Abstract
The early loss of photoreceptors in some retinal degenerations in mice has been shown to have a profound effect on vascular development of the retina. To better characterize this relationship, we have examined the formation of retinal blood vessels during the first month of life in 8 lines of transgenic rats with different ages of onset and rates of photoreceptor cell loss mediated by the expression of mutant rhodopsin (P23H and S334ter). The number of capillary profiles in the superficial plexus (SP) and deep capillary plexus (DCP) of the retina were quantified in retinal sections taken at postnatal day (P) 8, 10, 12, 15 and 30. In normal wild-type rats, the SP and DCP had mostly established mature, adult patterns by P15, as previously shown. In the transgenic rats, the loss of photoreceptors had relatively little effect on the SP. By contrast, the loss of photoreceptors during vascular development had a major impact on the DCP. In the two lines with early and most rapid photoreceptor loss, S334ter-7 and S334ter-3, where about 90% and 65%, respectively, of the photoreceptors were already lost by P15, the DCP either failed to form (S334ter-7) or the number of capillary profiles was less than 7% of controls (S334ter-3). In lines where almost all photoreceptors were still present at P15 (S334ter-4, S334ter-9, P23H-2 and P23H-3), the number of profiles in the DCP were the same as in wild-type controls at P30. In two lines with an intermediate rate of degeneration (S334ter-5 and P23H-1), where only about 25% of the photoreceptors were lost by P15, there was an intermediate number of vascular profiles in the DCP at P30. Thus, a very close relationship between the number of photoreceptors and vessel profiles in the DCP during its development exists in the transgenic rats, and the loss of photoreceptors results in the failure or inhibition of the DCP to develop. Several mechanisms may explain this relationship including changes in the level of physiological oxygen tension or alteration in the release of angiogenic factors that normally drive vessel development.
Analysis of older transgenic retinas up to one year of age revealed that 1) vascular profiles are lost from the DCP in essentially all lines once fewer than about 30-33% of photoreceptors remain; 2) in those lines where the DCP essentially did not develop (S334ter-7 and S334ter-3), the effect of photoreceptor absence was permanent, and there was no late vascularization of the DCP; 3) the number of capillary profiles in the SP remained no different from controls in any of the lines, despite long-standing loss of photoreceptors; and 4) neovascularization of the RPE by retinal capillaries occurred with a latency of 60-180 days after the loss of photoreceptors, except in S334ter-7 rats, where neovascularization essentially did not occur. Analysis of RCS rats was carried out for comparison.
Keywords: blood vessel development, retinal degeneration, rhodopsin mutations, transgenic, RCS, rat
1. Introduction
Understanding the relationship of vascular changes in retinal degenerative diseases remains an important challenge for treating these diseases. Patients with retinitis pigmentosa exhibit vascular attenuation, which correlates with the degeneration of photoreceptors (PRs) (Milam et al., 1998). Late complications of these diseases include the migration of retinal pigment epithelial (RPE) cells into the retina and the remodeling of vessels to form vascular complexes. Similar vascular changes have also been observed at late stages of retinal degeneration in rodent models such as the Pdebrd1/Pdebrd1 mouse, the RCS rat and other mouse models of retinitis pigmentosa (Blanks and Johnson, 1986; Hawes et al., 1999; LaVail, 1979; Villegas-Pérez et al., 1998).
It is widely appreciated that the development of retinal blood vessels is highly susceptible to environmental modification, particularly to changes in oxygen tension (Arden et al., 2005; Chen and Smith, 2007; Madan and Penn, 2003; Saint-Geniez and D'Amore, 2004; Stone et al., 1995). It has also been observed that rapid PR degeneration during the early postnatal period in mice alters blood vessel development (Blanks and Johnson, 1986; Matthes and Bok, 1984). The blood vessels of the rodent retina consist of a superficial plexus (SP) of vessels lying in the optic nerve fiber layer and a deep capillary plexus (DCP), which supplies the inner retina. The DCP fails to develop in Pdebrd1/Pdebrd1 mice that lose most PR nuclei during the developmental period of the DCP (Blanks and Johnson, 1986). In this model, vascular development follows a normal course until about postnatal day (P) 12, at which time there are fewer vessels in the DCP. The vessels disappear in the same central-to-peripheral fashion as the PRs. The SP is minimally affected in this model. In some other retinal degeneration models with slower rates of degeneration, such as the RCS rat and the pcd/pcd mouse, where there are normal numbers of PR cells during vasculogenesis of the retina, the DCP develops normally (Blanks and Johnson, 1985; Matthes and Bok, 1985).
The analysis of retinal degeneration mutants during the period of vascular development has provided clues to molecular influences on vasculogenesis (see Discussion), and the use of such mutants has provided a foundation for creative studies, such as the ability of bone marrow-derived stem cells to promote or inhibit angiogenesis (Otani et al., 2002). Moreover, analysis of the relationship between PR degeneration and vascular atrophy has revealed that by the use of hemopoietic stem cells, the stabilization and rescue of retinal blood vessels that would usually degenerate leads to a neurotrophic rescue of PR cells (Otani et al., 2004). Because of the potential usefulness of models with which to study the relationship of retinal degeneration to blood vessel development, we have analyzed 8 lines of mutant rhodopsin transgenic (Tg) rats, each with a different rate of retinal degeneration, by quantifying the relationship between the rate of PR degeneration and the formation of retinal blood vessels in the SP and DCP of these retinas.
Although the primary focus of this study was the relationship between PR degeneration and vasculogenesis of the retina, we have also examined older ages to follow vascular changes that occur both in retinas with early, rapid PR degeneration and in those retinas with slow retinal degenerations that occur after full development of the retinal vasculature. In addition, we have examined for comparison the widely studied RCS rat with inherited retinal dystrophy that has a relatively intermediate rate of degeneration (Bok and Hall, 1971; Dowling and Sidman, 1962; LaVail and Battelle, 1975).
2. Materials and methods
2.1 Animals and histological preparation
All animals were treated in accordance with the approved guidelines and procedures of the University of California, San Francisco IACUC. Tg rats were originally produced by Chrysalis DNX Transgenic Sciences (now Xenogen Biosciences) that carried one of two different rhodopsin mutations (Steinberg et al., 1996). These were P23H (single amino acid substitution at codon 23) and S334ter (a mouse opsin gene bearing a termination codon at residue 334, which results in a C-terminal truncated opsin protein lacking the last 15 amino acid residues and, thus, all of the phosphorylation sites of the molecule). Three lines of P23H and 5 lines of S334ter rats that have retinal degeneration phenotypes of different rates have been maintained from the original founders (http://www.ucsfeye.net/mlavailRDratmodels.shtml). Inbred RCS rats are from the original strain described by Sidman and Pearlstein (1965)
For histological studies, animals were euthanized with CO2. The rats were perfusion fixed with mixed aldehydes, the eyes were removed and bisected along the vertical meridian, postfixed in osmium tetroxide and embedded in an epoxy resin as described (LaVail and Battelle, 1975). One micron thick sections were cut through or near the optic nerve head with an ultramicrotome, and sections were mounted on slides and stained with toluidine blue.
