DNA (adapted from [9], see Results). (B) PCR products resolved in an agarose gel, detected by ethidium staining; (•) marks the expected PCR products. Contrast in the dysf-F + ETn-R2 lanes was adjusted for clarity.
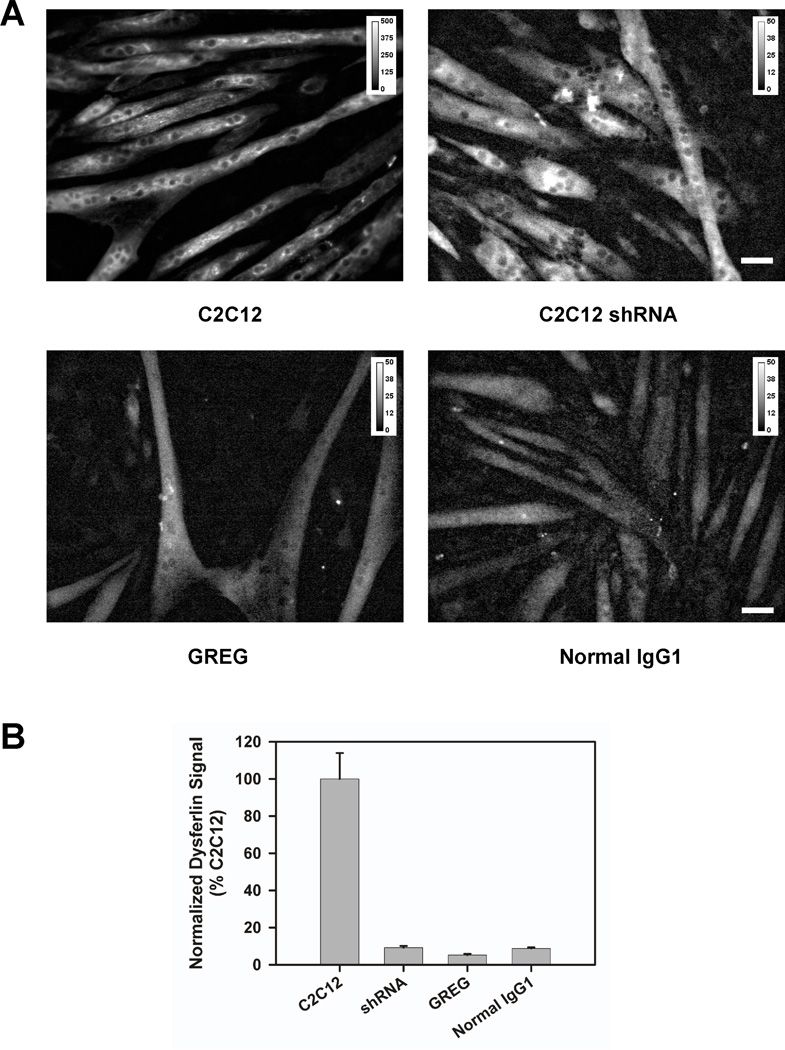
A quantitative immunofluorescence assay, using the Hamlet mAb to dysferlin, was used to assess whether there is any residual dysferlin expression in GREG myoblasts or myotubes [16]. The level of dysferlin expression, if present, was comparable to a non-specific antibody control (mouse IgG1); that is, below our detection threshold and significantly less than the positive controls, C2C12 myotubes (Figure 3).
Figure 3.
Quantitative immunofluorescence assay for dysferlin expression. (A) Immunofluorescent staining for dysferlin using Hamlet mAb in C2C12, C2C12 shRNA or GREG myotubes. Background control using normal mouse IgG1 in C2C12 myotubes. The dynamic range for image intensity in the C2C12 myotubes stained for dysferlin is ten-fold greater than in the other three images. Without rescaling, the C2C12 shRNA, GREG, and IgG1 control examples would appear black. (B) Quantization of fluorescence signal in (A) following Bezrukov et al. [16].
