Abstract
Background and Aims
Genome-wide association studies (GWAS) have identified several loci that are associated with body mass index (BMI = kg/m2). However, little is known regarding whether the genetic basis of BMI differs among children of diverse racial/ethnic backgrounds, how the cumulative effect of these genes influences weight, or the contribution of these variants to body composition. This study examined the association between 17 GWAS-identified loci located in 16 genes and body-composition phenotypes in a multiethnic pediatric sample and evaluated the association of a composite genetic risk score with these phenotypes.
Methods
Anthropometric measures of BMI, waist circumference and waist-to-hip ratio were obtained in a sample of 298 children. Lean and fat mass were obtained from dual-energy X-ray absorptiometry (DXA). Genotypes of 17 single nucleotide polymorphisms (SNPs) were tested for association with the phenotypic measures, adjusted by standard covariates and estimates of genetic admixture.
Results
Both SNPs rs8050136 and rs9939609 in FTO were associated with BMI and waist circumference in a direction opposite to that observed among adults, and an inverse association was detected between the risk variant in MC4R and total lean body mass. Lean body mass mediated the association between TMEM18 and BMI. The association between the genetic risk score and body composition differed according to ethnic/racial classification.
Conclusions
Our findings suggest that genetic associations with BMI among children are different from those in adults, that some loci may operate through lean body mass, and that genetic risk scores will not have universal applicability across ethnic/racial groups.
Keywords: GWAS, Obesity genes, Multiethnic children, Genetic risk score
Introduction
Genome-wide association studies (GWAS) have revealed several genomic loci that are reproducibly associated with body mass index (BMI), obesity, and adiposity (1–5). Because most of these associations were discovered mainly among adults of European descent and used only BMI as the phenotype, there is still a need to gain a better understanding of (a) how these candidate loci, individually and collectively, may be associated with obesity and related traits among children and individuals of various ancestral backgrounds and (b) how these loci may act through various body composition phenotypes to ultimately be implicated in overall body weight variation.
Following the majorGWAS, many groups have followedup on the identified loci in attempts to replicate the results in different samples and with related phenotypes. Among children (ages 0–18 years) of European ancestry, Zhao et al. found significant associations of FTO, TMEM18, GNPDA2, and MC4R with adjusted body weight (6). Other studies have examined associations across diverse racial/ethnic groups, failing to replicate all loci, suggesting, among other things, that the genetic etiology of obesity and obesity-related traits may vary among groups (7,8).
In search of a better understanding of the genetic etiology of obesity and given the small individual effect sizes for loci identified in GWAS, many researchers have aggregated information across loci to calculate a genetic risk score from the sum of an individual’s GWAS-identified risk alleles. These risk scores have been evaluated as contributors to increased adiposity under the assumption that risk alleles may act cumulatively in obesity risk, and they have been shown to be associated with obesity risk in children and adults (6,9,10).
The extent to which genetic factors underlie ethnic/racial differences in pediatric obesity-related traits continues to be an understudied topic. The present study tested the association of 17 SNPs in or near 16 obesity candidate genes with measures of BMI, total fat mass, total lean mass, waist circumference (WC) and waist-to-hip ratio (WHR) in a multiethnic sample of U.S. children self-classified as African-, European-, or Hispanic-American. We also compiled a genetic risk score for each individual based on the number of GWAS-identified risk alleles, and examined its relationship with these phenotypes.
Subjects and Methods
Study Participants
A total of 298 children, age 7–12 years (53% male), were recruited as part of a cross-sectional cohort study examining population differences in metabolic phenotypes among healthy children (no major illnesses or medical diagnoses). Race/ethnicity was determined by the parents of the subjects who could classify their children into one of the following categories: African-American (AA; n = 96), Hispanic American (HA; n = 78), European American (EA; n = 114), or biracial (n = 6). All children were pubertal stage ≤3 as assessed by a pediatrician according to the criteria of Marshall and Tanner (11). Informed assent and consent were obtained from children and parents, respectively, as approved and regulated by the University of Alabama at Birmingham Institutional Review Board. All measurements were taken between the years of 2004 and 2008 at the University of Alabama at Birmingham General Clinical Research Center (GCRC) and Department of Nutrition Sciences.