2.2 Quantification of Blood Vessel Profiles and Outer Nuclear Layer
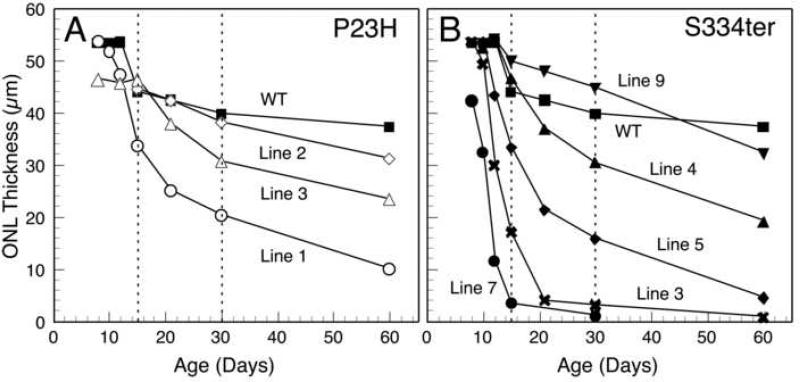
This was a retrospective quantitative study of histological slides prepared from the period 2000-2005 for the Tg rats and from the period 1972-1975 for the RCS rats, in the latter case from the study published previously by LaVail and Battelle (1975). Sections were chosen in which the rod outer segments, if present, and Müller cell processes crossing the inner plexiform layer were mostly continuous and in the plane of section to ensure that the sections were not oblique (Lewin et al., 1998). Capillary profiles in plastic-embedded sections of retinas from each of 3 lines of P23H (P23H-1, -2 and -3) and 5 lines of S334ter (S334ter-3, -4, -5, -7 and -9) Tg rats were counted and compared to age matched wild-type Sprague-Dawley rats. A single section was counted from each of 3-4 rats of each line and at each age, ranging from P8-P30, and from 1-4 rats of each line at each of several older ages, ranging up to 1 year. Capillary profiles were counted using a Nikon microscope with 40x objective and 10x eyepieces, providing a 440-μm field of view. Measurements commenced approximately 50 microns from the optic nerve and proceeded to the ora serrata. Depending on the length of the retina, approximately nine 440-μm adjacent fields on each side of the optic nerve head were counted. Three measurements of the ONL thickness were also made in each 440-μm field, as previously described (Faktorovich et al., 1992), so that 54 values were obtained; these were averaged to obtain a mean ONL thickness for each retina, and then these were averaged with the others of the same age to plot PR degeneration rates (Fig. 1).
Fig. 1.
Outer nuclear layer (ONL) thickness, which is proportional to the number of photoreceptor nuclei, in Sprague-Dawley rats and the different lines of Tg rats carrying the P23H (A) and S334ter (B) mutant rhodopsin transgenes. The values are based on 54 measurements of each retina from 3 or 4 rats at each age. For reference to light micrographs of plastic-embedded sections (Fig. 2), each photoreceptor nucleus is approximately 4.5 μm in diameter. Of note, the line with the earliest and most rapid photoreceptor degeneration, S334ter-7, already shows a significant (P < 0.05) loss of approximately 20% of ONL thickness (photoreceptor nuclei) as early as P8 (B). The dashed lines at P15 and P30 are the post-developmental ages examined for blood vessel counts.
Capillary profiles were identified by observing a single endothelial cell surrounding an empty lumen, basophilic staining of basement membrane around a lumen, or the presence of red blood cells within a lumen. In cases where an individual profile could not be conclusively distinguished from artifact, it was omitted from the count, but most profiles were readily identified by the aid of perfusion fixation which dilates the capillaries (Fig. 2). Capillary profiles were tabulated in the ganglion cell layer, the inner plexiform layer, the inner nuclear layer and the outer plexiform layer. The SP was defined by adding the profiles from the ganglion cell layer and the inner plexiform layers. The DCP of capillaries was defined by adding the profiles from the inner nuclear layer and outer plexiform layer. The total number of capillary profiles in each region was obtained for each section by adding the number in each of the 18 fields examined.
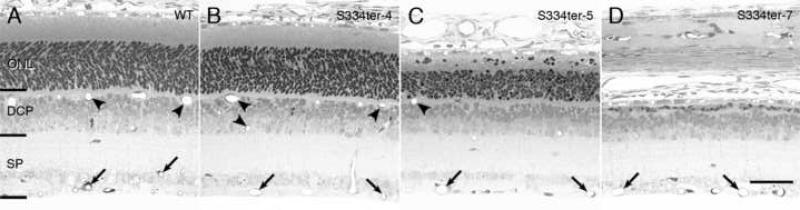
Fig. 2.
Histological sections from the retina of wild-type (WT) Sprague-Dawley rats (A) and selected Tg lines, S334ter-4 (B), S334ter-5 (C) and S334ter-7 (D) at P15, the age at which the mature retinal vascular distribution is reached. Brackets denote where vessels of the superficial plexus (SP) and deep capillary plexus (DCP) were counted. Several of the vessels in the SP are indicated at the arrows, and several are indicated in the DCP with arrowheads. Note that line S334ter-7 (D), which has severe loss of photoreceptor cells by P15, lacks any blood vessel profiles in the DCP. Line S334ter-5 (C), which has a slower course of degeneration, has a decreased number of blood vessel profiles in rats compared to the control (A). Line S334ter-4 (B), which has minimal loss of photoreceptors at P15, has a normal number of blood vessel profiles in the DCP. Magnification bar, 40 μm.
3. Results
3.1. Period of Analysis Based on Vascular Developmental Period
We have followed blood vessel development in the 8 lines of Tg rats during the first month of life for the following reasons relating to the normal temporal pattern of vasculogenesis in the rat as described by Engerman and Meyer (1965) and Cairns (1959). The normal development of retinal blood vessel development in the rat begins at the optic nerve head and spreads in a central-to-peripheral gradient. At birth the rat retina is avascular. Shortly after birth, 4-8 arterioles arise from the central retinal artery and radiate out symmetrically in the nerve fiber layer. Primitive capillaries then bud from these radiating arterioles between P1 and P2 to form the SP, which lies at the level of the nerve fiber layer. The development of the SP is almost complete by P8 (Engerman and Meyer, 1965), mostly complete by P10 (Cairns, 1959)(Cairns states P11 but counts the day of birth unconventionally as P1), with minor additional maturation in the far periphery of the retina through P16 (Cairns, 1959; Engerman and Meyer, 1965). The DCP, which supplies the inner nuclear layer of the retina, begins to form from buds off the superficial plexus between P6-P8. During the next week, or so, the DCP fully develops in most animals by P15-P16 (Cairns, 1959; Engerman and Meyer, 1965). Thus, we have focused on P15 as a key end-point to assess the DCP, but have examined retinas as old as P30, since in some studies of retinal degenerate mice there appears to be a further loss of blood vessels between P15 and P30 (Blanks and Johnson, 1985, 1986; Matthes and Bok, 1985).
3.2. Outer Nuclear Layer Thickness
The changes in ONL thickness for each of the lines at ages P8-P60 are shown in Fig. 1. The ONL thins in wild-type retinas due to stretching of the retina with eye growth during this period. Death and loss of PR cells results in additional thinning of the ONL in the Tg rats.
Two lines show very early and rapid degeneration, S334ter-7 and S334ter-3. The most severely affected is the S334ter-7 line, in which the ONL is already reduced by about 20% by P8, 40% by P10 and by just greater than 90% by P15 (Fig. 1B). Thus, the ONL is reduced to only a single row of PR nuclei, or less, by P15, as shown in Fig. 2D. The second most severely affected line is S334ter-3, in which the ONL is already reduced by about 65% by P15 (Fig. 1B).
Two lines of Tg rats have a somewhat slower loss of PRs, S334ter-5 (Fig. 1B) and P23H-1 (Fig. 1A). The ONL of both of these is reduced from wild-type control values by about 25% at P15.
The remaining lines all show little or no difference from wild-type control values for ONL thickness at P15. These are P23H-2 and P23H-3 (Fig. 1A) and S334ter-4 and S334ter-9 (Fig. 1B). By P30, however, lines P23H-3 and S334ter-4 have lost approximately 25% of their PRs (Fig. 1).
3.3 Development of Blood Vessels in the Superficial Plexus
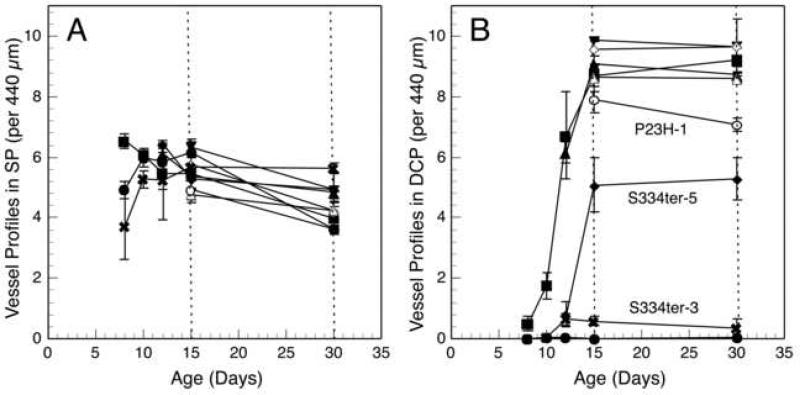
As noted earlier, the SP is mature in wild-type rats by P15 (Cairns, 1959; Engerman and Meyer, 1965). At this age, all 8 lines of Tg rats showed essentially the same number of capillary profiles in the SP as in wild-type rats (Fig. 3A). By P30, the number decreased by about 35% in the wild-type and in all 8 lines of Tg rats, all of which were statistically indistinguishable (Fig. 3A). This reduction was likely due to secondary pruning between P15 and P30. The only difference in the SP among all of the lines of rats was at the earliest age examined, P8, in the two lines with most rapid PR degeneration (S334ter-3 and S334ter-7), which had significantly fewer capillary profiles than controls (P < 0.05). However, this difference was no longer observed at older ages in these lines.
