Abstract
Background
Hurler syndrome (mucopolysaccharidosis type I/H, MPS I/H) is a lethal heritable enzymopathy that leads to accumulation of glycosaminoglycans (GAGs) and dysfunction of multiple organs of the body, including the heart.
Aim
As gender differences are common in heart disease and a murine model for mucopolysaccharidosis type I (MPSI) has been used for pre-clinical evaluations of strategies to correct heart valve disease in Hurler syndrome, we wished to determine the impact of gender on heart disease in the mucopolysaccharidosis type I murine model.
Methods
We have examined murine hearts by high resolution ultrasound biomicroscopy, measured tissue and urinary content of GAGs, and determined quantitative reverse transcribed ribonucleic acid polymerase chain reaction for metalloproteinase 9 and 12.
Results
Aortic insufficiency (AI) in conjunction with depressed myocardial function was observed significantly more often in MPSI males than MPSI females. Neither total body GAG burden nor myocardial GAG content was responsible for this difference. In contrast, expression of extracellular matrix tissue MMP-12, but not MMP-9, in the aorta was significantly elevated in MPSI males with AI when compared to MPSI females with AI.
Conclusions
Gender dimorphism occurs in cardiac valvular disease in MPSI mice. Male MPSI mice have an increased incidence of AI associated with an increase in MMP-12 aortic arch content. Evaluation of findings by gender is important in experimental treatment of murine models of disease so that gender-related variations in genetic penetrance are not mistaken for disease correction.
Keywords: mucopolysaccharidosis I, metalloproteinase, mouse, aorta, valve
Introduction
Population sex differences are commonly recognized in human cardiac disease(1–4) but have been only recently appreciated in murine models of cardiac disease (5,6). A murine model of mucopolysaccharidosis Type I (MPSI) with aortic dilation and valvar aortic insufficiency (AI) as part of its phenotype (7–11) has been used to develop strategies for correction of its human counterpart, known clinically as Hurler syndrome (mucopolysaccharidosis type I/H, MPS I/H). During the course of our studies it became apparent that gender differences were an important, and previously unreported, facet of this murine model.
In humans, Hurler syndrome is a lethal heritable inborn error of metabolism due to mutations in the α-L-iduronidase (IDUA, EC 3.2.1.76) gene (12). The resulting enzymopathy leads to progressive accumulation of glycosaminoglycans (GAGs), disruption of the normal function of the cellular lysosomes, and dysfunction of multiple organs of the body, including the heart. Within the human heart GAG deposition is responsible for thickening and subsequent stenosis and/or regurgitation of atrioventricular and semilunar valves, ventricular hypertrophy and marked myointimal proliferation of epicardial coronary arteries (13–15). Left untreated, death occurs within the first decade of life from cardiac or respiratory failure (16). In humans, hematopoietic stem cell transplantation significantly prolongs life and reverses some, but not all, of the effects of this disorder: ventricular hypertrophy regresses and late death from coronary artery disease has not been reported (17–20). Cardiac valvular thickening and insufficiency however continue to progress (17).
In order to develop therapeutic strategies that allow for the correction of valvular disease, we and others utilize the MPSI murine models, which mimic human cardiac disease, especially in the occurrence of aortic valve thickening and regurgitation (8–11). Accumulation of GAG in MPSI mice is known to increase with age (8) but, until now, gender differences have not been studied in this model.
Materials and Methods
Fifty-two aged (≥299 days) male and female IDUA deficient mice (C57BL/6J-MPSI; MPSI mice) developed by Dr. Lorne Clarke (University of British Columbia) were available for study (7). All animal studies were approved by the University of Minnesota Institutional Review Board and mice were maintained in accordance with established NIH guidelines.
Cardiac ultrasound was performed in mice under inhaled isoflurane anesthesia utilizing a 40 MHz probe and the Vevo 660 high resolution ultrasound biomicroscope (VisualSonics, Toronto, Ontario). M-mode images were obtained in short –axis for determination of shortening fraction. B-mode imaging of the aortic root was obtained in modified right parasternal long-axis view. Doppler interrogation was performed in long- and short-axis beneath the aortic valve for determination of aortic insufficiency and in the high right parasternal position for determination of aortic diastolic retrograde flow, a measure of severe aortic regurgitation.