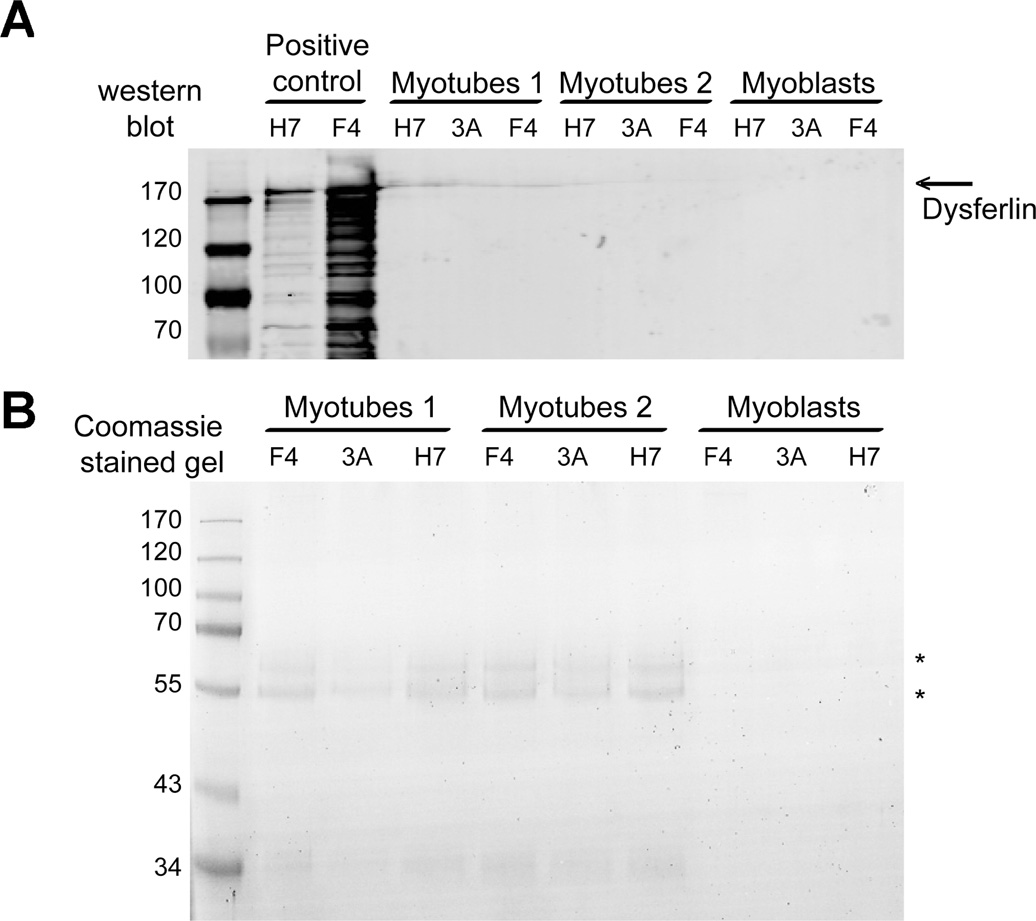
GREG myoblasts and myotubes were evaluated further for dysferlin expression using a sensitive, two-stage immuno-blotting assay [18]. Cell extracts were prepared in lysis buffer (see Methods). Dysferlin is first concentrated by immuno-precipitation with anti-dysferlin VhH heavy chain antibody fragments (HCAbs), and then detected by western blotting with Hamlet mAb. The immunoprecipitation step concentrates dysferlin from a larger volume of lysate than can be analyzed by one-dimenstion electrophoresis, and thus should be more sensitive than conventional western blotting. The F4 HCAb binds ≥ 90% of the dysferlin in the lysate [18]. No dysferlin was detected in the GREG cells (Figure 4A). In dysferlin-expressing cells, co-immunoprecipitating proteins in dysferlin complexes are detectable by Coomassie staining [18]. No co-immunoprecipitating proteins were detectable in dysferlin immunoprecipiates from GREG cells (Figure 4B) further supporting the hypothesis that dysferlin expression is either absent or below detection in GREG cells.