Phenotypic Measurements
In the first of two sessions completed by participants, pubertal status, anthropometric measurements, and body composition were assessed. Pubertal status was determined by a physical exam assessing reproductive maturity by assignment of a stage (I–V) for breast/genitalia development and pubic hair. The higher of the two numbers serves to designate pubertal status (Tanner stage). Height was measured without shoes to the nearest centimeter using a stadiometer (Heightronic 235; Quick Medical, Issaquah, WA). A GE Lunar Prodigy densitometer (GE LUNAR Radiation Corp., Madison, WI) was used to assess body composition by dual-energy x-ray absorptiometry (DXA) while lightly clothed participants were lying flat on their backs with arms at their sides as previously described (12). Total fat and lean mass were obtained from analyzing DXA scans using pediatric software (enCore 2002 v. 6.10.029) and adjusted by height2 (abbreviated as FM/h2 & LM/h2) as previously described (13). WC was measured just above the hip bone.
Genotyping
DNA was obtained from all study participants. Information on 13 variants identified and replicated by GWAS for BMI (3–5), three variants for WC and one for WHR (2,14) was obtained as listed in Table 1. DNA was genotyped using Illumina Golden Gate at the UAB Heflin Genotyping Core. Hardy-Weinberg equilibrium was tested for all SNPs separately in each racial/ethnic group, using the SNPassoc package (15) developed in R (16).
Table 1.
List of SNPs, risk allele, nearest gene, and associated traits from GWAS
| SNP | Risk allele | Gene | Trait |
|---|---|---|---|
| rs6265 | G | BDNF | BMI |
| rs7647305 | C | ETV5 | BMI |
| rs8050136 | A | FTO | BMI |
| rs9939609 | A | FTO | BMI |
| rs10938397 | G | GNPDA2 | BMI |
| rs29941 | C | KCTD15 | BMI |
| rs1424233 | A | MAF | BMI |
| rs17782313 | C | MC4R | BMI |
| rs2568958 | A | NEGR1 | BMI |
| rs1805081 | A | NPC1 | BMI |
| rs10913469 | C | SEC16B | BMI |
| rs7498665 | G | SH2B1 | BMI |
| rs7561317 | G | TMEM18 | BMI |
| rs10146997 | G | NRXN3 | WC, BMI |
| rs2605100 | G | LYPLAL1 | WHR |
| rs987237 | G | TFAP2B | WC |
| rs545854 | G | MRSA | WC |
BMI, body mass index; WC, waist circumference; WHR, waist-to-hip ratio.
Genetic Admixture Analysis
A total of 142 ancestry informative markers (AIMs) were genotyped for each subject by Prevention Genetics (Mashfield, WI) as described elsewhere (17). These markers were chosen because they exhibit large frequency differences among geographically separated human groups. Individual West African, Amerindian, and European genetic admixture estimates were obtained by maximum likelihood estimation (18), using the genotypes at each AIM and information on the allele frequencies of these AIMs in un-admixed parental populations (see Supplementary Material). Given allele frequencies at a locus in unadmixed parental populations, the probability of observing a marker genotype is computed for each locus for each individual in our sample. The logs of the individual locus probabilities at all loci are then summed. For every possible admixture proportion from 0 to 100, the probability of the observed genotype is computed. The admixture proportion that corresponds to the maximum combined probability across all loci is the one that is the maximum likelihood estimate of ancestry for that individual. These estimates were subsequently used as covariates in models as a way to control for population stratification.
Statistical Analyses
Using SNPAssoc in R, each SNP was individually tested for association with each trait, adjusting for age, Tanner stage, sex, racial/ethnic group (for entire sample). Analyses within each racial/ethnic group were additionally adjusted for African admixture (among AA), and Amerindian admixture (among HA). In order to limit the number of statistical tests performed and to be in concordance with the genetic modeling used to identify the 17 SNPs from GWAS, only the additive model was evaluated in the association analyses. Departures from Hardy-Weinberg equilibrium were tested for all SNPs separately for EA, HA and AA. To conform to the assumptions of regression, all models were evaluated for residual normality.