In 29 of 52 mice that had been previously assessed by cardiac ultrasound, cardiac apex and aortic arch tissue were available for analysis. GAG quantification was performed as previously described (8). Total RNA was extracted from the aortic arch using Invitrogen’s TRIzol reagent (Invitrogen Corp., Carlsbad, CA). Gene expression was analyzed by quantitative real-time PCR (ABI 7500 RT-PCR System; Applied Biosystems, Foster, CA) using primers and probes for: MMP9 and MMP12 (ABI Cat. No. Mm00442991_m1 and Mm00500554_m1), and an endogenous control, GAPDH (Mm99999915_m1). Fold difference was represented as RQ (2−(ΔΔCt )), where RQ is relative quantification, Δ is a change in value, and Ct is a threshold of PCR cycle.
Differences in the incidence of AI were compared by Fisher’s exact test; comparison of differences in ultrasound parameters and GAG content were made by unpaired t-testing; comparison of fold-differences in MMP9 and MMP12 were made by Mann-Whitney testing of nonparametric data, p<0.05 considered significant in all instances.
Results
Aortic Insufficiency and Depressed Myocardial Function are More Prevalent in Male than Female MPSI Mice
Fifty-two mice (26 males, 26 females) underwent evaluation for aortic insufficiency, left ventricular size and function by high resolution ultrasound biomicroscopy (Table 1). Although female mice were significantly older than males (p = 0.0299), aortic insufficiency (AI) was observed more often in MPSI males (20/26; 77%) than MPSI females (11/26; 42%) (p =0.0227) (Table 1). Within each gender, mice with AI were not significantly older than mice without AI (Table 1).
Table 1.
Gender Differences in Aortic Insufficiency in MPSI Mice.
| Male | Female | P value | |
|---|---|---|---|
| (N tested) | (N tested) | ||
| Age (±SD) | 334.7±33 days (26) | 362.4±48 days (26) | 0.0184 |
| AI absent | 339.3±43 days (6)* | 367.5±49 days (15)** | |
| AI present | 333.3±30 days (20) | 355.6±47 days (11) | |
| Weight (±SD) | 35.8 ± 4.2 gm | 28.2 ± 4.0 gm | 0.0001 |
| AI | 20/26 | 11/26 | 0.0227 |
| LVIDD (±SD) | 5.3±1 mm (26) | 4.2±0.8 (26) | 0.0002 |
| AI absent | 4.6±0.4 mm (6)† | 3.86±0.2 mm (15)†† | |
| AI present | 5,47±1.1 mm (20) | 4.72±1 mm (11) | |
| Shortening fraction (SF) | 22.3±6.4 (26) | 27.6±5.7 (26) | 0.0029 |
| AI absent | 26.7±4.5 (6)††† | 29.0±5.4 (15)‡ | |
| AI present | 21.0±6.3 (20) | 25.6±5.7 (11) |
p=0.6974 age males AI− compared to age males AI+;
p=0.5407 age females AI− compared to age females AI+;
p=0.0838 LVIDD males AI− compared to males AI+;
p=0.0046 LVIDD females AI− compared to females AI+;
p=0.0492 SF males AI− compared to SF males AI+;
p=0.1390 SF females AI− compared to SF females AI+. AI, aortic insufficiency.
Left ventricular internal diameter in diastole (LVIDD) paralleled mouse weight (Table 1), both being significantly greater for males when compared to females (p=0.0002 and 0.0001, respectively) (Table 1). The presence of AI significantly increased LVIDD in female mice (p=0.0046) and approached significance in male mice (p=0.0838)(Table 1). Left ventricular systolic function, as measured by shortening fraction, was significantly decreased in MPSI males compared to MPSI females (p=0.0029). While SF in mice without AI did not differ between males and females (Table 1), the presence of AI was associated with a significant decrease in SF for males (p=0.0492) but had no significant effect upon SF in female mice (p=0.1390) (Table 1).