Figure 4.
Immunoprecipitation of dysferlin followed by western blot detection (A) or Coomassie Blue staining (B). Dysferlin was immunoprecipitated with two specific VhH heavy chain antibody fragments (F4 and H7) and a nonspecific control (3A). Only the bound fractions from the immunoprecipitation were analyzed. Immunoprecipitation from GREG myotubes lysate was performed in duplicate (Myotubes 1 and 2). As positive control IM2 myotube lysate was used. The arrow denotes the dysferlin band. The asterisks denote background bands.
Myoferlin expression in GREG cells
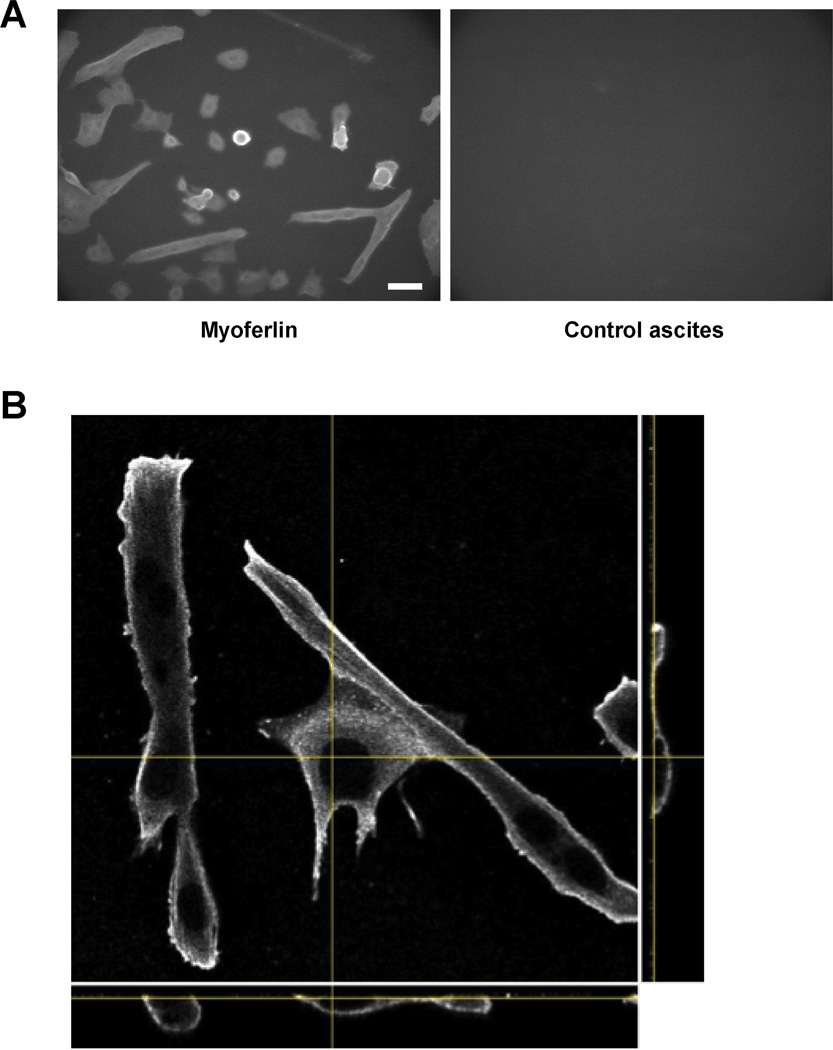
Myoferlin is a muscle-specific protein highly homologous to dysferlin (55% sequence identity). Myoferlin and dysferlin expression exhibit a temporal relationship in muscle fiber development. Myoferlin is highly expressed in myoblasts, while dysferlin is expressed mainly in myotubes [22]. Myoferlin knock-out mice exhibit impairment in myoblast fusion and muscle regeneration suggesting a role for myoferlin muscle development and maintenance [23]. Dysferlin and myoferlin form complexes in myotubes [18]; it is possible that the absence of dysferlin could affect the expression and localization of myoferlin. To assess whether the absence of dysferlin affects myoferlin expression, we assayed GREG myoblasts and myotubes for myoferlin expression, using a previously characterized antibody [24] (see Methods). Myoferlin was detected in both myoblasts and myotubes (Figure 5A). In widefield (low resolution) microscopy it appears to be concentrated at the plasma membrane, as would be expected for a membrane-anchored protein. To confirm localization of myoferlin at the membrane, we collected image z-stacks using a confocal microscope (Figure 5B). Myoferlin staining was concentrated at the cell surface, as viewed in all three image projections.