Fig. 3.
The number of blood vessel profiles in the (A) superficial plexus (SP), and (B) deep capillary plexus (DCP), (B) of Sprague-Dawley and Tg rats as a function of age. In the SP (A), the number of capillary profiles of S334ter-3 and S334ter-7 rats was significantly fewer than that of wild-type rats at P8 (P < 0.05), but neither these lines nor those of others differed significantly from wild-type at any other age. There was a slight reduction of capillaries from about P10-P30 in all lines, presumably a result of pruning of capillaries. In the DCP (B), very few capillaries are seen in the retinas with the fastest photoreceptor degeneration where most of the ONL is missing by P15 (S334ter-3 and S334ter-7), normal numbers of vessel profiles are seen in the more slowly degenerating retinas where almost all of the photoreceptors are present at P15 (S334ter-4, S334ter-9, P23H-2 and P23H-3), and intermediate numbers of vessel profiles are present in the retinas that show intermediate degrees of photoreceptor loss (S334ter-5 and P23H-1). Symbols for each of the lines are the same as in Fig. 1.
3.4 Development of Blood Vessels in the Deep Capillary Plexus
In wild-type rats, the number of capillary profiles in the DCP increases rapidly between P8 and P15, where it is almost at adult levels, with a slight increase to the full adult level by P30 (Fig. 3B). By contrast, the DCP is virtually missing at P15 and P30 in the line with earliest and most rapid PR loss, S334ter-7 (Figs. 2D and 3B). In this line, about 90% of the PRs are already lost by P15 (Fig. 1B). A total of only 1-2 capillary profiles was seen in the DCP in all of the 18 fields in all of the 3-4 retinal sections examined at each age. In the S334ter-3 retinas, which have the second fastest degeneration with about 65% of the PRs lost by P15 (Fig. 1B), only a very small number of capillary profiles was found in the DCP. These represented only about 6-7% of the number seen in wild-type control retinas (Fig. 3B).
In lines where almost all PRs are still present at P15 (S334ter-4, S334ter-9, P23H-2 and P23H-3) (Fig. 1), the number of profiles in the DCP at P15 is the same as that in wild-type controls (Fig. 3B). An example of the histological appearance of one of these lines, S334ter-4 (Fig. 2B), can be compared to that of a wild-type control (Fig. 2A), and the DCP is indistinguishable in the two. In two lines with an intermediate rate of degeneration (S334ter-5 and P23H-1), where about 25% of the PRs are lost by P15 (Fig. 1), there was an intermediate number of vascular profiles in the DCP at P15 and P30 (Fig. 3B). The intermediate number of DCP capillary profiles in S334ter-5 retinas is shown in Fig. 2C and can be compared to that in a wild-type control retina in Fig. 2A.
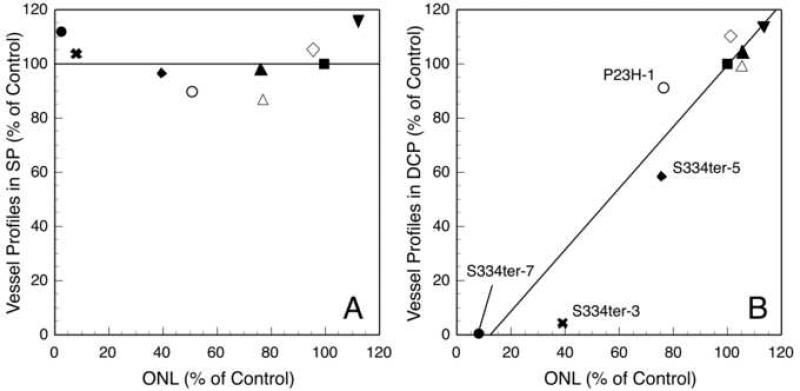
Figure 4 shows a correlation of the number of capillary profiles in the SP (Fig. 4A) and the DCP (Fig. 4B) versus the ONL thickness for each different line as normalized to control. In the SP, there is no correlation between the thickness of the ONL and the number of vessel profiles (Fig. 4A). By contrast, when the number of capillary profiles in the DCP is plotted versus the ONL thickness, there is a positive linear correlation between the two (Fig. 4B).
Fig. 4.
For each line, the percentage of the number of blood vessel profiles in the (A) superficial plexus (SP), and (B) deep capillary plexus (DCP) compared to control rats at P15 plotted versus the percentage ONL thickness compared to control rats at P15. Note there is no correlation between vessels in the SP and ONL thickness (A) at P15 (linear correlation, R=0.008). However, there is a linear correlation between the number of blood vessels in the DCP and ONL thickness (B) at P15 (linear regression, R=0.95, 95% confidence interval (0.88-1.51)). Symbols for each of the lines are the same as in Fig. 1.
3.5 Blood Vessel Changes at Older Ages
We have quantified the vessel profiles in the retinas of wild-type and each of the 8 Tg lines at several ages up to a year, and the results are presented in Table 1, along with the counts at P30 for comparison with the fully developed SP and DCP at this age.
Table 1.
Number of capillary profiles in the retinas of mutant rhodopsin transgenic and RCS rats
| Line | Age | # Vessel Profiles (per 440μm) |
n | ONL (% of WT)b & Debrisc | BV in RPEd | |
|---|---|---|---|---|---|---|
| DCP | SP | |||||
| SD (WT) | P30 | 9.2 | 4.0 | 4 | 100 | 0 |
| P120 | 8.3 | 3.8 | 3 | 100 | 0 | |
| P240 | 8.8 | 4.2 | 3 | 100 | 0 | |
| P365 | 8.0 | 4.2 | 3 | 100 | 0 | |
| S334ter-9 | P30 | 9.5 | 4.9 | 3 | 100 | 0 |
| P120 | 9.1 | 3.7 | 2 | 85a | 0 | |
| P240 | 8.0 | 3.8 | 2 | 89a | 0 | |
| P365 | 7.8 | 4.0 | 2 | 75a | 0 | |
| P23H-2 | P30 | 9.7 | 4.2 | 3 | 96 | 0 |
| P60 | 8.2 | 4.1 | 2 | 84a | 0 | |
| P120 | 7.7 | 3.6 | 4 | 80a | 0 | |
| P240 | 8.3 | 4.2 | 3 | 77a | 0 | |
| P365 | 7.0 | 4.2 | 4 | 61a | 0 | |
| P23H-3 | P30 | 8.6 | 4.2 | 4 | 77a | 0 |
| P120 | 8.6 | 3.4 | 2 | 40a | 0 | |
| P240 | 5.8a | 4.3 | 3 | 28a | 0 | |
| P300 | 4.5a | 3.9 | 2 | 0a | 0, 12 (0.33) | |
| P365 | 3.9a | 4.1 | 3 | 0a | 30, 0, 11 (0.76) | |
| S334ter-4 | P30 | 8.7 | 4.8 | 3 | 76a | 0 |
| P120 | 8.2 | 3.8 | 3 | 34a | 0 | |
| P180 | 3.8a | 4.0 | 2 | 0a | 0 | |
| P240 | 5.4a | 4.9 | 3 | 0a | 9, 7, 2 (0.33) | |
| P365 | 2.6a | 3.7 | 3 | 0a | 37, 21, 28 (1.59) | |
| P23H-1 | P30 | 7.0a | 3.6 | 3 | 51a | 0 |
| P60 | 4.1a | 5.0 | 3 | 27a | 0 | |
| P120 | 5.0a | 4.8 | 3 | 0a | 0 | |
| P180 | 3.6a | 3.9 | 3 | 0a | 0, 0, 14 (0.26) | |
| P240 | 2.2a | 3.9 | 3 | 0a | 26, 55, 17 (1.81) | |
| P365 | 0.3a | 3.4 | 2 | 0a | 14, 16 (0.83) | |
| S334ter-5 | P30 | 5.3a | 4.9 | 3 | 40a | 0 |
| P60 | 2.9a | 4.0 | 3 | 0a | 0 | |
| P90 | 3.4a | 3.9 | 3 | 0a | 0 | |
| P120 | 1.8a | 3.3 | 2 | 0a | 0 | |
| P240 | 1.6a | 3.1 | 2 | 0a | 13, 20 (0.92) | |
| P365 | 0.4 | 3.2 | 2 | 0a | 5, 5 (0.28) | |
| S334ter-3 | P30 | 0.4a | 5.6 | 3 | 0a | 0 |
| P60 | 0.2a | 3.5 | 3 | 0a | 0 | |
| P90 | 0.4a | 3.8 | 4 | 0a | 3, 7, 13, 2 (0.35) | |
| P120 | 0.6a | 3.7 | 3 | 0a | 6, 5, 0 (0.20) | |
| P180 | 0.8a | 3.9 | 1 | 0a | 9 (0.5) | |
| P365 | 0.1a | 3.8 | 2 | 0a | 0, 4 (0.11) | |
| S334ter-7 | P30 | 0.0a | 3.6 | 4 | 0a | 0 |
| P90 | 0.3a | 4.4 | 2 | 0a | 0 | |
| P120 | 0.0a | 4.2 | 3 | 0a | 0 | |
| P365 | 0.1a | 3.6 | 2 | 0a | 0 | |
| RCS | P30 | 9.1 | 4.3 | 3 | 72a, Dc | 0 |
| P53 | 10.0 | 4.6 | 3 | 0a, Dc | 0 | |
| P75 | 8.6 | 3.6 | 3 | 0a, Dc | 0 | |
| P85 | 5.2a | 3.9 | 2 | 0a, no Dc | 30, 12 (1.17) | |
| P96 | 3.7a | 2.5 | 1 | 0a, no Dc | 18 (1.0) | |
| P162 | 4.1a | 3.1 | 1 | 0a, no Dc | 22 (1.22) | |
| P217 | 2.4a | 4.1 | 1 | 0a, no Dc | 52 (2.89) | |
Significantly different than Sprague Dawley (SD) wild-type (WT); Student's t-test (P < 0.05 or less).