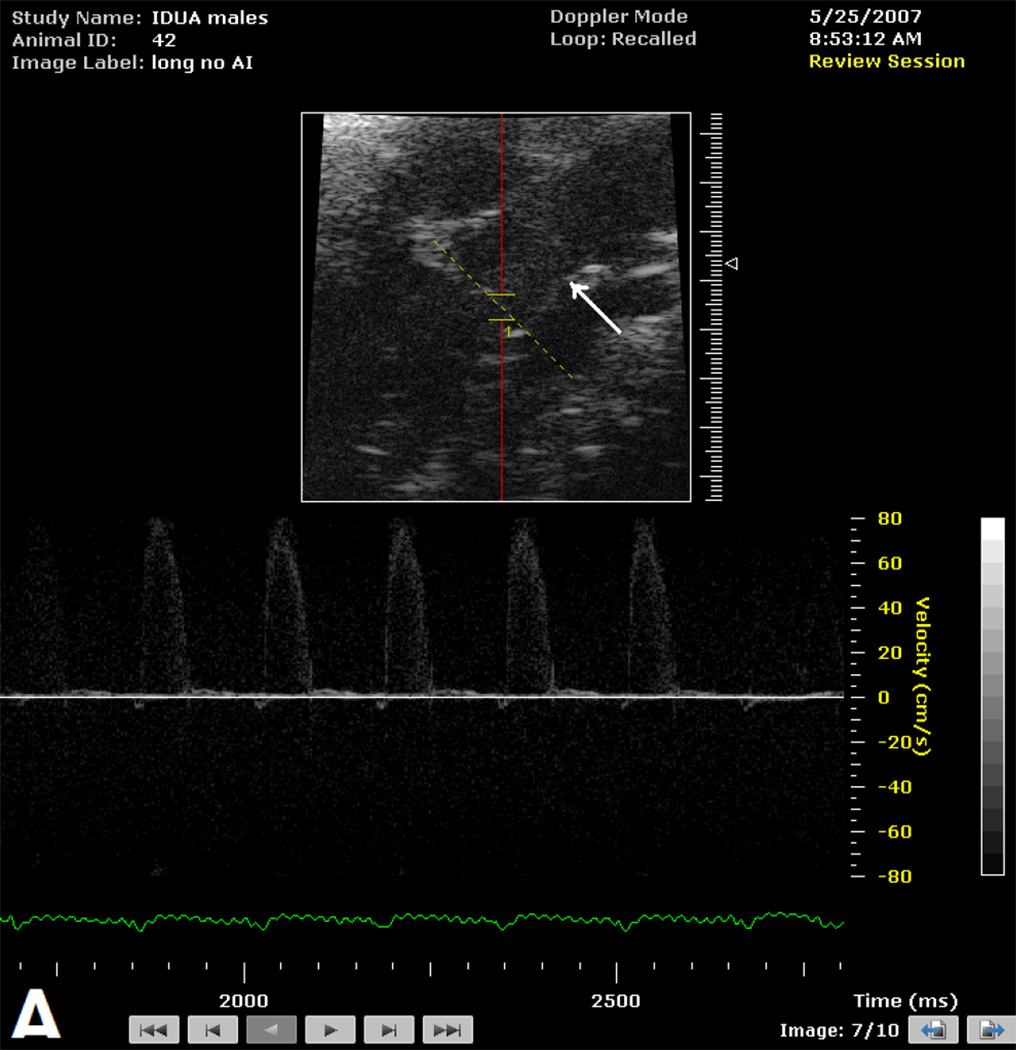
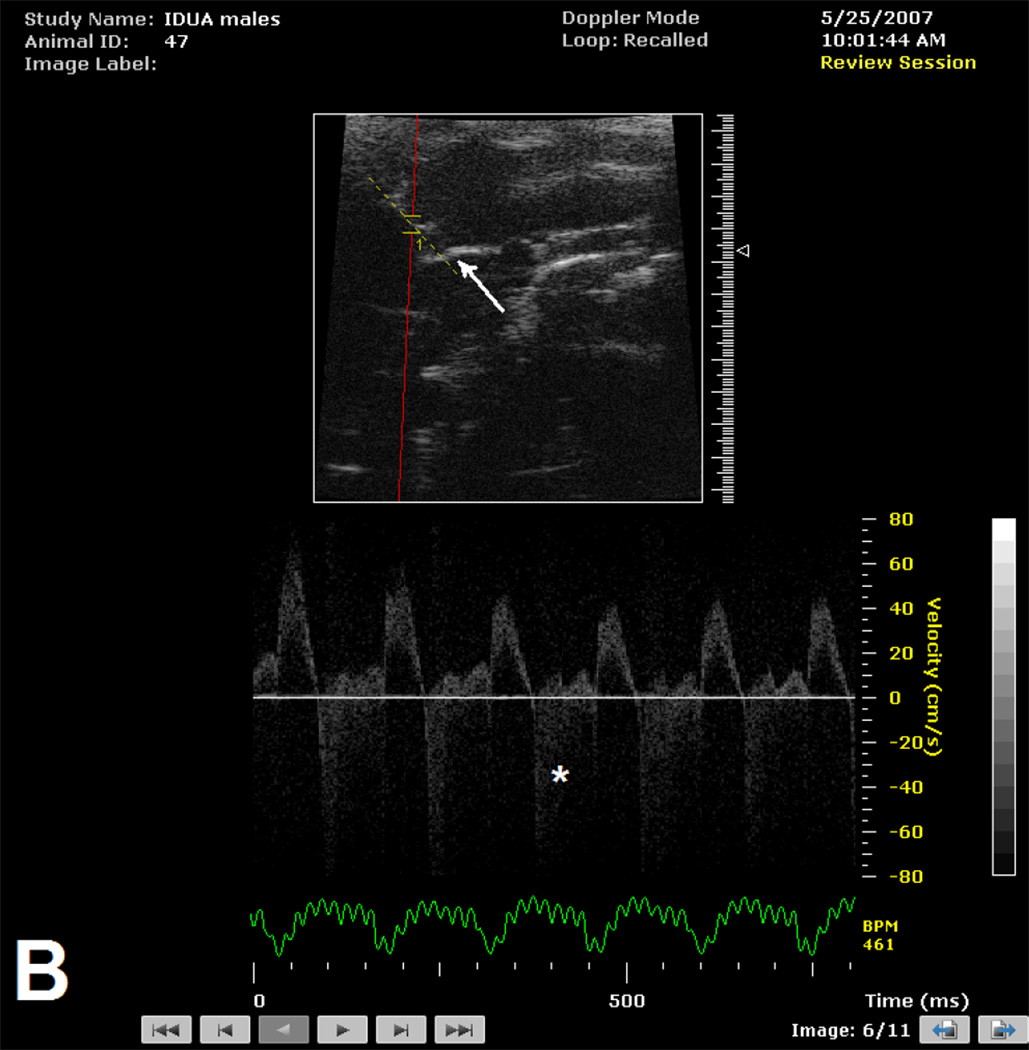
The presence of aortic insufficiency was associated with a specific appearance of the aortic root (Figure 1) which included effacement of the sino-tubular ridge and dilation of the ascending aorta. This pattern was observed in 19 of 20 male and 5 of 6 female mice with aortic insufficiency. The aortic root was not adequately imaged to comment upon appearance in 2 mice (I each, male and female).
Figure 1.
B-mode image of aortic root and Doppler interrogation of aortic valve in male mice > 300 days of age without (Figure 1A) and with (Figure 1B) aortic insufficiency. Note normal appearance of sino-tubular ridge (white arrow) in absence (Figure 1A) of aortic insufficiency and effacement of sino-tubular ridge (white arrow) in the presence (Figure 1B) of high velocity aortic insufficiency signal (*).
