Abstract
We aimed to assess whether modulation of the dorsolateral prefrontal cortex (DLFPC) with noninvasive brain stimulation, namely transcranial direct current stimulation (tDCS), modifies food craving in healthy subjects. We performed a randomized sham-controlled cross-over study in which 23 subjects received sham and active tDCS (anode left/cathode right and anode right/cathode left) of the DLPFC. Subjects were exposed to food and also watched a movie of food associated with strong craving. Desire for food consumption was evaluated by visual analogue scales (VAS) and food consumption before and after treatment. In addition we measured visual attention to food using an eye tracking system. Craving for viewed foods as indexed by VAS was reduced by anode right/cathode left tDCS. After sham stimulation, exposure to real food or food-related movie increased craving; whereas after anode left/cathode right tDCS, the food-related stimuli did not increase craving levels, as revealed by the VAS scale. Moreover, compared with sham stimulation, subjects fixated food-related pictures less frequently after anode right/cathode left tDCS and consumed less food after both active stimulation conditions. These changes were not related to mood changes after any type of tDCS treatment. The effects of tDCS on food craving might be related to a modulation of neural circuits associated with reward and decision-making.
Keywords: Food craving, Smoking, Substance addiction, Brain stimulation, Transcranial direct current stimulation, Brain polarization
Introduction
Increasing evidence suggests that the control of eating origins in neural networks associated with decision-making (Pignatti et al., 2006). Although several factors influence the decision of food consumption, such as levels of blood sugar, hormonal changes, food availability, emotional state (including anxiety and depression), physical activity, memory, this information is finally processed in the neural networks associated with decision-making such as the prefrontal cortex, resulting in a final action. Therefore one possible approach to regulate food craving might be to interfere with this decision-making process by changing the activity of the dorsolateral prefrontal cortex (DLPFC).
Several studies from our and other groups have already shown that the prefrontal cortex modulates drug craving and decision-making. For instance, noninvasive brain stimulation, namely repetitive transcranial magnetic stimulation (rTMS), of the DLPFC significantly reduces smoking (Eichhammer et al., 2003; Fregni et al., in press), cocaine (Camprodon, Martinez-Raga, Alonso-Alonso, Shih, & Pascual-Leone, 2007) and alcohol (Boggio et al., 2008) craving. Indeed, one of the most important areas participating in the cue-associated anticipation and planning of drug use involves DLPFC, an area involved in planning and memory (Wilson, Sayette, & Fiez, 2004). For food craving, it was shown that high-frequency (10 Hz) rTMS of the left dorsolateral prefrontal cortex decreases food craving in women with frequent cravings for food. Specifically, the results of this study demonstrated that food craving during exposure to foods remained constant in the active treatment group but increased in the sham treatment group (Uher et al., 2005) after exposure to real food. Finally, we showed that stimulation of the dorsolateral prefrontal cortex is associated with decreased risk-taking in the BART task (Fecteau et al., 2007a). Indeed it has been shown that craving (particularly for cocaine) is associated with specific sensations similar to those of individuals engaged in risky behavior (Goeders, 2002).
In the present study, we tested whether modulation of prefrontal cortex with another technique of noninvasive brain stimulation, transcranial direct current stimulation (tDCS), modulates food craving-related behavior. We chose this technique because it modulates brain activity significantly in a safe, powerful and painless way and its effects can last for more than an hour (Nitsche & Paulus, 2000, 2001). It is a technically simple tool in which a continuous weak electric current is applied to the brain via large electrodes that are placed on the scalp of the subject. The effects of tDCS depend on the direction of the electric current, anodal stimulation increases brain activity and excitability and cathodal stimulation reduces it (Nitsche et al., 2003; Antal et al., 2001). Several well-conducted studies in animals and humans confirmed the behavioral and neurophysiological effects of tDCS (Bindman, Lippold, & Redfearn, 1964; Purpura & McMurtry, 1965). In fact, in humans, it has been shown that: anodal stimulation increases cortical excitability in the motor and visual cortex and cathodal stimulation decreases it (Nitsche & Paulus, 2000, 2001). Furthermore the effects of 13 min of tDCS on cortical excitability can last up to 90 min after the end of the stimulation (Nitsche & Paulus, 2001), most probably due to changes of NMDA receptor-efficacy (Nitsche et al., 2003). TDCS, as used in current protocols, is safe in humans as shown by neuropsychological testing (Fregni, Boggio, Lima et al., 2006; Iyer et al., 2005), EEG assessment (Iyer et al., 2005), a neuroimaging study (Nitsche et al., 2004) and brain metabolites evaluation (Nitsche & Paulus, 2001). Finally, recent modeling studies have shown that the amount of electric current going to the brain is large enough to induce a modulation of brain activity (Miranda, Lomarev, & Hallett, 2006; Wagner et al., 2007).