To search for endophenotypes that may mediate the genetic effect of associated SNPs on BMI, we tested the other related phenotypes in mediation models using Structural Equation Modeling with MPLUS v. 6.1 (http://statmodel.com). Various path models were fit and evaluated using the chi-square goodness of fit test, the AIC model selection criteria, total explained variance (R2) of dependent variables, and a two-tailed Wald test to obtain p values for path coefficients for individual effects.
A genetic obesity risk score was calculated by summing the number of risk alleles that each individual possesses. Risk alleles were defined as those that were associated with higher BMI in previous GWAS studies. Because the two FTO variants are in LD, only one (rs9939609) was used in the risk score calculation. To account for missing genotypes, we divided the sum of risk alleles by the number of loci included in the score. We then compared the mean risk score across the three racial/ethnic groups using ANOVA. Finally, the risk score was included as a predictor in a linear regression model, considering various obesity-related phenotypes as outcomes and comparing direction and statistical significance of associations among ethnic/racial groups. All analyses were performed in R (16).
Results
HWE
Among EA, markers that depart from HWE include rs7498665 in SH2B1 ( p = 0.025). Among AA, no SNPs exhibited departures from HWE. Among HA, rs987237 in TFAP2B exhibited a departure from HWE ( p = 0.027).
Genetic Associations
Statistical analyses evaluating BMI identified a significant relationship between BMI and the two variants in FTO (rs8050136 and rs9939609) and the TMEM18 SNP (Table 2). Interestingly, the associations with both FTO SNPs are in the opposite direction compared to those found in GWAS. The protective FTO alleles, according to GWAS, are also associated with greater WC in our sample. Of the 16 loci tested, eight associations with BMI are in the expected direction based on previous GWAS, whereas the other eight are in the opposite direction. In the entire sample, we find no significant associations between any of the SNPs and FM/H2. For this trait, 10/16 loci are in the same direction as that identified for BMI in GWAS. We find that six loci (BDNF, NPC1, TMEM18, NRXN3, MC4R, and SH2B1) are nominally associated with LM/H2 (Table 2). For three of these (NPC1, TMEM18, and SH2B1) the obesity risk allele (as per GWAS) is the one that is associated with increased lean body mass in our sample.
Table 2.
Loci nominally associated with various obesity-related traits.
| Phenotype | Entire Sample | AA | EA | HA |
|---|---|---|---|---|
| BMI | FTO (rs8050136; 0.012; C) | None | MRSA (0.034; C) | None |
| FTO (rs9939609; 0.028; T) | NEGR1 (0.04; A) | |||
| TMEM18 (0.013, G) | TMEM18 (0.038; G) | |||
| FM/H2 | None | None | NEGR1 (0.008; A ) | GNPDA2 (0.022; A) |
| MRSA (0.021; C) | ||||
| LYPLAL1(0.047; A) | ||||
| LM/H2 | NRXN3 (0.042; A) | MC4R (0.0033; A) | BDNF(0.017; A) | FTO (rs8050136; 0.022; C) |
| MC4R (0.00065; A) | SH2B1(0.021; G) | FTO (rs9939609; 0.031; T) | ||
| BDNF(0.033; A) | NPC1(0.0070; A) | |||
| NPC1(0.024; A) | GNPDA2 (0.030; G) | |||
| SH2B1(0.049; G) | ||||
| TMEM18(0.016; G) | ||||
| WC | FTO (rs8050136; 0.0168; C) | ETV5 (0.047; G) | NEGR1 (0.035; A) | None |
| FTO (rs9939609; 0.0197; T) | ||||
| TMEM18 (0.042; G) | ||||
| WHR | None | None | None | TFAP2B (0.012; G) |
| NPC1 (0.033; G) |
WC: waist circumference; WHR: waist to hip ratio.
Note: p-value and trait increasing allele underlined if consistent with risk allele from GWAS, in parentheses.
Covariates included - BMI: age, sex, tanner stage, ethnicity (for entire sample only); FM/H2 (fat mass/height2) & LM/H2 (lean mass/height2): age, sex, tanner stage, ethnicity; WC: age, sex, tanner stage, ethnicity; WHR: age, sex, tanner stage, ethnicity; AA: additionally adjusted by African genetic admixture; HA: additionally adjusted by Native American genetic admixture.