Zero indicates that photoreceptor cells are missing from some or all regions of the retina; other regions of the retina may have photoreceptor cells present.
D indicates outer segment debris present and mostly confluent around eye; no D indicates substantial regions where debris is no longer present.
Zero indicates no blood vessels invading the RPE. Where numbers are given, the total number present in each retinal section (1 section per rat) is given, and the average per 440-μm field (18 per retinal section) of all rats is in parentheses.
DCP, deep capillary plexus, SP, superficial plexus; n = number of rats (1 section per rat); ONL, outer nuclear layer; BV, blood vessel profiles; RPE, retinal pigment epithelium
The number of capillary profiles in the SP of the inner retina is very slightly lower at the oldest ages than at younger ages, but it is not statistically different from the number seen in wild-type control retinas at any age (Table 1). Thus, even long-standing loss of PR cells does not alter the number of capillary profiles of the SP.
By contrast, the number of capillary profiles in the DCP may be reduced depending upon the degree of PR cell loss. In the slowest PR degenerations, lines S334ter-9 and P23H-2, where the ONL is reduced to only 75% and 61% of control values at P365, respectively, there is no significant loss of capillary profiles in the DCP (Table 1).
The two lines with the next slowest degenerations, P23H-3 and S334ter-4, showed normal numbers of capillary profiles during vascular development through P30 (Fig. 3B), when the ONL thickness was about 75% of normal (Fig. 1). In the P23H-3 rats, the number of capillary profiles in the DCP was still not different than normal when the ONL was 40% of normal at P120. However, there was a significant loss of vessel profiles in the DCP when the ONL reached 28% of normal at P240, and thereafter when most PRs had disappeared (Table 1). (It should be noted that even when there appeared to be a complete loss of PRs, a few PR nuclei could always be found in the retinal sections of all of the lines of rats.) Similarly, in S334ter-4 rats the number of DCP vessel profiles was still no different from normal at P120 when the ONL was 34% of control, but about 60 days later (P180) where the ONL had lost most PRs, there was a large, significant drop in the number of profiles in the DCP, with an even greater loss at P365 (Table 1).
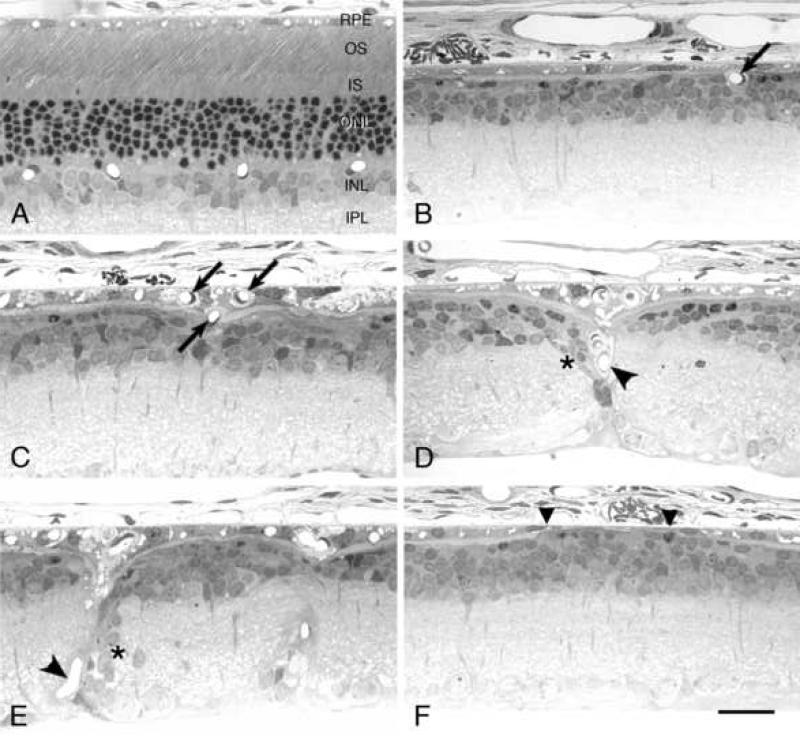
The two lines of rats with the next fastest rate of PR degeneration that showed a moderate reduction of DCP vessel profile development at P15 and P30, P23H-1 and S334ter-5 (Fig. 3B), demonstrated a further and progressive loss of DCP profiles with age (Table 1). Likewise, in the lines with the most rapid PR degeneration, where very few (S334ter-3) or virtually no (S334ter-7) DCP vessel profiles were formed, the profile number remained extremely low or virtually non-existent, respectively, for the entire year. So, the effect of PR absence was permanent, and there was no late vascularization of the DCP in these rapidly degenerating lines. The inner nuclear layer (INL) remained relatively intact compared to normal (Fig. 5A) in these (Figs. 5B and F) and the other (Figs. 5C-E) lines, except where NV of the RPE occurred (see below). In general, the INL of the wild-type and all lines of Tg rats contained about 3-5 rows of nuclei in the posterior retina, 2-4 rows in the equatorial region, and 2-3 rows in the far periphery at P120 and P365.
Fig. 5.
Histological sections from the posterior or equatorial retinal regions of a wild-type Sprague-Dawley rat (A) at P365 and selected Tg lines at different ages. S334ter-3 at P120 (B) and P23H-3 at P365 (C) illustrating retinal capillary profiles (arrows) appearing to enter (B) or having entered (C) the retinal pigment epithelium (RPE) from the DCP. P23H-1 at P277 (D) and P23H-3 at P365 (E) showing capillary profiles with a trajectory toward the RPE from the inner retina (arrowheads). In both cases, strands of nuclei (asterisks) from the inner nuclear layer (INL) have been displaced along the blood vessel. S334ter-7 at P60 (F) illustrating attenuation of the RPE (between the triangles). OS, outer segment; IS, inner segment; ONL, outer nuclear layer; IPL, inner plexiform layer. Magnification bar, 25 μm.