In this study, we tested the hypothesis that bilateral stimulation of prefrontal cortex with tDCS is suited to reduce food craving. Therefore a placebo-tDCS-controlled, randomized, double-blind, crossover study was performed.
Methods
Study subjects
Subjects were recruited by local advertising in websites, flyers and notices distributed throughout local universities. We used the same inclusion criteria as the study of Uher et al. (2005): subjects had frequent (≥3 times/day) and strong urges to eat one of the foods we chose for our experiment (see the list). We included healthy subjects aged between 18 and 55 years. Subjects were excluded if they had any neuropsychiatric disorder, current or past history of alcohol or other drugs abuse, were taking any psychiatric medication or were pregnant. Finally, we excluded subjects with eating disorder as clinically assessed and according to the DSM-IV criteria for eating disorders.
Twenty-three subjects (mean age of 23.7 ± 7.2, 21 females) were enrolled in this study and 21 completed the entire study (3 different sessions of treatment); 2 subjects did not complete the study – performing only the first session (sham tDCS) in one case and two sessions (shamtDCS and anode right/cathode left) in the other case – the main reason in both cases was that school work precluded them to return to the other stimulation sessions.
This study was performed at Universidade Presbiteriana Mackenzie (Sao Paulo, Brazil). The subjects gave written informed consent for the study, and approval was obtained from the local and also national research ethics committee (SISNEP number CAAE-0004.0.272.000-07). The study was carried out in conform to the principles of the Declaration of Helsinki.
Study protocol
This study was a crossover study in which subjects received three different types of bilateral stimulation of DLPFC with tDCS: (1) active anode left/cathode right tDCS, (2) active anode right/cathode left tDCS and (3) sham tDCS. A 48-h intersession-interval was used to avoid the potential of any carry-over effects due to stimulation. The order of stimulation was randomized and counterbalanced across subjects using a Latin square design. Participants and the evaluating investigators (except the investigators that applied tDCS) were blinded to the treatment arm.
All stimulation sessions were carried out by the same researchers and at the same time of the day. In addition, subjects were instructed to come 3 h after breakfast or lunch. Demographic and food habits profile data were collected at baseline. The following instruments of evaluation were used:
-
Baseline evaluation: subjects were instructed to complete a visual analogue scale (VAS) with 16 items evaluating mood and a visual analogue scale to measure food craving that consists of four items (similarly to the study of Uher et al., 2005):
Urge to eat (0–10 with 0 corresponding to no urge to eat and 10 corresponding to extremely strong urge to eat)
Appearance (0–10 with 0 corresponding to very poor appearance and 10 corresponding to the best food appearance)
Smell (0–10 with 0 corresponding to very unpleasant smell and 10 corresponding to very pleasant smell).
Taste (0–10 with 0 corresponding to an extremely awful taste and 10 corresponding to the best taste) – note that this item was only evaluated after treatment when subjects were allowed to eat ad-libitum (i.e., subjects were invited to eat as much as they wanted of the foods we offered to them).
-
Food craving: subjects were then exposed to food. They were seated in front of a large table containing different types of food (the choice was based on the local (Sao Paulo, Brazil) preferences) and also a 5-min movie showing scenes of food that usually elicit craving (e.g., cakes, sweets):
coconut candy (called “beijinho” in Brazil)
homemade chocolate candy (called “brigadeiro” in Brazil)
peanut candy (called “pacoca”)
chocolate cookies
chocolate cake
chocolate candies (“bis” and “confete”)
toast
cheese
salami
Subjects were assessed again regarding their food craving after this exposure.
Subjects underwent tDCS treatment for 20 min (as detailed below).
The procedure of the pre-treatment was repeated: initial craving evaluation, food exposure to provoke craving and new assessment of craving and also mood using VAS; however, at this point subjects were left in the room alone and allowed to eat “ad-libitum”. We then assessed the quantity each subject had eaten and they were asked to rate the taste of the food from 0 to 10 (“0”, the worst food ever; “10”, the best food ever).
Eye tracking
As another measurement of craving, we analyzed eye movements during visual scanning strategies. The eye movements were recorded with the equipment Tobii eye-tracking (Tobii Technology Inc., Falls Church, VA) that allows analysis of saccadic movements (mean length and duration) and fixation (localization and duration). The advantage of this system is that it has an easy method of calibration and the eye trackers are integrated into a 17″ TFT monitor.
The subjects were seated in front of a computer (50 cm from the screen) with an eye-tracking device, and asked to watch. They already knew that no questions would be asked, and the only task was to watch some pictures. The subjects were presented with powerpoint slides cointaining four different pictures (each one in a different quadrant), one of them being a picture of food associated with craving. The position of the food picture varied across the slides. In addition, they were informed that there were no rules, neither right nor wrong ways to do the task. For the eye gaze, we analyzed two parameters (fixation point time, and number of fixations).