Figure 5.
Myoferlin expression and localization in GREG cells. Cultures containing GREG myoblasts and early differentiating myotubes were prepared for immunofluorescent staining as in Fig. 3. Myoferlin was detected using mouse mAb Cov-7D2, in ascites; normal mouse ascites served as a control. Myoferlin was detected in both myoblasts and myotubes. (A) Low resolution (widefield) image of myoferlin (left) staining in myoblasts and myotubes; (right) control ascites. Scale bar is 200 µm. (B) Localization of myoferlin in myotubes and myoblasts by confocal microscopy; yellow lines indicate position of each panel in the image stack. Central panel, x-y projection; lower panel, x-z projection; right panel, y-z projection. Myoferlin staining is concentrated at the plasma membrane. Scale bar is 10 µm.
Membrane repair activity in GREG myotubes
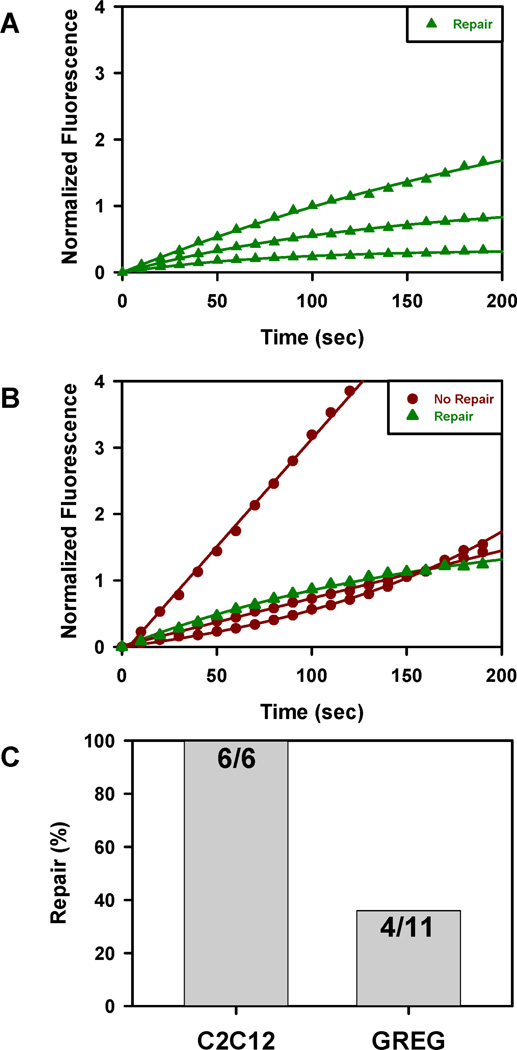
We compared membrane repair activity in GREG and C2C12 myotubes using a standard laser wounding assay (see Methods). In an effort to minimize cell-to-cell variability and phototoxicity, we determined the minimum wound area (~8 µm2) and level of laser energy (scan repetitions) required to produce consistent membrane wounding; the wound site was located at the edge of the myotubes. Both C2C12 and GREG myotubes incorporated FM1-43 dye following laser wounding (Figure 6A, B). For the same laser parameters, the magnitude of the response varied in both cell types. However, the uptake kinetics in all wounded C2C12 myotubes was always consistent with membrane repair as indicated by a plateau in the fitting of the normalized response. The GREG myotubes exhibited greater variability in FM1-43 uptake than C2C12 myotubes; the majority of the responses was consistent with an impairment of membrane resealing as indicated by either a linear or super linear model describing the normalized response. While GREG myotubes are capable of membrane resealing, the overall percentage (36%) was significantly lower than C2C12 myotubes (100%) and consistent with a dysferlin dependent defect in membrane resealing (Figure 6C). It should be noted that the C2C12 cell are derived from the C3H mouse strain; differences in resealing activity between GREG and C2C12 could be due to differences at other genetic loci besides dysferlin.