3.6 Neovascularization of the Retinal Pigment Epithelium
Neovascularization (NV) of the RPE from retinal capillaries is widely recognized to occur following PR cell loss in rodents with environmentally induced and inherited retinal degenerations (Reviewed by Nishikawa and LaVail, 1998), so we examined the temporal pattern of RPE NV in the 8 lines of Tg rats. NV of the RPE occurred only after loss of PR nuclei, so the most slowly degenerating lines (S334ter-9 and P23H-2), showed no NV of the RPE since they still had numerous PRs surviving at the oldest ages examined (Table 1). The RPE of each of the other lines of Tg rats became vascularized by retinal capillaries (Figs. 5C-E), except for line S334ter-7, which had almost none (Table 1). In those retinas where the RPE was vascularized, the degree of NV was highly variable, with some animals at a given age showing none, whereas others had significant NV (Table 1). In each case, the period between almost complete loss of PRs from at least a part of the retina and the time of RPE NV was about 60-90 days, with an approximately 180-day latency in S334ter-5 rats (Table 1). The retinal capillaries that invaded the RPE appeared to come from both the DCP, immediately adjacent to the RPE (Fig. 5B), or from the inner retina in the SP (Figs. 5D-E). In some profiles of vessels emanating from the inner retina, strands of cells from the INL had migrated or become displaced along the vessel path (Figs. 5D-E), as seen abundantly in the RCS rat (Gerstein and Dantzker, 1969; Villegas-Pérez et al., 1998). The two lines of rats, S334ter-3 and S334ter-7, that had the most rapid PR degeneration (Fig. 1B) and almost no development of the DCP (Fig. 3B), showed relatively few vascular profiles in the RPE in the case of S334ter-3, or almost none in the case of S334ter-7 (Table 1).
3.7 Attenuation of the Retinal Pigment Epithelium
With increasing age following PR cell loss in rodent retinal degenerations, some of the RPE becomes attenuated. In the case of three mouse mutants, including P23H, the percent of the total RPE attenuated to 15% of the normal thickness of the RPE cells slowly rises to about 20-25% by one year of age (Nishikawa and LaVail, 1998). Using the same criteria, we examined the 8 lines of Tg rats, and while observing some RPE attenuation (Fig. 5F), each of the lines showed less than 5% of the total RPE length at every age up to P365.
3.8 Comparison with the RCS Rat
Analysis of the vascular development and degeneration in the RCS rat with inherited retinal dystrophy has previously been carried out by Matthes and Bok (1985), although different methods were used that did not distinguish the SP from the DCP. Their key findings were that this mutant rat develops a full vascular bed at 1 month of age that was maintained until 4 months, and that there was a significant drop in blood vessel density between 4-6 months that continued to at least 1 year of age (Matthes and Bok, 1985). For comparison with the mutant rhodopsin Tg rats, we have examined key ages of RCS rats with the same methods used for the 8 lines of Tg rats.
In the developing RCS retina, the ONL has a rate of PR cell loss similar to that of S334ter-5 rats. At the end of vascular development at P15, the ONL is identical to that of wild-type rats, and by P30 it is reduced to 72% of normal (LaVail and Battelle, 1975) (Table 1). By P53, there are foci where the ONL has lost virtually all PR nuclei, and the loss progresses rapidly, so that only scattered PR nuclei are present by P75 and later (LaVail and Battelle, 1975). A characteristic of retinal dystrophy in the RCS rat is the early appearance of an outer segment debris zone resulting from the failure of the RPE to phagocytize PR outer segments (Bok and Hall, 1971) due to a defect of the Mertk gene expressed in the RPE (D'Cruz et al., 2000) and persistence of the debris after PR cell loss (LaVail and Battelle, 1975). The debris is mostly persistent at P55, with a few foci of an obliterated debris layer at P75, and by P96 the debris is missing from at least the posterior half of the retina (LaVail and Battelle, 1975). Our current observations are consistent with these historical descriptions, with the addition that the retinas at P85 are virtually identical with the descriptions of those at P96, with the debris missing from the posterior half of the retina.
Like the 8 lines of Tg rats, the SP of the RCS rats developed normally and remained essentially indistinguishable from wild-type throughout the period examined (Table 1). Likewise, the DCP develops normally and remains so through P75; thereafter, beginning about P85, the number of vessel profiles of the DCP becomes progressively reduced (Table 1). Thus, unlike the Tg rats, the number of vascular profiles in the DCP remains at normal levels after the loss of most PR cells; however, the number of vascular profiles in the DCP is reduced when the outer segment debris zone is cleared (Table 1).
In RCS rats, NV of the RPE by retinal capillaries has been observed by many investigators (Gerstein and Dantzker, 1969; LaVail, 1979; Villegas-Pérez et al., 1998), and in preliminary observations, it appeared that the invasion of the RPE occurred only after the removal of the outer segment debris zone (LaVail, 1979). In the present study, this was confirmed (Table 1). The most illuminating observation was in P85 RCS rats, where extensive areas of debris removal were first encountered. Of the 36 440-μm fields examined in these sections, 13 still had outer segment debris, and no NV of the RPE was present in these fields. Of the remaining 23 fields in these same sections where the debris had been removed, 74% of the fields showed NV of the RPE. At older ages, NV of the RPE became progressively greater in the RCS rats (Table 1).
4. Discussion
4.1 Vascular Development in Retinal Degenerations
In the analysis of 8 lines of mutant rhodopsin Tg rats, we have found a very close relationship during the developmental period of the DCP between the number of PRs and blood vessel profiles in the DCP. The DCP fails to develop when most of the PR cells are lost during the developmental period of the DCP, and there is a partial failure of the DCP to develop when there is a partial loss of PRs during the developmental period.
In the initial studies that demonstrated abnormally reduced vascular development in Pdebrd1/Pdebrd1 mice, the suggested mechanisms were the possible release of toxic metabolites during the degenerative process (Blanks and Johnson, 1986; Matthes and Bok, 1984) or the possible increased oxygen tension resulting from loss of PR cells (Matthes and Bok, 1984). The latter possibility was based on the observations of Michaelson (1954) and Ashton (1968) who had shown that the developing retinal vasculature was reduced in hyperoxic conditions. While the idea that harmful degeneration products cannot be excluded, an enormous amount of experimental data has been generated on the influence of oxygen tension on retinal vasculature (Reviewed by Arden et al., 2005; de Gooyer et al., 2006), and on the primary retinal angiogenic factor that regulates the development and maintenance of retinal vasculature, vascular endothelial growth factor (VEGF) (Reviewed by Chen and Smith, 2007; Saint-Geniez and D'Amore, 2004; Schlingemann and van Hinsbergh, 1997).
Retinal oxygen tension is a key regulator of retinal vascular growth and survival, mediated by the alteration of VEGF expression (Alon et al., 1995; Nomura et al., 1995; Pierce et al., 1995). A “physiological” level of hypoxia caused by an increasing demand for oxygen by developing neuronal activity induces VEGF and vasculogenesis in the retina (Chan-Ling et al., 1995; Stone et al., 1995). In fact, a hypoxia-induced, transient wave of VEGF expression from central to peripheral retina within retinal astrocytes, then from inner to outer retina within Müller glial cells, directs the local development of retinal vessels (Stone et al., 1995). By contrast, hyperoxia reduces retinal VEGF and, as noted above, results in the reduction or obliteration of developing retinal vessels (Alon et al., 1995; Ashton, 1968; Michaelson et al., 1954; Nomura et al., 1995; Pierce et al., 1995; Yamada et al., 1999), as well as in adult mouse retinas (Yamada et al., 1999).