Instruments of evaluation
As aforementioned, we used a visual analogue scale to measure food craving (subjects were asked to rate the desire to eat) and mood changes. Mood was assessed (at baseline—T0 and at the end of the study—T3) as it was a potential confounder in this study as stimulation of DLPFC is an effective treatment for major depression, and thus, can change mood (Fregni, Boggio, Nitsche et al., 2006; Pridmore, Bruno, Turnier-Shea, Reid, & Rybak, 2000). We used a visual analogue scale (VAS—that ranged from 0 to 10) to assess 16 different domains of mood, encompassing different domains such as: calm/restless; alert/drowsy; apathetic/dynamic; confused/lucid; strong/weak; sharp/blunt; satisfied/unfulfilled; worried/unconcerned; fast mind/slow mind; tense/relaxed; attentive/neglectful; inept/competent; happy/sad; hostile/friendly; interested/indifferent; quiet/sociable. Subjects were asked to rate from 0 to 10, their state, 0 corresponding to 100% to the left pole (e.g., calm) and 10 corresponding to 100% of the other pole (e.g., restless). This scale is reliable and we used it in other studies (Boggio et al., 2008; Fregni et al., in press). We assessed adverse effects at the end of each section asking subjects about the most common adverse effects after tDCS (such as headache, scalp burning, tingling, dizziness). Common adverse effects of tDCS are based on our experience and previous studies (see Iyer et al., 2005; Nitsche et al., 2003). Finally, we also measured the calories each subject had ingested after each session of treatment (we chose processed food that had information on calories in its label).
Transcranial direct current stimulation
Direct current was transferred by a saline-soaked pair of surface sponge electrodes and delivered by a specially developed, battery-driven, constant current stimulator (for more technical details, please contact Sergio A. Boggio at sboggio@colband.com.br) with a maximum output of 10 mA. We used electrodes of 35 cm2 (Nitsche & Paulus, 2000).
As aforementioned participants received three different types of treatment:
Anodal stimulation of the left DLPFC and cathodal stimulation of the right DLPFC (referred in the text as “anode left/cathode right”). The anode electrode was placed over F3 (using EEG 10/20 system) and the cathode electrode over F4.
Anodal stimulation of right DLPFC and cathodal stimulation of the left DLPFC (referred in the text as “anode right/cathode left”). The anode electrode was placed over F4 (using EEG 10/20 system) and the cathode electrode over F3.
Sham stimulation of DLPFC (referred as “sham stimulation”). For sham stimulation, the electrodes were placed at the same positions as in active stimulation; however, the stimulator was turned off after 30 s of stimulation. Therefore, the subjects felt the initial itching sensation associated with turning on the device, but received no current stimulation for the rest of the treatment period. A recent study showed that this method of sham stimulation is reliable (Gandiga, Hummel, & Cohen, 2006).
The principal target for treatment was the DLFPC cortex based on prior TMS studies showing that modulation of this area results in a decrease in smoking and also food craving (Johann et al., 2003; Uher et al., 2005) and other studies suggesting that the activity in this area is significantly associated with food craving (see review Alonso-Alonso & Pascual-Leone, 2007).
A constant current of 2 mA intensity was applied for 20 min. Stimulation with 2 mA (for a single session) has been shown to be safe in healthy volunteers (Iyer et al., 2005).
Statistical analysis
Analyses were done with Stata statistical software (version 9.2, StataCorp., College Station, Texas). The following analyses were made:
Main outcome: the main outcome of this study was whether craving after craving-cues exposure (movies and food exposure) was decreased by active tDCS as compared to sham tDCS. For this analysis we used a mixed model ANOVA in which the main outcome was food craving as indexed by VAS and the independent fixed-effects variables were condition of stimulation (sham, anode left/cathode right and anode right/cathode left), time (pre- and post-stimulation) and the independent random-effects variable was subject number in order to account for the within-subject variability in this cross-over study design. According to our main hypothesis, we expected to detect a significant time × condition interaction. Post hoc analyses to compare specific groups of stimulation were performed with a Bonferroni correction for multiple comparisons.
Secondary outcomes: We measured several secondary outcomes;
Food smell: we performed a similar analysis; however using VAS scores for smell as the dependent variable and same independent variables (condition of stimulation, time, condition by time and subject number).
Food appearance: a similar analysis was performed, but using VAS scores for appearance as the dependent variable and same independent variables (condition of stimulation, time, condition by time and subject number).
For food taste and ingested calories; we performed a different model as there was no main effect of time because subjects were just allowed to eat at the end of experiment; therefore in this model the independent variables were: condition of stimulation (fixed effects) and subject number (random-effects).