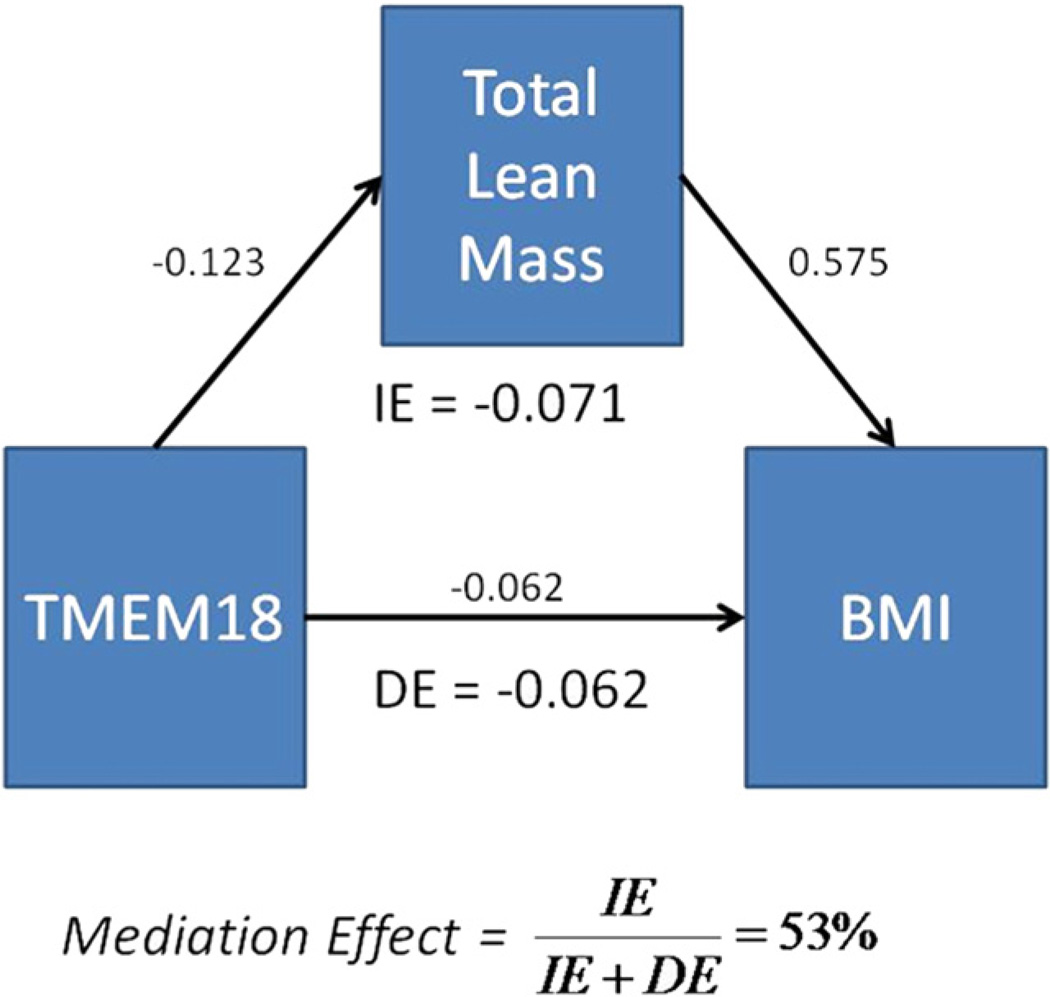
Genetic Mediation Model for TMEM18 on BMI Through Lean Mass
Because of the significant effect of the TMEM18 SNP on BMI and LM/H2 (adjusted total lean mass), we proposed a mediation model shown in Figure 1. The interpretation of this model is that the effect of the TMEM18 risk allele on increased BMI may be due to effects on the creation or maintenance of lean mass. Of course, the possibility of confounding factors in this cross-sectional study requires that this model, even if supported statistically in this dataset, be confirmed in an independent controlled study. Our questions were as follows: 1) Does this model appropriately fit the data (as measured here by a nonsignificant chisquared goodness of fit statistic and significant path coefficients for model terms)? 2) If so, what is the degree of mediation? And, finally, 3) Is the mediation model ethnicgroup- specific? First we fit a complete mediation model with only the indirect effect of TMEM18 on BMI through lean mass and adjusted for the following covariates: age, sex, Tanner stage, and ethnicity for both BMI and LM/H2. The model had an acceptable fit ( p value of χ2 goodness of fit statistic of 0.197) and significant p values for the path coefficients ( pBMI_LM <0.001, pLM_TMEM18 = 0.014), with 40% and 32% explained variance in BMI and LM/H2, respectively (see Table 3). We then tested the full model by adding a direct effect from TMEM18 to BMI (Figure 1). The resulting model fit did not improve substantially over the previous model with the AIC statistic remaining virtually the same, and the p value for the path coefficient from TMEM18 to BMI was not significant ( p = 0.196). Thus, we concluded that the full mediation model (with no indirect TMEM18->BMI effect) was supported by our data. It is, however, possible that a partial mediation model is the true model, but that the direct effect of TMEM18->BMI may not be significant due to the limited sample size of this study. To estimate the degree of mediation in a model with direct and indirect effects, the full model was estimated (Figure 1), and the mediation percentage was 53% (defined as the mediated effect divided by the total effect of TMEM18 on BMI). As a contrast, when fitting a mediation model for fat mass rather than lean mass, a highly significant goodness of fit p value is obtained (indicating a poor model fit), and the path coefficients are not significant for all of the paths (data not shown). Thus, lean mass appears to have a specific influence in mediating the effect of TMEM18 on BMI.
Figure 1.