Figure 6.
Uptake of FM1-43 dye in cultured myotubes following laser wounding at the same energy parameters. (A) Dysferlin-positive C2C12 myotubes; (B) Dysferlin-negative GREG myotubes. Normalized fluorescence calculated as ΔF/F-1. (C) Fraction of wounded myotubes which resealed, based on kinetics of dye uptake (see Results).
Discussion
We have isolated a myogenic cell line (GREG) from the dysferlin-deficient A/J mouse strain. The myoblasts actively proliferate in growth medium (> 50 population doublings) and fuse into long, multinucleated myotubes in differentiation medium. They are morphologically similar to primary mouse myotubes, express muscle-specific proteins, and exhibit muscle fiber functionality, i.e. membrane depolarization and contractility (unpublished results).
The GREG cell line retains the retroposon insertion in the A/J dysferlin gene and has no detectable dysferlin expression. Apparently, dysferlin is not required for either myoblast proliferation or fusion into myotubes in this cell line. GREG myotubes are defective in plasma membrane repair, as previously observed in dysferlin-deficient muscle fibers [6]. Under the wounding conditions used, the extent of the membrane repair defect in GREG myotubes is variable; approximately 34% of the GREG myotubes exhibit membrane repair, compared with 100% of dysferlin-positive C2C12 myotubes. The variable extent of the membrane repair deficiency in GREG myotubes may be related to the process of myoblast fusion and differentiation. Both C2C12 and GREG myoblasts exhibit membrane repair (unpublished results), but membrane repair capacity appears to be lost as GREG myoblasts fuse into myotubes, suggesting that dysferlin provides some additional functionality required for membrane repair in developing muscle fibers. This could be a consequence of myotube morphology (i.e. surface to volume ratio), a lack of excess membrane, or the formation of new membrane and protein structures within the developing myotube.
A curious aspect of human dysferlin-deficiency syndromes is the great variability in 1) age of onset (early adolescence to late middle age), 2) muscles affected (proximal vs. distal), and 3) severity (mild weakness to loss of ability to stand or walk) [3, 4]. This suggests that the patient’s genetic background is an important factor in disease progression, and that some degree of compensation for dysferlin deficiency may occur through altered expression of other genes. Dysferlin-deficiency phenotypes in mutant mice are relatively mild compared with humans (i.e. the mice are not immobilized) [9, 25], which suggests that genetic compensation for dysferlin-deficiency may be present in these disease models. The cell-to-cell variation in membrane repair function observed in GREG myotubes could be a manifestation of such compensation. The GREG cell line is a mixed population, similar to the original C2 myoblast cell line [12]. The isolation of clonal lines of GREG cells (i.e. the C2C12 subclone of C2) [26] could provide a means to assess whether the variability is stable (i.e. epigenetic) and correlated with expression of specific genes.