Whole Body and Heart GAG Content Does Not Correlate with Valve Disease
As GAG storage is currently considered to be the primary consequence of the enzymopathy in MPSI, we investigated whether the presence of AI was related to increased systemic (assessed as urinary GAG) or local GAG content. Urinary GAG content was not significantly different in MPSI males versus MPSI females. Neither was urinary GAG content different in MPSI males without AI versus MPSI males with AI, MPSI females without AI versus MPSI females with AI (all p > 0.05, Table 2). Similarly, heart muscle GAG content was not significantly different between any of the experimental groups (all p > 0.05, Table 2). We concluded that neither total body GAG burden nor myocardium GAG accumulation were likely responsible for the increased incidence of AI in males versus females with MPSI.
Table 2.
Gender Differences in GAG in MPSI mice
| GAG | ||
|---|---|---|
| Urine GAG/creatinine1 |
Heart GAG/protein |
|
| (N tested) | (N tested) | |
| Male | 16.8±7.6 (23)* | 31.4±8.1 (10)* |
| AI absent | 15.1±7.4 (7)** | 30.2±14.6 (3)** |
| AI present | 17.6±7.7 (16) | 31.9±5.2 (7) |
| Female | 17.8±7.3 (21)* | 33.8±9 (7)* |
| AI absent | 15.8±4.2 (12)** | 33.5±10 (3)** |
| AI present | 20.6±9.6 (9) | 34.0±10 (4) |
| WT2 | 1 (5)*** | 2 (11)*** |
p-value > 0.05 all MPSI males compared to MPSI females
p-value > 0.05 AI− compared to AI+ within same gender.
p-value < 0.05 when compared to any mutant group
No significant differences in creatinine values among the cohorts were observed.
No gender differences were observed in WT mice, therefore the data from males and females were pooled. AI, aortic insufficiency; WT, wild type.
Matrix metalloproteinase-12 is significantly greater in male than female MPSI mice
When compared to wild-type controls, aortic arch mRNA levels of both MMP-9 and MMP-12 were significantly elevated in both male and female MPSI mice (Table 2) as has been previously reported by others (21). No differences were observed in aortic arch expression of MMP-9 in MPSI mice related to gender or the presence or absence of AI. In contrast, aortic arch MMP-12 expression was significantly elevated in MPSI males with AI when compared to females with AI (p < 0.05, Table 3).
Table 3.
Gender Differences in ECM Enzyme Expression1 in MPSI Mice
| MMP9 | MMP12 | |||
|---|---|---|---|---|
| Apex (N) | Aorta (N) | Apex (N) | Aorta (N) | |
| Males | 6.7±2.8 (9)* | 5.4±2.5 (7)* | 13.4±9.2 (10)† | 74.5±56.6 (9)† |
| AI+ | 6.6±3 (8)** | 4.6±1.5 (6)** | 12.7±5.8 (8)†† | 73.4±60.4 (8)††† |
| Females | 5.9±2.4 (6) | 4.5±3.2 (6) | 7.8±5.8 (6) | 36.4±19.7 (6) |
| AI+ | 5.2±0.5 (3) | 3.6±1.2 (3) | 6.5±3 (3)** | 5.7±2.6 (3) |
p-value >0.05 MMP9 in apex or aorta of MPSI males vs MPSI females
p-value >0.05 MMP9 in apex or aorta of MPSI males AI+ vs. MPSI females AI+
p-value >0.05 MMP12 in apex, and >0.05 in aorta of MPSI males vs MPSI females
p-value >0.05 MMP12 in apex of MPSI males AI+ vs MPSI females AI+
p-value = 0.0242 MMP12 in aorta of MPSI males AI+ vs MPSI females AI+ For both MMP9 and MMP 12: p-value < 0.05 WT mice vs. any mutant group.
Normalized to expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Fold difference in comparison to wild-type mRNA expression is shown.
AI, aortic insufficiency; WT, wild type.
Discussion
Gender differences, such as the increased incidence of both coronary artery disease and abdominal aortic aneurysm in men (1–4) are well described in human cardiovascular pathophysiology but similar differences have only recently been appreciated in intact murine models of disease (5,6). Aortic root dilation has long been known to be the commonest definable cause of aortic regurgitation in aging humans (22). The incidence of aortic regurgitation is directly related to aortic root diameter (23) and associated with male gender (3). To our knowledge, the aging MPSI mouse is the only model reported to date with spontaneously occurring aortic root dilation and subsequent aortic valvar insufficiency (8,9).