Mediation model for genetic risk of TMEM18 for BMI mediated through adjusted total lean mass. Normalized path coefficients, total direct and indirect effects of TMEM18 on BMI, and percent mediation are displayed. (A color figure can be found in the online version of this article.)
Table 3.
Normalized path coefficients
| Path coefficients | All | AA | EA | HA |
|---|---|---|---|---|
| TMEM18->LM | −0.124 (<0.014) | −0.092 (0.337) | −0.146 (0.068) | −0.142 (0.107) |
| LM->BMI | 0.586 (0.001) | 0.617 (<0.001) | 0.587 (<0.001) | 0.557 (<0.001) |
p values in parentheses for full mediation model (model illustrated in Figure 1 with direct effect from TMEM18 to BMI removed) in full sample and each ethnic group. Path coefficients for covariates to both BMI and LM including sex, age, Tanner stage, and ethnic group (for full sample), African admixture (for AA group) and native American admixture (for HA group) are not shown.
Because the full mediation model (Figure 1 with direct effect from TMEM18->BMI removed) was the best-fitting model, we show the normalized path coefficients and p values for this model for the full sample and also for each ethnicity (covariate path coefficients are not shown) in Table 3. It can be seen that the path coefficient for TMEM18>LM is only significant in the full sample, although it is suggestive in the EA and HA samples. Also, the path coefficient effect directions are consistent across ethnicities and similar in magnitude, indicating that this mediation model may be applicable across multiple ethnicities.
Genetic Obesity Risk Score
First we examined the mean risk score in the three ethnic/ racial groups. Significant differences (ANOVA; p = 1.7 × 10−8) existed between HA (1.177), AA (1.170) and EA (1.066). The genetic risk score for obesity was then tested for association with BMI, FM/H2, and LM/H2 using the same covariates as in the single marker associations. In the entire sample we find that the genetic risk score is not significantly associated with BMI ( p = 0.87), and the direction of association is negative (Table 4). Within ethnic/racial groups, we did not find any significant associations between genetic risk score and BMI. However, interestingly, we find that the direction of association is negative only among HA ( p = 0.066) and positive among AA ( p = 0.87) and EA ( p = 0.54). We find a similar pattern for FM/H2. The genetic risk score is significantly negatively associated with FM/H2 ( p = 0.0068) among HA. For LM/H2, we find no significant associations with the genetic obesity risk score. However, we do find the same general pattern in which the direction of association among HA is the opposite of that found among EA and AA.
Table 4.