Craving induction: we performed a model in which we included all the conditions and measured only food craving before the exposure to craving cues and after this exposure (but before treatment) in order to evaluate whether our method to induce craving was effective. In this model, the independent variables were time of stimulation and subject number.
Eye tracking analysis: the main variable here was number of fixations and mean duration of fixations. We performed a similar model with two main fixed effects (condition of stimulation and time of stimulation).
Mood: for the mood analysis; we performed initially a MANOVA as we expected a correlation between mood domains; if there was a significant effect; we then performed individual ANOVAs.
There were two dropouts after one session of tDCS (sham tDCS) and the few missing data were considered missing at random. Statistical significance refers to a two-tailed p-value < 0.05.
Results
Subjects tolerated tDCS well. The adverse effects were mild and similar in the three conditions of stimulation (Table 1 lists the adverse effects). The most frequent adverse effects were scalp burning, headache, local itching, burning sensation and somnolence. They were not significantly different across the three conditions of stimulation (p = 0.98).
Table 1.
Adverse effects
| Sham tDCS | Right cathode/left anode | Right anode/left cathode | |
|---|---|---|---|
| Headache | 2 | 3 | 3 |
| Scalp pain | 2 | 3 | 1 |
| Itching | 2 | 1 | 2 |
| Burning sensation | 3 | 4 | 2 |
| Sonolence | 3 | 3 | 2 |
There was no significant difference in the number of adverse effects across groups of stimulation (p = 0.98).
Food craving – VAS – main outcome
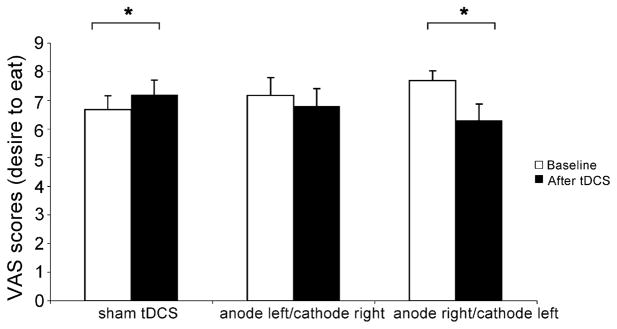
The mixed ANOVA model showed a significant interaction term time by condition (F[2,62] = 7.1, p = 0.0017). Interestingly, craving was significantly reduced only after anode right/cathode left stimulation (reduction by 17.9%, p = 0.0034). After sham stimulation there was a significant increase in craving (by 7.8%, p = 0.011) and after anode left/cathode right, there was no significant change in craving levels (p = 0.41)— see Fig. 1. Although it seems that anode left/cathode right and anode right/cathode left induced different effects, the comparison between these two conditions was not significantly different (p > 0.05).
Fig. 1.
Food craving levels at baseline and after treatment for each condition (sham tDCS, anode left/cathode right tDCS and anode right/cathode left tDCS). Columns represent mean craving and error bars represent standard error of mean (S.E.M). *Statistically significant when comparing craving between pre-stimulation vs. post-stimulation.
Food craving—other assessments (secondary outcomes)
For the food smell and appearance, the mixed ANOVA models showed no significant interaction time by condition (F[2,59] = 0.59, p = 0.55 and F[2,64] = 2.58, p = 0.08, respectively); suggesting that active tDCS did not change how the subjects rated the smell and appearance of food.
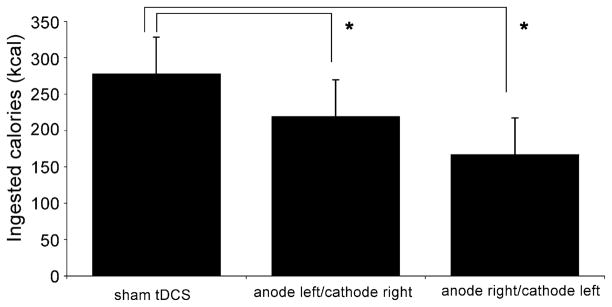
For the food taste and ingested calories; however, there was a significant difference across groups of stimulation. Mixed ANOVA models revealed a significant main effect of condition for taste (F[2,31] = 9.71, p = 0.0005) and for ingested calories (F[2,39] = 4.94, p = 0.012). Post hoc tests revealed that caloric ingestion after active treatments was significantly lower than sham stimulation (p = 0.033 for anode left/cathode right and p = 0.0036 for anode right/cathode left, as compared to sham stimulation). Similar results were obtained for taste; a significant difference between sham and anode left/cathode right (p = 0.015) and sham versus anode right/cathode left (p = 0.01) (Fig. 2). This result was similar to the analysis of VAS (desire to eat).
Fig. 2.