In the case of retinal degenerations, as the retina thins due to loss of PRs, it might be expected that the inner retina would become hyperoxic, particularly since the surviving choroidal circulation has little or no capacity to autoregulate, regardless of oxygen demand (Bill and Sperber, 1990; Delaey and Van De Voorde, 2000). In fact, the inner retina is normally hypoxic (de Gooyer et al., 2006; Yu and Cringle, 2001), but following PR cell loss in the retinas of Rho-/- mice, the inner retina becomes less hypoxic, or relatively hyperoxic, and there is a concomitant reduction in the expression of VEGF (de Gooyer et al., 2006). Similarly, in Pdebrd1/Pdebrd1 mice that have a loss of PRs and loss of the DCP (Blanks and Johnson, 1986; Matthes and Bok, 1984), there is a reduced level of VEGF in the inner retina (Lahdenranta et al., 2001; Yamada et al., 1999). It should be noted that the retinas of RCS rats (Yu et al., 2000) and P23H rhodopsin Tg rats (Yu et al., 2004) showed little change in the oxygen tension of the inner retina following PR loss. However, numerous studies show that the suppression of neovascularization by laser treatment results in hyperoxia of the inner retina, and that in these areas VEGF is down-regulated (Reviewed by Penn et al., 2000).
Penn et al. (2000) carried out a remarkable experimental demonstration of the relationship of retinal oxygen tension and vasculogenesis in an inherited retinal degeneration, a transgenic mouse model of autosomal dominant retinitis pigmentosa (I-255/256 rhodopsin mutation) with a rapid loss of most PR cells by P20, including some loss of PRs before DCP maturation at P15. In this mutant, There was some loss of vascular profiles of the DCP by P20, but an even greater loss by P26. Penn et al. tested the hypothesis that the DCP loss was due to retinal hyperoxia following the loss of PRs by placing the mice in an ambient hypoxic environment. With this procedure, they were able to reverse retinal capillary atrophy and stimulate new retinal capillary growth, causing a increase in the DCP density of almost 100% (Penn et al., 2000).
From the foregoing, if during the development of retinal vasculature, the loss of PR cells results in an increase of inner retinal hyperoxia, a suppression of VEGF expression and an inhibition or suppression of capillary development, then a prediction for the present study would be that the earlier and faster the loss of PRs occurred, the greater the loss of the DCP would occur in the Tg rat lines. This is precisely what we found. The DCP is mature by P15, and in the earliest and fastest degenerating lines of Tg rats, S334ter-7 and S334ter-3, about 90% and 65%, respectively, of the PRs were already lost by this age. In these rapidly degenerating lines, the DCP either failed to form (S334ter-7) or the number of capillary profiles was less than 7% of control values (S334ter-3) when examined at P15 and P30. In lines with slower rates of degeneration, where almost all PRs were still present at P15 (S334ter-4, S334ter-9, P23H-2 and P23H-3), the number of profiles in the DCP was the same as in wild-type controls at P15 and P30. In two lines with an intermediate rate of degeneration (S334ter-5 and P23H-1), where about 25% of the PRs were lost by P15, there was an intermediate number of vascular profiles in the DCP. Thus, there is a very close relationship between the number of PRs and vessel profiles in the DCP during its development in the Tg rats (Fig. 4B), and the loss of PRs during that time results in the failure or inhibition of the DCP to develop. The RCS rat showed the same relationship. PR cell death begins at approximately P16-P20 (LaVail and Battelle, 1975), after the formation of the DCP, and the number of vascular profiles in the DCP at P30 is the same as in wild-type retinas (Table 1).
By the same reasoning, the SP would presumably not be affected, because this part of the retinal vasculature is mostly developed by P8-10, before any substantial PR cell loss in the Tg rats (Fig. 1). However, in the earliest and most rapid degenerating lines, S334ter-7 and S334ter-3, there were slightly fewer capillary profiles at P8, but this was a transient effect because the number very quickly caught up with that of the other lines of rats and was not different from wild-type at any age after P8. We cannot be certain of the cause of this transient effect, but these two lines of Tg rats have greater numbers of pyknotic PR nuclei in the ONL than normal at P4 and P6, 4 days earlier than any other line (LaVail, unpublished observations), and the ONL is actually reduced to 80% of normal by P8 in the fastest degenerating line, S334ter-7 (Fig. 1B). It is possible that those factors influencing the loss of DCP in the two most rapidly degenerating lines have an effect on the SP at the earliest age, but it is also possible that the two vaso-obliterative events have different causes. For example, the vasculogenesis of the SP may not be subject to the same influences, such as oxygen tension, as the DCP at this age. The changes at this early age are obviously complex, with as yet undetermined vaso-formative and vaso-obliterative factors competing.
Thus, the Tg rats with different rates of PR degeneration appear to follow the pattern of an increase of inner retinal hyperoxia as oxygen-consuming and space-taking PRs are lost, which presumably suppresses expression of VEGF or other vasoactive agent(s), such as adenosine (Lutty and McLeod, 2003) and other growth factors (Saint-Geniez and D'Amore, 2004), and thereby inhibits or abolishes DCP vessel growth. While this presumed mechanism has yet to be demonstrated in the Tg rats, it is clear that these animals provide a model system with which to study influences on or results of retinal vascular development, with its different rates of PR loss and corresponding incremental degrees of vascularization, and in an eye larger than that of a mouse if needed for certain experimental approaches.
4.2 Vascular Changes Following the Developmental Period in Retinal Degenerations
One of the reasons to explore vascular changes at later stages of the Tg rat retinas is that a hallmark of mid- to late-stage human retinitis pigmentosa is attenuation of retinal capillaries, along with migration of pigmented cells to form “bone spicule” pigmentation associated with retinal vessels as viewed funduscopically (Reviewed in Li et al., 1995; Milam et al., 1998). A pale, waxy optic nerve head is also frequently observed funduscopically (Li et al., 1995; Milam et al., 1998). The optic nerve head palor is due to the loss of at least some retinal ganglion cells (Stone et al., 1992) which, in turn, may be due to the narrowing and occlusion of retinal vessels by the thickening of the extracellular matrix around them by the retinal pigmented cells (Li et al., 1995), corresponding to regions of reduced blood flow (Grunwald et al., 1996). These changes may occur many years to decades into the disease process, and while the temporal features cannot be known for certain because of the lack of many young donor eyes, at least some photoreceptors degenerate long before these changes occur (Geltzer and Berson, 1969; Li et al., 1995; Milam et al., 1998).
Animal models such as the Tg rats, by contrast, allow for temporal analysis of vascular and other changes that cannot be made at present in human retinas. We have followed the vascular changes of each of the lines to one year of age, and while vascular attenuation cannot be accurately judged using the plastic section technology in this retrospective study, frank loss of capillaries can be precisely assessed. There are 4 lines of Tg rats that have established a complete vascular network at P15 before the loss of PR cells, S334ter-9, P23H-2, P23H-3 and S334ter-4, so in this respect they are similar to retinitis pigmentosa, albeit PR degeneration occurs earlier than in the human condition. The slowest degenerating lines, S334ter-9 and P23H-2, still have 75% and 61% of their PRs at a year of age and show no vascular abnormalities of the DCP, so they establish that if a significant number of PRs survive, vascular changes in the retina do not occur, at least in these rodent retinas.
However, in the somewhat more rapidly degenerating P23H-3 and S334ter-4 retinas when the ONL is reduced to below about 30-33%, loss of capillary profiles begins to occur in the DCP, with progressively greater reduction as more PRs are lost (i.e., thinning of the ONL). In these lines, no capillary loss in the DCP occurs when the ONL is 34% of control or greater (Table 1). This appears to be a consistent phenomenon, even when other retinas are considered. For example, in the two Tg lines with intermediate rates of degeneration where about 25% of the PRs are lost by P15 during DCP development, P23H-1 and S334ter-5, there is only a relatively small loss in capillary profiles in the DCP by P30, when the ONL of these retinas are 51% and 40%, respectively. By P60, when the ONL has dropped to 27% and 0% of normal, respectively, there is a very large drop in the number of capillary profiles in the DCP (Table 1).
Thus, just as the loss of PRs during the development of the DCP results in the developmental inhibition or loss of retinal capillaries, so does the loss of PRs at later stages. We have found a consistent threshold of about 30-33% of surviving PRs exists, below which the loss of retinal capillaries in the DCP is seen. It may be that the same mechanisms are involved at later stages as during development. As PRs are lost, a threshold mass of oxygen-consuming and space-taking retinal PRs might prevent the deleterious inner retinal hyperoxia from altering the DCP.