Association between genetic risk score and obesity-related traits
| Phenotype | All | AA | EA | HA |
|---|---|---|---|---|
| BMI | −0.19 (0.87) | 0.33 (0.87) | 1.04 (0.54) | −4.45 (0.066) |
| FM/H2 | −0.015 (0.88) | 0.081 (0.61) | 0.16 (0.27) | −0.53 (0.0068) |
| LM/H2 | 0.020 (0.67) | −0.053 (0.52) | −0.0019 (0.98) | 0.11 (0.20) |
BMI, body mass index; FM/H2, fat mass/height2; LM/H2, lean mass/height2; AA, African-American; EA, European-American; HA, Hispanic-American. β coefficients, and p in parentheses, are shown.
Discussion
This study tested whether SNPs associated with BMI in large-scale GWAS are associated with BMI, fat mass, lean mass, WC and WHR in children. The study also tested whether a risk score composed of information across all loci identified by GWAS is associated with body composition traits among children.
Our findings indicate that both FTO SNPs are significantly associated with BMI and WC. However, for both phenotypes the association was contrary to expected such that the allele reported as ‘‘protective’’ in the adult literature was associated with higher phenotypic levels in our pediatric sample. These observations provide a platform for the development of new hypotheses to further understand the role of genes and population differences throughout the lifespan and support the findings of others. For example, previous research has reported a significant inverse association between the FTO variant rs9939609 and BMI among infants (19). Our association tests, after controlling for the genetic heterogeneity of ancestry associated with ethnic/racial classification, were concordant with adult findings in only 50% of the tests performed. Furthermore, the relationship between the risk score factors and the phenotypes were in the opposite direction than expected in the entire sample, and further inspection reveals that HA were influencing the negative association. Therefore, it may be possible that, to the extent to which genetic associations respond to phenotypic expression, at early stages of the lifespan the effect of the genes evaluated in the study does not exert a significant influence in the acquisition of body fat, and that the genetic risk score may not be broadly applicable to all ethnic/racial groups.
We find that TMEM18, a variant that was previously found to have the second strongest effect on BMI after FTO, is nominally significantly associated with BMI. Interestingly, this SNP is nominally significantly associated with LM/H2 but not with FM/H2, suggesting that variation in this gene is associated with BMI through an effect on lean mass and not through fat mass. A mediation model in which LM/H2 mediates the effect of TMEM18 on BMI has been shown to fit the data well and to show consistency across ethnicities in size and direction of the path coefficients. The function of the gene TMEM18 and its connection to obesity is as yet poorly understood. Thus, this study, which points to a mechanism involving lean tissue creation or maintenance, provides a potential avenue for exploring the functional role of this gene in obesity.
Our study is strengthened by the use of a multiethnic cohort of children, a population poorly understood in the field of obesity genetics, and by the use of genetic admixture to account for the ancestral component underlying ethnic/race classification and to reduce Type I error rates in the association analyses. The use of endophenotypes that are surrogates of risk factors for obesity and its comorbidities and the utilization of a more precise measure of lean and fat mass also provide strength to our study design. However, the study is limited by its sample size, its ability to confirm complex relationships underlying genes, development and populations, and the limited inclusion of environmental factors.
In summary, the results of this study provide support to the notion that BMI associations among adults are different from those in children and may operate through lean body mass rather than fat, regardless of ethnic/race group. It also suggests that genetic risk scores will not have universal applicability across ethnic/racial groups. Our results raise the need for further research investigating the role of genetics, body composition, and population differences in obesity throughout the lifespan.
Supplementary Material
Acknowledgments
The authors would like to thank the study participants and their families. Financial support was provided by the following NIH grants: R01-DK067426, T32-Hl007457, R01 DA025095, 5P30DK056336-09, CA47888, and P60-DK079626.
Footnotes
Disclaimer: The opinions expressed are those of the authors and not necessarily those of the NIH or any other organization with which the authors are affiliated.