Total of caloric consumption after each condition of treatment (sham tDCS, anode left/cathode right tDCS and anode right/cathode left tDCS). Columns represent mean caloric consumption and error bars represent standard error of mean (S.E.M). *Statistically significant when comparing active conditions vs. sham stimulation.
Craving induction
For craving induction analysis, the mixed model ANOVA showed a significant effect of time (F[1,64] = 7.53, p = 0.0078), suggesting that our strategy significantly increased craving. Indeed craving was increased by an average of 24.7%.
Eye tracking
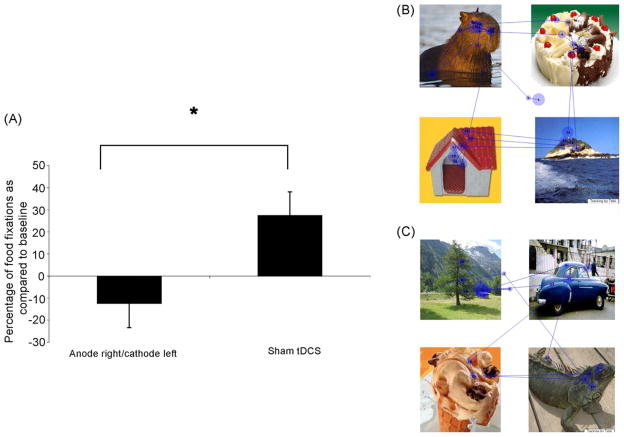
For the eye tracking analysis, we found a significant difference between sham and anode right/cathode left—the interaction time by condition was significant (F[1,29] = 5.12, p = 0.0313). Indeed after active stimulation the fixation in food decreased by 12.4% (±49.3%) as compared to an increase after sham stimulation by 27.5% (±47.3%) (Fig. 3).
Fig. 3.
A. Change in the number of fixations in the food (index food fixations/total number of fixations) after sham tDCS and anode right/cathode left tDCS. Columns represent mean fixation change (%) and error bars represent standard error of mean (S.E.M). *Statistically significant when comparing active condition vs. sham stimulation. (B and C) Visual tracking analysis for a subject who received active anode right/cathode left stimulation. The numbers represent the order of fixation and the size of the circle the duration of fixation. (B) is the task before stimulation and (C) after stimulation.
Mood assessment
For mood assessment, the MANOVA model (including all the 16 different domains as dependent variables) revealed that the interaction term time by condition was not significant (W = 0.78, F[32,156] = 0.62, p = 0.94); therefore suggesting that mood changes did not confound our results.
Discussion
The results of our study demonstrate that active anode right/cathode left DLPFC stimulation reduced food craving significantly. After sham stimulation, craving was significantly increased by presentation of food-related cues, whereas after anode left/cathode right tDCS, craving levels did not change. These results suggest that both active tDCS conditions had an effect in reducing food craving. Moreover, subjects consumed a smaller amount of food after both active stimulation conditions and, also, had significantly less fixation time in food pictures after anode right/cathode left tDCS. There were no significant mood changes after any type of tDCS treatment. Finally the results showed that tDCS was well tolerated. The adverse effects were mild and equally distributed across the three conditions of stimulation.
Several animal studies have shown that anodal stimulation increases neuronal firing and that cathodal stimulation results in reversed effects (Bindman et al., 1964). Therefore based on this evidence, we speculate that either an increase in the right and decrease in the left DLPFC activity or vice-versa leads to craving reduction. It is interesting to note that that while right anodal DLPFC stimulation led to a decrease in food craving beyond baseline levels, left anodal DLPFC stimulation did not reduce craving levels as compared to baseline. Although the comparison of anode right/cathode left versus anode left/cathode right stimulation was not significant, most probably because the study was underpowered for such an analysis; there might be a hemispheric laterality for food craving; and it might be speculated that the effects of left stimulation versus right stimulation might be qualitatively different. Whereas the right hemisphere might suppress desire to eat in general (or hunger), the left hemisphere might have a selective effect on food craving—decreasing the craving for particular foods. A previous study showed that implantable gastric stimulator (IGS) – which induces stomach expansion via electrical stimulation of the vagus nerve – induces a larger change in the activity of the right hemisphere as compared to the left (Wang et al., 2006). IGS is used to suppress appetite in obese patients. In addition, this laterality hypothesis for food craving is supported by recent studies showing that modulation of the right prefrontal cortex modulates risk-behavior – as risky behavior induces similar physiological responses as craving behavior (Goeders, 2002). In our recent study with 36 healthy subjects, we observed that after anode right/cathode left stimulation over the DLPFC, subjects adopted a strong risk-averse response (Fecteau et al., 2007b) and a previous rTMS study showed that low-frequency rTMS over the right DLPFC also decreases risk-taking (Knoch, Pascual-Leone, Meyer, Treyer, & Fehr, 2006). Therefore it is conceivable that modulation of the right hemisphere inhibits reward-related neural circuits (such as the circuit related to food intake); therefore decreasing appetite and other reward-related behaviors. In a recent review, Alonso-Alonso et al. discussed the role of the right hemisphere in food intake and obesity (Alonso-Alonso, 2007).