The major finding of this study is that the high levels of MMP-12 expression in the aortic arch of male MPSI mice, not GAG accumulation, correlated with an increased incidence of aortic insufficiency and depressed myocardial function when compared to female MPSI mice. We recognize that our study is limited by the lack of histological and detailed immunohistochemical analyses. Nevertheless, defective elastin assembly due to excess GAG accumulation has been implicated in the pathogenesis of aortic dilation in human Hurler disease (24). In additional support of this contention, enhanced activation of MMP-12 and cathepsin, two elastin degrading proteins, have recently been demonstrated in the dilated aortas of 6 month old MPSI mice (gender not specified) when compared to wild type mice (21).
While a full understanding of the pathophysiology of aortic dilation, either human or murine, is unknown and likely to be complex, the available evidence implicates sex-steroids as a contributor (25–27). Matrix metalloproteinase-9 and TIMP-1 have been found to be 10-fold greater in male than female rat aortic smooth muscle cells when stimulated with interleukin-1 beta consistent with the increased ease of aneurysm formation in male, rather than female, rats (6). In other studies, orchiectomy, but not ovariectomy, attenuates the formation of abdominal aortic aneurysms in rats (28). To date, gender differences have not been reported in the cardiac phenotype of human MPS I, likely because the most common phenotype begins and completes its natural history within the first decade of life.
The targeted disruption of a single gene, resulting in the development of the Clarke MPSI mouse model, theoretically limits the phenotypic differences that are present in human MPSI with its extensive genomic variability (29, 30). While this approach has allowed elucidation of a reproducible cardiac phenotype, it is remarkable that, in spite of the single gene defect, not all male mice developed aortic regurgitation, a fact that likely implicates other non-genetic factors in the development of aortic regurgitation and the clinical phenotype.
In summary, our findings of increased incidence of AI and depressed cardiac function in male MPSI mice have been correlated not with GAG burden but rather with an increased incidence of aortic MMP-12 within the aortic wall. Our study highlights the importance of evaluating the effect of gender in murine models of disease, especially when considering therapies aimed at correction.
Acknowledgements
This work has been supported by NIH R01 HL49997 and the Children’s Cancer Research Fund, MN.
Footnotes
There are no relevant conflicts of interest to disclose.
Scientific contribution: JT, EB and BRB designed the study and interpreted the data. MR, BP, and RTE performed the experiments and collected the data. JT drafted the manuscript. EB, PJO, and BRB revised the manuscript.
References
- 1.Mak KH, Ma S, Heng D, Tan CE, Tai ES, Topol EJ, et al. Impact of sex, metabolic syndrome, and diabetes mellitus on cardiovascular events. Am J Cardiol. 2007;100:227–233. doi: 10.1016/j.amjcard.2007.02.090. [DOI] [PubMed] [Google Scholar]
- 2.Kurtulmus N, Bos S, Arslan S, Kurt T, Tukek T, Ince N. Differences in risk factors for acute coronary syndromes between men and women. Acta Cardiol. 2007;62:251–255. doi: 10.2143/ac.62.3.2020813. [DOI] [PubMed] [Google Scholar]
- 3.Singh JP, Evans JC, Levy D, Larson MG, Freed LA, Fuller DL, Lehman B, Benjamin EJ. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study) Am J Cardiol. 1999;83:897–902. doi: 10.1016/s0002-9149(98)01064-9. [DOI] [PubMed] [Google Scholar]