References
- 1.Frayling TM, Timpson NJ, Weedon MN, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindgren CM, Heid IM, Randall JC, et al. Genome-wide association scan meta-analysis identifies three loci influencing adiposity and fat distribution. PLoS Genet. 2009;5:e1000508. doi: 10.1371/journal.pgen.1000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyre D, Delplanque J, Chevre JC, et al. Genome-wide association study for early-onset and morbid adult obesity identifies three new risk loci in European populations. Nat Genet. 2009;41:157–159. doi: 10.1038/ng.301. [DOI] [PubMed] [Google Scholar]
- 4.Thorleifsson G, Walters GB, Gudbjartsson DF, et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat Genet. 2009;41:18–24. doi: 10.1038/ng.274. [DOI] [PubMed] [Google Scholar]
- 5.Willer CJ, Speliotes EK, Loos RJ, et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet. 2009;41:25–34. doi: 10.1038/ng.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao J, Bradfield JP, Li M, et al. The role of obesity-associated loci identified in genome-wide association studies in the determination of pediatric BMI. Obesity (Silver Spring ) 2009;17:2254–2257. doi: 10.1038/oby.2009.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wing MR, Ziegler JM, Langefeld CD, et al. Analysis of FTO gene variants with obesity and glucose homeostasis measures in the multiethnic Insulin Resistance Atherosclerosis Study cohort. Int J Obes (Lond) 2010 Nov 23; doi: 10.1038/ijo.2010.244. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu L, Xi B, Zhang M, et al. Associations of six single nucleotide polymorphisms in obesity-related genes with BMI and risk of obesity in Chinese children. Diabetes. 2010;59:3085–3089. doi: 10.2337/db10-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.den Hoed M, Ekelund U, Brage S, et al. Genetic susceptibility to obesity and related traits in childhood and adolescence: influence of loci identified by genome-wide association studies. Diabetes. 2010;59:2980–2988. doi: 10.2337/db10-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elks CE, Loos RJ, Sharp SJ, et al. Genetic markers of adult obesity risk are associated with greater early infancy weight gain and growth. PLoS Med. 2010;7:e1000284. doi: 10.1371/journal.pmed.1000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marshall WA, Tanner JM. Growth and physiological development during adolescence. Annu Rev Med. 1968;19:283–300. doi: 10.1146/annurev.me.19.020168.001435. [DOI] [PubMed] [Google Scholar]
- 12.Goran MI, Driscoll P, Johnson R, et al. Cross-calibration of bodycomposition techniques against dual-energy X-ray absorptiometry in young children. Am J Clin Nutr. 1996;63:299–305. doi: 10.1093/ajcn/63.3.299. [DOI] [PubMed] [Google Scholar]
- 13.Wells JC, Cole TJ. Adjustment of fat-free mass and fat mass for height in children aged 8 y. Int J Obes Relat Metab Disord. 2002;26:947–952. doi: 10.1038/sj.ijo.0802027. [DOI] [PubMed] [Google Scholar]
- 14.Heard-Costa NL, Zillikens MC, Monda KL, et al. NRXN3 is a novel locus for waist circumference: a genome-wide association study from the CHARGE Consortium. PLoS Genet. 2009;5:e1000539. doi: 10.1371/journal.pgen.1000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez JR, Armengol L, Sole X, et al. SNPassoc: an R package to perform whole genome association studies. Bioinformatics. 2007;23:644–645. doi: 10.1093/bioinformatics/btm025. [DOI] [PubMed] [Google Scholar]
- 16.R Development Core Team. R: a language and environment for statistical computing
- 17.Halder I, Shriver M, Thomas M, et al. A panel of ancestry informative markers for estimating individual biogeographical ancestry and admixture from four continents: utility and applications. Hum Mutat. 2008;29:648–658. doi: 10.1002/humu.20695. [DOI] [PubMed] [Google Scholar]
- 18.Hanis CL, Chakraborty R, Ferrell RE, et al. Individual admixture estimates: disease associations and individual risk of diabetes and gallbladder disease among Mexican-Americans in Starr County, Texas. Am J Phys Anthropol. 1986;70:433–441. doi: 10.1002/ajpa.1330700404. [DOI] [PubMed] [Google Scholar]
- 19.Sovio U, Mook-Kanamori DO, Warrington NM, et al. Association between common variation at the FTO locus and changes in body mass index from infancy to late childhood: the complex nature of genetic association through growth and development. PLoS Genet. 2011;7:e1001307. doi: 10.1371/journal.pgen.1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.