Several neuroimaging studies have shown that activity in the DLPFC is critically associated with other types of craving— previous studies investigating neural responses to cues in nicotine abusers demonstrated that the anterior cingulate, amygdala, insula, orbitofrontal and dorsolateral prefrontal cortex, are associated with craving (Wilson et al., 2004). In a study in which smoking craving was induced by a video, cigarette smokers responded to smoking stimuli with increased craving and activation in bilateral dorsolateral prefrontal cortices and other areas such as anterior cingulate, medial and orbital prefrontal cortex (McBride, Barrett, Kelly, Aw, & Dagher, 2006). The data of our study agree with these previous findings as modulation of either left or right DLPFC activity modulates craving.
Although our results give additional evidence to support the relationship between DLPFC activity and craving; the exact mechanisms by which DLPFC modulation by tDCS decrease craving are still unknown. DLPFC is one of the main areas of the prefrontal cortex associated with executive function. Therefore this area relates to the ability of determining actions, assessing future consequences of current activities, predicting outcomes and also social control. Therefore one possible mechanism by which DLPFC stimulation decreased craving is an increase of ‘social control’—in other words, subjects became more capable to suppress their urges. Thus DLPFC stimulation might have led to inhibition of excitatory neural networks of memory associated with food craving and also neural networks associated with appetite control. As aforementioned, right hemisphere activity is related to appetite suppression (Wang et al., 2006). In a study with obese patients, Wang et al. showed that implantable gastric stimulator (IGS) system, which generates electric signals to induce the expansion of the fundus, and thus increase satiety, leads to decreased food intake and reduced body weight in obese subjects and is associated with an increased activity in the right hemisphere structures predominantly, particularly the prefrontal cortex and hippocampus. Although stimulation in our study did not reach the hippocampus directly, it is conceivable that this area was indirectly stimulated through prefrontal-limbic neural pathways (see Lang et al., 2005).
Another alternative explanation is that stimulation of the prefrontal cortex stimulated dopaminergic pathways. Specifically, mesolimbic DA projections into striatum are hypothesized to regulate food intake by modulating appetitive motivational processes. Dopaminergic modulation through cortical stimulation has been shown before with tDCS (Nitsche et al., 2006) and rTMS (Strafella, Paus, Barrett, & Dagher, 2001).
Our results showing that DLPFC stimulation changes the number of fixations in food as compared to sham stimulation suggests that one potential mechanism to decrease craving might be a top-down mechanism in which modulation of the prefrontal cortex leads to a change in the modulation of the frontal eye fields—an area responsible for saccadic movements and therefore suggesting that a decrease in craving might be due to an attentional shift to non-food stimuli.
Two previous studies with a similar design demonstrated that both left and right DLPFC anodal stimulation results in significant decreases in smoking (Fregni et al., in press) and alcohol (Boggio et al., 2008) craving. Although the results of the present study showed a larger effect for anode right/cathode left tDCS; after anode left/cathode right, there were no significant changes in craving in comparison with sham stimulation that was associated with a significant craving increase. Indeed this result is similar to a previous research with 28 subjects showing that after excitability enhancing high-frequency rTMS of the left DLPFC, there were no food craving levels increase in comparison to sham rTMS in which there was a significant increase in craving levels (Uher et al., 2005). Therefore our study extends the results of this previous rTMS investigation as we showed that craving level is not only maintained after excitability-enhancing anodal tDCS of left DLPFC but can also be reduced after excitability-enhancing anodal tDCS of right DLPFC.
It is noteworthy that there were no significant mood changes in this study despite the fact that DLPFC was stimulated. At a first glance, this seems contradictory to our previous pilot study in which we showed that anodal tDCS over left DLPFC induces mood improvement (Fregni, Boggio, Nitsche et al., 2006). However this study was performed in depressed patients (and five sessions of tDCS were applied). Thus the impact of tDCS on DLPFC on mood is likely to differ between healthy subjects and depressed patients; this is in line with neuroimaging studies showing differential patterns of brain activity in the prefrontal cortex in depressed patients as compared to healthy controls. In addition, it has been shown that rTMS of the DLPFC can induce contrary effects in healthy subjects as compared to depressed patients such as that high-frequency rTMS of the left DLPFC induces mood worsening in healthy subjects (Pascual-Leone, Catala, & Pascual-Leone Pascual, 1996).