One additional model is consistent with this suggestion. In the RCS rat retinas, the number of capillary profiles in the DCP is maintained at normal levels for about 20 days after the ONL is reduced to a single row, or less, unlike the Tg rats (see immediately above). However, the RCS retina has a mass of outer segment debris that is thought by many to be a barrier to diffusion from the RPE, and thereby lead to PR cell death. In this case, though, the debris layer may serve to prevent hyperoxia of the DCP, since the number of capillary profiles of the DCP drops below normal just as the debris zone is removed at P85 (Table 1).
Several additional observations are noteworthy in the present study of the later stages. The INL, which is vascularized by the DCP, does not appear to be affected to a significant degree in any of the Tg lines, despite the loss of some or nearly all of the capillaries of the DCP (except where it is disrupted by capillaries invading the RPE). It may be as pointed out in RCS retinas (Gerstein and Dantzker, 1969), that with the loss of PR cell mass, the choroidal blood may be acting as the chief supplier of the INL as it had previously done for the outer layers of the retina. It should be noted, though, that remodeling changes not generally visible by conventional light microscopy are occurring in the INL and other retinal layers, at least at later stages of the older retinas examined (Jones et al., 2003; Jones and Marc, 2005).
The SP of the Tg and RCS rat retinas is not significantly different from normal at any age, from P15 to P365, except where it is perturbed and disrupted by blood vessels invading the RPE. Thus, unlike the capillary profiles of the DCP, loss of PR cells at any age never alters the numbers of capillary profiles of the SP, at least up to a year of age.
The relatively short lifespan of rodent models and the lack of many human histopathological specimens at very early stages of retinitis pigmentosa make it difficult to know whether the rather rapid loss of vascular profiles in the DCP following PR cell loss occurs in human retinas. At very least, this is a possibility that can be sought in the future, and if so, the major vascular changes in the inner retina of retinitis pigmentosa patients (Li et al., 1995) would represent a second wave of changes that occurs at a much later time.
One additional feature of retinal vasculature followed in the present study was NV of the RPE by retinal capillaries. NV of the RPE is a feature common to most, if not all, inherited and environmentally induced retinal degenerations in rodents (Reviewed by Nishikawa and LaVail, 1998). A period of several weeks to several months typically occurs between loss of PRs and NV of the RPE, but a P23H mutant rhodopsin Tg mouse showed a much earlier NV, occurring just as PR nuclei were lost (Nishikawa and LaVail, 1998). For this reason, we suspected NV of the RPE might occur soon after the loss of PR cells, at least in the P23H lines. As expected from previous studies, NV occurred only after the loss of all PRs in a region of retina in the Tg rats, so it was not seen in the two slowest lines that still retained PRs at a year of age. Moreover, unlike the rapid invasion of the RPE by retinal capillaries in the P23H Tg mouse (Nishikawa and LaVail, 1998), there was a latency of 60-90 days, and approximately 180 days in one line, following PR cell loss before retinal capillaries invaded the RPE in the Tg rats. In the S334ter-7 line, where the DCP essentially failed to develop, almost no NV of the RPE occurred. In RCS rats, invasion of the RPE by retinal capillaries required not only the loss of PR nuclei, but also the outer segment debris layer. However, once the debris was missing, NV of the RPE appeared to occur within days. Thus, there are clear differences in the stimuli for retinal capillaries to invade the RPE, and these remain to be determined.
Acknowledgements
We would like to thank Cathy Lau-Villacorta, Jose Velarde and Nancy Lawson for technical assistance. This work was supported by the Foundation Fighting Blindness, That Man May See, Inc., Research to Prevent Blindness, and by NIH grants EY001919, EY006842 and EY002162.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alon T, Hemo I, Itin A, Pe'er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat. Med. 1995;1:1024–1028. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- Arden GB, Sidman RL, Arap W, Schlingemann RO. Spare the rod and spoil the eye. Br. J. Ophthalmol. 2005;89:764–769. doi: 10.1136/bjo.2004.062547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton N. Some aspecrs of the comparative pathology of oxygen toxicity in the retina. Br. J. Ophthalmol. 1968;52:505–531. [PubMed] [Google Scholar]
- Bill A, Sperber GO. Control of retinal and choroidal blood flow. Eye. 1990;4(Pt 2):319–325. doi: 10.1038/eye.1990.43. [DOI] [PubMed] [Google Scholar]
- Blanks JC, Johnson LV. Vascular atrophy associated with photoreceptor degeneration in mutant mice. In: LaVail MM, Hollyfield JG, Anderson RE, editors. Retinal Degeneration: Experimental and Clinical Studies. Alan R. Liss, Inc.; New York: 1985. pp. 189–207. [Google Scholar]
- Blanks JC, Johnson LV. Vascular atrophy in the retinal degenerative rd mouse. J. Comp. Neur. 1986;254:543–553. doi: 10.1002/cne.902540407. [DOI] [PubMed] [Google Scholar]
- Bok D, Hall MO. The role of the pigment epithelium in the etiology of inherited retinal dystrophy in the rat. J. Cell Biol. 1971;49:664–682. doi: 10.1083/jcb.49.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns JE. Normal development of the hyaloid and retinal vessels in the rat. Br. J. Ophthalmol. 1959;43:385–393. doi: 10.1136/bjo.43.7.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan-Ling T, Gock B, Stone J. The effect of oxygen on vasoformative cell division. Evidence that ‘physiological hypoxia’ is the stimulus for normal retinal vasculogenesis. Invest. Ophthalmol. Vis. Sci. 1995;36:1201–1214. [PubMed] [Google Scholar]
- Chen J, Smith LE. Retinopathy of prematurity. Angiogenesis. 2007;10:133–140. doi: 10.1007/s10456-007-9066-0. [DOI] [PubMed] [Google Scholar]
- D'Cruz PM, Yasumura D, Weir J, Matthes M, Abderrahim H, LaVail MM, Vollrath D. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum. Mol. Genet. 2000;9:645–651. doi: 10.1093/hmg/9.4.645. [DOI] [PubMed] [Google Scholar]
- de Gooyer TE, Stevenson KA, Humphries P, Simpson DA, Curtis TM, Gardiner TA, Stitt AW. Rod photoreceptor loss in Rho-/- mice reduces retinal hypoxia and hypoxia-regulated gene expression. Invest. Ophthalmol. Vis. Sci. 2006;47:5553–5560. doi: 10.1167/iovs.06-0646. [DOI] [PubMed] [Google Scholar]
- Delaey C, Van De Voorde J. Regulatory mechanisms in the retinal and choroidal circulation. Ophthalmic Res. 2000;32:249–256. doi: 10.1159/000055622. [DOI] [PubMed] [Google Scholar]
- Dowling JE, Sidman RL. Inherited retinal dystrophy in the rat. J. Cell Biol. 1962;14:73–109. doi: 10.1083/jcb.14.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engerman RL, Meyer RK. Development of retinal vasculature in rats. Am. J. Ophthalmol. 1965;60:628–641. doi: 10.1016/0002-9394(65)92251-8. [DOI] [PubMed] [Google Scholar]
- Faktorovich EG, Steinberg RH, Yasumura D, Matthes MT, LaVail MM. Basic fibroblast growth factor and local injury protect photoreceptors from light damage in the rat. J. Neurosci. 1992;12:3554–3567. doi: 10.1523/JNEUROSCI.12-09-03554.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geltzer AI, Berson EL. Fluorescein angiography of hereditary retinal degenerations. Arch Ophthalmol. 1969;81:776–782. doi: 10.1001/archopht.1969.00990010778004. [DOI] [PubMed] [Google Scholar]