- 4.Lederle FA, Johnson GR, Wilson SE, Chute EP, Littooy FN, Bandyk D, Krupski WC, Barone GW, Archer CW, Ballard DJ. Prevalence and association of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) Veterns Affairs Cooperative Study Group. Ann Int Med. 1997;126:441–449. doi: 10.7326/0003-4819-126-6-199703150-00004. [DOI] [PubMed] [Google Scholar]
- 5.Cavasin MA, Tao Z, Menon S, Yang XP. Gender differences in cardiac function during early remodeling after acute myocardial infarction in mice. Life Sci. 2004;75:2181–2192. doi: 10.1016/j.lfs.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 6.Woodrum DT, Ford JW, Ailawadi G, Pearce CG, Sinha I, Eagleton MJ, Henke PK, Stanley JC, Upchurch GR. Gender differences in rat aortic smooth muscle cell matrix metalloproteinase-9. J Am Coll Surg. 2005;201:398–404. doi: 10.1016/j.jamcollsurg.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Clarke LA, Russell CS, Pownall S, Warrington CL, Borowski A, Dimmick JE, et al. Murine mucopolysaccharidosis type I: targeted disruption of the murine alpha-L-iduronidase gene. Hum Mol Genet. 1997;6:503–511. doi: 10.1093/hmg/6.4.503. [DOI] [PubMed] [Google Scholar]
- 8.Braunlin E, Mackey-Bojack S, Panoskaltsis-Mortari A, Berry JM, McElmurry RT, Riddle M, et al. Cardiac functional and histopathologic findings in humans and mice with mucopolysaccharidosis type I: implications for assessment of therapeutic interventions in Hurler syndrome. Pediatr Res. 2006;59:27–32. doi: 10.1203/01.pdr.0000190579.24054.39. [DOI] [PubMed] [Google Scholar]
- 9.Jordan MC, Zheng Y, Ryazantsev S, Rozengurt N, Roos KP, Neufeld EF. Cardiac manifestations in the mouse model of mucopolysaccharidosis I. Mol Genet Metab. 2005;86:233–243. doi: 10.1016/j.ymgme.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Domenico C, Villani GR, Di Napoli D, Reyero EG, Lombardo A, Naldini L, et al. Gene therapy for a mucopolysaccharidosis type I murine model with lentiviral-IDUA vector. Hum Gene Ther. 2005;16:81–90. doi: 10.1089/hum.2005.16.81. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Xu L, Hennig AK, Kovacs A, Fu A, Chung S, et al. Liver-directed neonatal gene therapy prevents cardiac, bone, ear, and eye disease in mucopolysaccharidosis I mice. Mol Ther. 2005;11:35–47. doi: 10.1016/j.ymthe.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 12.Bach G, Friedman R, Weissmann B, Neufeld EF. The defect in the Hurler and Scheie syndromes: deficiency of α-L-iduronidase. Proc Natl Acad Sci U S A. 1972;69:2048–2051. doi: 10.1073/pnas.69.8.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Renteria VG, Ferrans VJ, Roberts WC. The heart in the Hurler syndrome: gross, histologic and ultrastructural observations in five necropsy cases. Am J Cardiol. 1976;38:487–501. doi: 10.1016/0002-9149(76)90468-9. [DOI] [PubMed] [Google Scholar]
- 14.Brosius FC, 3rd, Roberts WC. Coronary artery disease in the Hurler syndrome. Qualitative and quantitative analysis of the extent of coronary narrowing at necropsy in six children. Am J Cardiol. 1981;47:649–653. doi: 10.1016/0002-9149(81)90550-6. [DOI] [PubMed] [Google Scholar]
- 15.Dangel JH. Cardiovascular changes in children with mucopolysaccharide storage diseases and related disorders--clinical and echocardiographic findings in 64 patients. Eur J Pediatr. 1998;157:534–538. doi: 10.1007/s004310050872. [DOI] [PubMed] [Google Scholar]
- 16.Krovetz LJ, Schiebler GL. Cardiovascular manifestations of the genetic mucopolysaccharidoses. Birth Defects. 1972;8:192–196. [Google Scholar]