This study has some limitations. First, we did not assess subjects blinding (i.e., asking if they could guess the treatment received). Although we showed, in previous studies, that using our parameters of stimulation, blinding is reliable (Boggio et al., 2008), this can be viewed as a limitation in this study. Second, we used a subjective tool to assess craving (VAS) that might be influenced by several factors. However we also performed objective craving-related measurements such as food consumption and eye tracking analysis.
Conclusion
In conclusion, our study demonstrates that anodal tDCS of the DLPFC can suppress food craving. This finding extends the results of a previous study using rTMS to inhibit craving as it suggests that excitability enhancing strategies of brain stimulation of the right hemisphere might be more effective to reduce food craving and therefore opens an avenue for the exploration of noninvasive brain stimulation for eating disorders.
Acknowledgments
This work was supported by a grant from the Harvard Medical School Scholars in Clinical Science Program (NIH K30 HL04095) to F.F. The authors are thankful to MackPesquisa (Sao Paulo, Brazil) that supported part of this study. The authors are thankful to Barbara Bonnetti for the administrative support.
References
- Alonso-Alonso M, Pascual-Leone A. The right brain hypothesis for obesity. Journal of American Medical Association. 2007;297(16):1819–1822. doi: 10.1001/jama.297.16.1819. [DOI] [PubMed] [Google Scholar]
- Antal A, Nitsche MA, Paulus W. External modulation of visual perception in humans. Neuroreport. 2001;12(16):3553–3555. doi: 10.1097/00001756-200111160-00036. [DOI] [PubMed] [Google Scholar]
- Bindman LJ, Lippold OC, Redfearn JW. The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of long-lasting after-effects. Journal of Physiology. 1964;172:369–382. doi: 10.1113/jphysiol.1964.sp007425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggio PS, Sultani N, Fecteau S, Merabet L, Mecca T, Pascual-Leone A, et al. Prefrontal cortex modulation using transcranial DC stimulation reduces alcohol craving: Adouble-blind, sham-controlled study. Drug Alcohol Dependence. 2008;92(1–3):55–60. doi: 10.1016/j.drugalcdep.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Camprodon JA, Martinez-Raga J, Alonso-Alonso M, Shih MC, Pascual-Leone A. One session of high frequency repetitive transcranial magnetic stimulation (rTMS) to the right prefrontal cortex transiently reduces cocaine craving. Drug Alcohol Dependence. 2007;86(1):91–94. doi: 10.1016/j.drugalcdep.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Eichhammer P, Johann M, Kharraz A, Binder H, Pittrow D, Wodarz N, et al. High-frequency repetitive transcranial magnetic stimulation decreases cigarette smoking. Journal of Clinical Psychiatry. 2003;64(8):951–953. doi: 10.4088/jcp.v64n0815. [DOI] [PubMed] [Google Scholar]
- Fecteau S, Pascual-Leone A, Zald DH, Liguori P, Theoret H, Boggio PS, et al. Activation of prefrontal cortex by transcranial direct current stimulation reduces appetite for risk during ambiguous decision making. Journal of Neuroscience. 2007a;27(23):6212–6218. doi: 10.1523/JNEUROSCI.0314-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecteau S, Knoch D, Fregni F, Sultani N, Boggio P, Pascual-Leone A. Diminishing risk-taking behavior by modulating activity in the prefrontal cortex: a direct current stimulation study. Journal of Neuroscience. 2007b;27(46):12500–12505. doi: 10.1523/JNEUROSCI.3283-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Lima MC, Ferreira MJ, Wagner T, Rigonatti SP, et al. A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain. 2006a;122(1/2):197–209. doi: 10.1016/j.pain.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Nitsche MA, Marcolin MA, Rigonatti SP, Pascual-Leone A. Treatment of major depression with transcranial direct current stimulation. Bipolar Disorder. 2006b;8(2):203–204. doi: 10.1111/j.1399-5618.2006.00291.x. [DOI] [PubMed] [Google Scholar]
- Fregni F, Liguori P, Fecteau S, Nitsche M, Pascual-Leone A, Boggio PS. Cortical Stimulation of the Prefrontal Cortex With Transcranial DC Stimulation reduces Smoking Cue-Provoked Craving: A Randomized, Sham-Controlled Study. Journal of Clinical Psychiatry. doi: 10.4088/jcp.v69n0105. (in press) [DOI] [PubMed] [Google Scholar]
- Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): A tool for double-blind sham-controlled clinical studies in brain stimulation. Clinical Neurophysiology. 2006;117(4):845–850. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Goeders NE. Stress and cocaine addiction. Journal of Pharmacology Experimental Therapeutics. 2002;301(3):785–789. doi: 10.1124/jpet.301.3.785. [DOI] [PubMed] [Google Scholar]