- Gerstein DD, Dantzker DR. Retinal vascular changes in hereditary visual cell degeneration. Arch. Ophthalmol. 1969;81:99–105. doi: 10.1001/archopht.1969.00990010101014. [DOI] [PubMed] [Google Scholar]
- Grunwald JE, Maguire AM, DuPont J. Retinal hemodynamics in retinitis pigmentosa. Am. J. Ophthalmol. 1996;122:502–508. doi: 10.1016/s0002-9394(14)72109-9. [DOI] [PubMed] [Google Scholar]
- Hawes NL, Smith RS, Chang B, Davisson M, Heckenlively JR, John SW. Mouse fundus photography and angiography: a catalogue of normal and mutant phenotypes. Mol. Vis. 1999;5:22. [PubMed] [Google Scholar]
- Jones BW, Watt CB, Frederick JM, Baehr W, Chen CK, Levine EM, Milam AH, LaVail MM, Marc RE. Retinal remodeling triggered by photoreceptor degenerations. J. Comp. Neurol. 2003;464:1–16. doi: 10.1002/cne.10703. [DOI] [PubMed] [Google Scholar]
- Jones BW, Marc RE. Retinal remodeling during retinal degeneration. Exp Eye Res. 2005;81:123–137. doi: 10.1016/j.exer.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Lahdenranta J, Pasqualini R, Schlingemann RO, Hagedorn M, Stallcup WB, Bucana CD, Sidman RL, Arap W. An anti-angiogenic state in mice and humans with retinal photoreceptor cell degeneration. Proc. Natl. Acad. Sci. U.S.A. 2001;98:10368–10373. doi: 10.1073/pnas.181329198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVail MM, Battelle BA. Influence of eye pigmentation and light deprivation on inherited retinal dystrophy in the rat. Exp. Eye Res. 1975;21:167–192. doi: 10.1016/0014-4835(75)90080-9. [DOI] [PubMed] [Google Scholar]
- LaVail MM. The retinal pigment epithelium in mice and rats with inherited retinal degeneration. In: Zinn KM, Marmor MF, editors. The Retinal Pigment Epithelium. Harvard University Press; Cambridge: 1979. pp. 357–380. [Google Scholar]
- Lewin AS, Drenser KA, Hauswirth WW, Nishikawa S, Yasumura D, Flannery JG, LaVail MM. Ribozyme rescue of photoreceptor cells in a transgenic rat model of autosomal dominant retinitis pigmentosa. Nature Med. 1998;4:967–971. doi: 10.1038/nm0898-967. [DOI] [PubMed] [Google Scholar]
- Li ZY, Possin DE, Milam AH. Histopathology of bone spicule pigmentation in retinitis pigmentosa. Ophthalmology. 1995;102:805–816. doi: 10.1016/s0161-6420(95)30953-0. [DOI] [PubMed] [Google Scholar]
- Lutty GA, McLeod DS. Retinal vascular development and oxygen-induced retinopathy: a role for adenosine. Prog. Ret. Eye Res. 2003;22:95–111. doi: 10.1016/s1350-9462(02)00058-7. [DOI] [PubMed] [Google Scholar]
- Madan A, Penn JS. Animal models of oxygen-induced retinopathy. Front. Biosci. 2003;8:d1030–1043. doi: 10.2741/1056. [DOI] [PubMed] [Google Scholar]
- Matthes MT, Bok D. Blood vascular abnormalities in the degenerative mouse retina (C57BL/6J-rd le). Invest. Ophthalmol. Vis. Sci. 1984;25:364–369. [PubMed] [Google Scholar]
- Matthes MT, Bok D. Blood vascular abnormalities in animals with inherited retinal degeneration. In: LaVail MM, Hollyfield JG, Anderson RE, editors. Retinal Degeneration: Experimental and Clinical Studies. Alan R. Liss, Inc.; New York: 1985. pp. 209–237. [Google Scholar]
- Michaelson IC, Herz N, Lewkowitz E, Kertesz D. Effect of increased oxygen on the development of the retinal vessels; an experimental study. Br. J. Ophthalmol. 1954;38:577–587. doi: 10.1136/bjo.38.10.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milam AH, Li ZY, Fariss RN. Histopathology of the human retina in retinitis pigmentosa. Prog. Ret. Eye Res. 1998;17:175–205. doi: 10.1016/s1350-9462(97)00012-8. [DOI] [PubMed] [Google Scholar]
- Nishikawa S, LaVail MM. Neovascularization of the RPE: temporal differences in mice with rod photoreceptor gene defects. Exp. Eye Res. 1998 doi: 10.1006/exer.1998.0538. In press. [DOI] [PubMed] [Google Scholar]
- Nomura M, Yamagishi S, Harada S, Hayashi Y, Yamashima T, Yamashita J, Yamamoto H. Possible participation of autocrine and paracrine vascular endothelial growth factors in hypoxia-induced proliferation of endothelial cells and pericytes. J. Biol. Chem. 1995;270:28316–28324. doi: 10.1074/jbc.270.47.28316. [DOI] [PubMed] [Google Scholar]
- Otani A, Kinder K, Ewalt K, Otero FJ, Schimmel P, Friedlander M. Bone marrow-derived stem cells target retinal astrocytes and can promote or inhibit retinal angiogenesis. Nat. Med. 2002;8:1004–1010. doi: 10.1038/nm744. [DOI] [PubMed] [Google Scholar]
- Otani A, Dorrell MI, Kinder K, Moreno SK, Nusinowitz S, Banin E, Heckenlively J, Friedlander M. Rescue of retinal degeneration by intravitreally injected adult bone marrow-derived lineage-negative hematopoietic stem cells. J. Clin. Invest. 2004;114:765–774. doi: 10.1172/JCI21686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn JS, Li S, Naash MI. Ambient hypoxia reverses retinal vascular attenuation in a transgenic mouse model of autosomal dominant retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 2000;41:4007–4013. [PubMed] [Google Scholar]
- Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc. Natl. Acad. Sci. U.S.A. 1995;92:905–909. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Geniez M, D'Amore PA. Development and pathology of the hyaloid, choroidal and retinal vasculature. Int. J. Dev. Biol. 2004;48:1045–1058. doi: 10.1387/ijdb.041895ms. [DOI] [PubMed] [Google Scholar]
- Schlingemann RO, van Hinsbergh VW. Role of vascular permeability factor/vascular endothelial growth factor in eye disease. Br. J. Ophthalmol. 1997;81:501–512. doi: 10.1136/bjo.81.6.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman RL, Pearlstein R. Pink-eyed dilution (p) gene in rodents: increased pigmentation in tissue culture. Dev. Biol. 1965;12:93–116. doi: 10.1016/0012-1606(65)90023-0. [DOI] [PubMed] [Google Scholar]
- Steinberg RH, Flannery JG, Naash M, Oh P, Matthes MT, Yasumura D, Lau-Villacorta C, Chen J, LaVail MM. Transgenic rat models of inherited retinal degeneration caused by mutant opsin genes. Invest. Ophthalmol. Vis. Sci. 1996;37:S698. [Google Scholar]
- Stone J, Itin A, Alon T, Pe'er J, Gnessin H, Chan-Ling T, Keshet E. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J. Neurosci. 1995;15:4738–4747. doi: 10.1523/JNEUROSCI.15-07-04738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JL, Barlow WE, Humayun MS, Jr, d.J.E., Milam AH. Morphometric analysis of macular photoreceptors and ganglion cells in retinas with retinitis pigmentosa. Arch. Ophthalmol. 1992;110:1634–1639. doi: 10.1001/archopht.1992.01080230134038. [DOI] [PubMed] [Google Scholar]
- Villegas-Pérez MP, Lawrence JM, Vidal-Sanz M, LaVail MM, Lund RD. Ganglion cell loss in RCS rat retina: a result of compression of axons by contracting intraretinal vessels linked to the pigment epithelium. J. Comp. Neur. 1998;392:58–77. [PubMed] [Google Scholar]
- Yamada H, Yamada E, Hackett SF, Ozaki H, Okamoto N, Campochiaro PA. Hyperoxia causes decreased expression of vascular endothelial growth factor and endothelial cell apoptosis in adult retina. J. Cell Physiol. 1999;179:149–156. doi: 10.1002/(SICI)1097-4652(199905)179:2<149::AID-JCP5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Yu DY, Cringle SJ, Su EN, Yu PK. Intraretinal oxygen levels before and after photoreceptor loss in the RCS rat. Invest. Ophthalmol. Vis. Sci. 2000;41:3999–4006. [PubMed] [Google Scholar]
- Yu DY, Cringle SJ. Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Prog. Retin. Eye Res. 2001;20:175–208. doi: 10.1016/s1350-9462(00)00027-6. [DOI] [PubMed] [Google Scholar]
- Yu DY, Cringle S, Valter K, Walsh N, Lee D, Stone J. Photoreceptor death, trophic factor expression, retinal oxygen status, and photoreceptor function in the P23H rat. Invest. Ophthalmol. Vis. Sci. 2004;45:2013–2019. doi: 10.1167/iovs.03-0845. [DOI] [PubMed] [Google Scholar]