- 17.Braunlin EA, Stauffer NR, Peters CH, Bass JL, Berry JM, Hopwood JJ, et al. Usefulness of bone marrow transplantation in the Hurler syndrome. Am J Cardiol. 2003;92:882–886. doi: 10.1016/s0002-9149(03)00909-3. [DOI] [PubMed] [Google Scholar]
- 18.Vinallonga X, Sanz N, Balaguer A, Miro L, Ortega JJ, Casaldaliga J. Hypertrophic cardiomyopathy in mucopolysaccharidoses: regression after bone marrow transplantation. Pediatr Cardiol. 1992;13:107–109. doi: 10.1007/BF00798216. [DOI] [PubMed] [Google Scholar]
- 19.Gatzoulis MA, Vellodi A, Redington AN. Cardiac involvement in mucopolysaccharidoses: effects of allogeneic bone marrow transplantation. Arch Dis Child. 1995;73:259–260. doi: 10.1136/adc.73.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braunlin EA, Rose AG, Hopwood JJ, Candel RD, Krivit W. Coronary artery patency following long term successful engraftment 14 years after bone marrow transplantation in a patient with Hurler Syndrome. Am J Cardiol. 2001;88:1075–1077. doi: 10.1016/s0002-9149(01)01999-3. [DOI] [PubMed] [Google Scholar]
- 21.Ma X, Tittiger M, Knutsen RH, Kovacs A, Schaller L, Mecham RP, et al. Upregulation of elastase proteins results in aortic dilatation in mucopolysaccharidosis I mice. Mol Genet Metab. 2008;94:298–304. doi: 10.1016/j.ymgme.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roman MJ, Devereux RB, Niles NW, Hochresiter C, Kligfield P, Sato N, Spitzer MC, Borer JS. Aortic root dilation as a cause of isolated, severe aortic regurgitation. Ann Int Med. 1987;106:800–807. doi: 10.7326/0003-4819-106-6-800. [DOI] [PubMed] [Google Scholar]
- 23.Seder JD, Burke JF, Pauletto FJ. Prevalence of aortic regurgitation by color flow Doppler in relation to aortic root size. J Am Soc Echo. 1990;3:316–319. doi: 10.1016/s0894-7317(14)80315-5. [DOI] [PubMed] [Google Scholar]
- 24.Hinek A, Wilson SE. Impaired elastogenesis in Hurler disease: dermatan sulfate accumulation linked to deficiency in elastin-binding protein and elastic fiber assembly. Am J Pathol. 2000;156:925–938. doi: 10.1016/S0002-9440(10)64961-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. NEJM. 1999;340:1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 26.Grandas OH, Mountain DJ, Kirkpatrick SS, Rudrapatna VS, Cassada DC, Stevens SL, et al. Effect of hormones on matrix metalloproteinases gene regulation in human aortic smooth muscle cells. J Surg Res. 2008;148:94–99. doi: 10.1016/j.jss.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 27.diNatoli AK, Medley TL, Ahimastos AA, Drew BG, Thearle DJ, Dilley RJ, et al. Sex steroids modulate human aortic smooth muscle cell matrix protein deposition and matrix metalloproteinase expression. Hypertension. 2005;46:1129–1134. doi: 10.1161/01.HYP.0000187016.06549.96. [DOI] [PubMed] [Google Scholar]
- 28.Henriques TA, Huang J, D'Souza SS, Daugherty A, Cassis LA. Orchiectomy, but not ovariectomy, regulates angiotension II-induced vascular disease in apolipoprotein E-deficient mice. Endocrinolgoy. 2004;145:3866–3872. doi: 10.1210/en.2003-1615. [DOI] [PubMed] [Google Scholar]
- 29.Beesley CE, Meaney CA, Greenland G, Adams V, Vellodi A, Young EP, et al. Mutational analysis of 85 mucopolysaccharidosis type I families: frequency of known mutations, identification of 17 novel mutations and in vitro expression of missense mutations. Hum Genet. 2001;109:503–511. doi: 10.1007/s004390100606. [DOI] [PubMed] [Google Scholar]
- 30.Pastores GM, Arn P, Beck M, Clarke JT, Guffon N, Kaplan P, et al. The MPS I registry: design, methodology, and early findings of a global disease registry for monitoring patients with Mucopolysaccharidosis Type I. Mol Genet Metab. 2007;91:37–47. doi: 10.1016/j.ymgme.2007.01.011. [DOI] [PubMed] [Google Scholar]