- Iyer MB, Mattu U, Grafman J, Lomarev M, Sato S, Wassermann EM. Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology. 2005;64(5):872–875. doi: 10.1212/01.WNL.0000152986.07469.E9. [DOI] [PubMed] [Google Scholar]
- Johann M, Wiegand R, Kharraz A, Bobbe G, Sommer G, Hajak G, et al. Transcranial magnetic stimulation for nicotine dependence. Psychiatrische Praxis. 2003;30(Suppl 2):S129–S131. [PubMed] [Google Scholar]
- Knoch D, Pascual-Leone A, Meyer K, Treyer V, Fehr E. Diminishing reciprocal fairness by disrupting the right prefrontal cortex. Science. 2006;314(5800):829–832. doi: 10.1126/science.1129156. [DOI] [PubMed] [Google Scholar]
- Lang N, Siebner HR, Ward NS, Lee L, Nitsche MA, Paulus W, et al. How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? European Journal of Neuroscience. 2005;22(2):495–504. doi: 10.1111/j.1460-9568.2005.04233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride D, Barrett SP, Kelly JT, Aw A, Dagher A. Effects of expectancy and abstinence on the neural response to smoking cues in cigarette smokers: An fMRI Study. Neuropsychopharmacology. 2006;31(12):2728–3278. doi: 10.1038/sj.npp.1301075. [DOI] [PubMed] [Google Scholar]
- Miranda PC, Lomarev M, Hallett M. Modeling the current distribution during transcranial direct current stimulation. Clinical Neurophysiology. 2006;117(7):1623–1629. doi: 10.1016/j.clinph.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Lampe C, Antal A, Liebetanz D, Lang N, Tergau F, et al. Dopaminergic modulation of long-lasting direct current-induced cortical excitability changes in the human motor cortex. European Journal of Neuroscience. 2006;23(6):1651–1657. doi: 10.1111/j.1460-9568.2006.04676.x. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Liebetanz D, Antal A, Lang N, Tergau F, Paulus W. Modulation of cortical excitability by weak direct current stimulation— technical, safety and functional aspects. Supplement Clinical Neurophysiology. 2003;56:255–276. doi: 10.1016/s1567-424x(09)70230-2. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Niehaus L, Hoffmann KT, Hengst S, Liebetanz D, Paulus W, et al. MRI study of human brain exposed to weak direct current stimulation of the frontal cortex. Clinical Neurophysiology. 2004;115(10):2419–2423. doi: 10.1016/j.clinph.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. Journal of Physiology. 2000;527(Pt 3):633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57(10):1899–1901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Catala MD, Pascual-Leone Pascual A. Lateralized effect of rapid-rate transcranial magnetic stimulation of the prefrontal cortex on mood. Neurology. 1996;46(2):499–502. doi: 10.1212/wnl.46.2.499. [DOI] [PubMed] [Google Scholar]
- Pignatti R, Bertella L, Albani G, Mauro A, Molinari E, Semenza C. Decision-making in obesity: A study using the gambling task. Eating and Weight Disorders. 2006;11(3):126–132. doi: 10.1007/BF03327557. [DOI] [PubMed] [Google Scholar]
- Pridmore S, Bruno R, Turnier-Shea Y, Reid P, Rybak M. Comparison of unlimited numbers of rapid transcranial magnetic stimulation (rTMS) and ECT treatment sessions in major depressive episode. International Journal of Neuropsychopharmacology. 2000;3(2):129–134. doi: 10.1017/S1461145700001784. [DOI] [PubMed] [Google Scholar]
- Purpura DP, McMurtry JG. Intracellular activities and evoked potential changes during polarization of motor cortex. Journal of Neurophysiology. 1965;28:166–185. doi: 10.1152/jn.1965.28.1.166. [DOI] [PubMed] [Google Scholar]
- Strafella AP, Paus T, Barrett J, Dagher A. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. Journal of Neuroscience. 2001;21(15):RC157. doi: 10.1523/JNEUROSCI.21-15-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R, Yoganathan D, Mogg A, Eranti SV, Treasure J, Campbell IC, et al. Effect of left prefrontal repetitive transcranial magnetic stimulation on food craving. Biological Psychiatry. 2005;58(10):840–842. doi: 10.1016/j.biopsych.2005.05.043. [DOI] [PubMed] [Google Scholar]
- Wagner T, Fregni F, Fecteau S, Grodzinsky A, Zahn M, Pascual-Leone A. Transcranial direct current stimulation: A computer-based human model study. Neuroimage. 2007;35(3):1113–1124. doi: 10.1016/j.neuroimage.2007.01.027. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Yang J, Volkow ND, Telang F, Ma Y, Zhu W, et al. Gastric stimulation in obese subjects activates the hippocampus and other regions involved in brain reward circuitry. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(42):15641–15645. doi: 10.1073/pnas.0601977103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: A neurocognitive analysis. Nature Neuroscience. 2004;7(3):211–214. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]