Acknowledgments
This work was supported by the intramural program of the NICHD, NIH; and the Jain Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bansal D, Campbell KP. Dysferlin and the plasma membrane repair in muscular dystrophy. Trends Cell Biol. 2004;14:206–213. doi: 10.1016/j.tcb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Glover L, Brown RH., Jr Dysferlin in membrane trafficking and patch repair. Traffic. 2007;8:785–794. doi: 10.1111/j.1600-0854.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen K, Bassez G, Krahn M, Bernard R, Laforet P, Labelle V, Urtizberea JA, Figarella-Branger D, Romero N, Attarian S, Leturcq F, Pouget J, Levy N, Eymard B. Phenotypic study in 40 patients with dysferlin gene mutations: high frequency of atypical phenotypes. Arch Neurol. 2007;64:1176–1182. doi: 10.1001/archneur.64.8.1176. [DOI] [PubMed] [Google Scholar]
- 4.Weiler T, Bashir R, Anderson LV, Davison K, Moss JA, Britton S, Nylen E, Keers S, Vafiadaki E, Greenberg CR, Bushby CR, Wrogemann K. Identical mutation in patients with limb girdle muscular dystrophy type 2B or Miyoshi myopathy suggests a role for modifier gene(s) Hum Mol Genet. 1999;8:871–877. doi: 10.1093/hmg/8.5.871. [DOI] [PubMed] [Google Scholar]
- 5.Selcen D, Stilling G, Engel AG. The earliest pathologic alterations in dysferlinopathy. Neurology. 2001;56:1472–1481. doi: 10.1212/wnl.56.11.1472. [DOI] [PubMed] [Google Scholar]
- 6.Bansal D, Miyake K, Vogel SS, Groh S, Chen CC, Williamson R, McNeil PL, Campbell KP. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature. 2003;423:168–172. doi: 10.1038/nature01573. [DOI] [PubMed] [Google Scholar]
- 7.Lennon NJ, Kho A, Bacskai BJ, Perlmutter SL, Hyman BT, Brown RH., Jr Dysferlin interacts with annexins A1 and A2 and mediates sarcolemmal wound-healing. J Biol Chem. 2003;278:50466–50473. doi: 10.1074/jbc.M307247200. [DOI] [PubMed] [Google Scholar]
- 8.McNeil AK, Rescher U, Gerke V, McNeil PL. Requirement for annexin A1 in plasma membrane repair. J Biol Chem. 2006;281:35202–35207. doi: 10.1074/jbc.M606406200. [DOI] [PubMed] [Google Scholar]
- 9.Ho M, Post CM, Donahue LR, Lidov HG, Bronson RT, Goolsby H, Watkins SC, Cox GA, Brown RH., Jr Disruption of muscle membrane and phenotype divergence in two novel mouse models of dysferlin deficiency. Hum Mol Genet. 2004;13:1999–2010. doi: 10.1093/hmg/ddh212. [DOI] [PubMed] [Google Scholar]
- 10.Roche JA, Lovering RM, Bloch RJ. Impaired recovery of dysferlin-null skeletal muscle after contraction-induced injury in vivo. Neuroreport. 2008;19:1579–1584. doi: 10.1097/WNR.0b013e328311ca35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roche JA, Lovering RM, Roche R, Ru LW, Reed PW, Bloch RJ. Extensive mononuclear infiltration and myogenesis characterize recovery of dysferlin-null skeletal muscle from contraction-induced injuries. Am J Physiol Cell Physiol. 2010;298:C298–C312. doi: 10.1152/ajpcell.00122.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yaffe D, Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977;270:725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- 13.Yasin R, van Beers G, Bulien D, Thompson EJ. A quantitative procedure for the dissociation of adult mammalian muscle into mononucleated cells. Exp Cell Res. 1976;102:405–408. doi: 10.1016/0014-4827(76)90056-2. [DOI] [PubMed] [Google Scholar]
- 14.Belanto JJ, Diaz-Perez SV, Magyar CE, Maxwell MM, Yilmaz Y, Topp K, Boso G, Jamieson CH, Cacalano NA, Jamieson CA. Dexamethasone induces dysferlin in myoblasts and enhances their myogenic differentiation. Neuromuscul Disord. 2010;20:111–121. doi: 10.1016/j.nmd.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson JM, Vandre DD. Antigen retrieval in cells and tissues: enhancement with sodium dodecyl sulfate. Histochem Cell Biol. 2001;116:119–130. doi: 10.1007/s004180100299. [DOI] [PubMed] [Google Scholar]
- 16.Bezrukov L, Blank PS, Polozov IV, Zimmerberg J. An adhesion-based method for plasma membrane isolation: evaluating cholesterol extraction from cells and their membranes. Anal Biochem. 2009;394:171–176. doi: 10.1016/j.ab.2009.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang Y, Verheesen P, Roussis A, Frankhuizen W, Ginjaar I, Haldane F, Laval S, Anderson LV, Verrips T, Frants RR, de Haard H, Bushby K, den Dunnen J, van der Maarel SM. Protein studies in dysferlinopathy patients using llama-derived antibody fragments selected by phage display. Eur J Hum Genet. 2005;13:721–730. doi: 10.1038/sj.ejhg.5201414. [DOI] [PubMed] [Google Scholar]
- 18.de Morree A, Hensbergen PJ, van Haagen HH, Dragan I, Deelder AMt, Hoen PA, Frants RR, van der Maarel SM. Proteomic analysis of the dysferlin protein complex unveils its importance for sarcolemmal maintenance and integrity. PLoS One. 2010;5:e13854. doi: 10.1371/journal.pone.0013854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rutgers KS, van Remoortere A, van Buchem MA, Verrips CT, Greenberg SM, Bacskai BJ, Frosch MP, van Duinen SG, Maat-Schieman ML, van der Maarel SM. Differential recognition of vascular and parenchymal beta amyloid deposition. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNeil PL, Miyake K, Vogel SS. The endomembrane requirement for cell surface repair. Proc Natl Acad Sci U S A. 2003;100:4592–4597. doi: 10.1073/pnas.0736739100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sternberg SR. Biomedical image processing. IEEE Computer. 1983;16:22–34. [Google Scholar]
- 22.Davis DB, Doherty KR, Delmonte AJ, McNally EM. Calcium-sensitive phospholipid binding properties of normal and mutant ferlin C2 domains. J Biol Chem. 2002;277:22883–22888. doi: 10.1074/jbc.M201858200. [DOI] [PubMed] [Google Scholar]
- 23.Doherty KR, Cave A, Davis DB, Delmonte AJ, Posey A, Earley JU, Hadhazy M, McNally EM. Normal myoblast fusion requires myoferlin. Development. 2005;132:5565–5575. doi: 10.1242/dev.02155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robinson JM, Ackerman WEt, Behrendt NJ, Vandre DD. While dysferlin and myoferlin are coexpressed in the human placenta, only dysferlin expression is responsive to trophoblast fusion in model systems. Biol Reprod. 2009;81:33–39. doi: 10.1095/biolreprod.108.074591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bittner RE, Anderson LV, Burkhardt E, Bashir R, Vafiadaki E, Ivanova S, Raffelsberger T, Maerk I, Hoger H, Jung M, Karbasiyan M, Storch M, Lassmann H, Moss JA, Davison K, Harrison R, Bushby KM, Reis A. Dysferlin deletion in SJL mice (SJL-Dysf) defines a natural model for limb girdle muscular dystrophy 2B. Nat Genet. 1999;23:141–142. doi: 10.1038/13770. [DOI] [PubMed] [Google Scholar]
- 26.Blau HM, Chiu CP, Webster C. Cytoplasmic activation of human nuclear genes in stable heterocaryons. Cell. 1983;32:1171–1180. doi: 10.1016/0092-8674(83)90300-8. [DOI] [PubMed] [Google Scholar]