Abstract
Previous studies have shown that human prostate cancer cells constitutively generate 5-lipoxygenase (5-LOX) metabolites from arachidonic acid, and inhibition of 5-LOX blocks production of 5-LOX metabolites and triggers apoptosis in prostate cancer cells. This apoptosis is prevented by exogenous metabolites of 5-LOX, suggesting an essential role of 5-LOX metabolites in the survival of prostate cancer cells. However, downstream signaling mechanisms which mediate the survival-promoting effects of 5-LOX metabolites in prostate cancer cells are still unknown. Recently, we reported that MK591, a specific inhibitor of 5-LOX activity, induces apoptosis in prostate cancer cells without inhibition of Akt, or ERK, two well-characterized regulators of pro-survival mechanisms, suggesting the existence of an Akt and ERK-independent survival mechanism in prostate cancer cells regulated by 5-LOX. Here, we report that 5-LOX inhibition-induced apoptosis in prostate cancer cells occurs via rapid inactivation of protein kinase C-epsilon (PKCε), and that exogenous 5-LOX metabolites prevent both 5-LOX inhibition-induced down-regulation of PKCε and induction of apoptosis. Interestingly, pre-treatment of prostate cancer cells with diazoxide (a chemical activator of PKCε), or KAE1-1 (a cell-permeable, octa-peptide specific activator of PKCε) prevents 5-LOX inhibition-induced apoptosis, which indicates that inhibition of 5-LOX triggers apoptosis in prostate cancer cells via down-regulation of PKCε. Altogether, these findings suggest that metabolism of arachidonic acid by 5-LOX activity promotes survival of prostate cancer cells via signaling through PKCε, a pro-survival serine/threonine kinase.
Keywords: 5-Lipoxygenase, Prostate cancer, Apoptosis, PKC-epsilon
1. Introduction
Prostate cancer is the most common form of malignancy and second leading cause of cancer-related deaths in men in the United States [1]. Significant progress has been made to control localized prostate cancer by surgery and radiation therapy. However, management of metastatic prostate cancer still remains a major problem for which no satisfactory measure is available. Prostate cancer initially responds to androgen-ablation therapy, however, androgen-independent disease almost always develops which eventually metastasizes to distant organs and assumes a lethal phenotype [2]. No cure is currently available for metastatic, androgen-independent prostate cancer. Lack of proper understanding about critical molecular mechanisms in prostate cancer cells is delaying development of effective therapeutic regimen against prostate cancer. Thus, exploration and characterization of novel mechanisms, that are specific and critical for prostate cancer cells, are of utmost significance to develop effective strategies to control this disease.
Both epidemiological studies and experiments with laboratory animals repeatedly suggested a link between consumption of high-fat diets and occurrence of clinically evident prostate cancer [3–8], indicating that dietary fatty acids and their metabolic products may play an important role in the promotion and/or progression phases of prostate cancer presumably via regulation of growth and survival characteristics of prostate cancer cells. Arachidonic acid, an omega-6, polyunsaturated fatty acid, was found to stimulate prostate cancer cell growth via metabolic conversion through the 5-LOX pathway [9–11]. Later, it was observed that prostate cancer cells constitutively generate 5-LOX metabolites, and inhibition of 5-LOX blocks production of 5-LOX metabolites and triggers apoptosis both in androgen-sensitive as well as androgen-independent prostate cancer cells [12,13]. This apoptosis is prevented by exogenous 5(S)-HETE (5-hydroxyeicosatetraenoic acid), and more effectively by its dehydrogenase-derivative 5-oxoETE, suggesting a critical role of 5-LOX metabolites in the survival of prostate cancer cells. It is interesting to note that under normal health condition, expression of 5-LOX is restricted to specific immune cells such as neutrophils, eosinophils, basophils and macrophages (not in T cells) where it plays a role in chemotaxis [14,15], whereas the vast majority of non-immune parenchyma body cells do not express 5-LOX unless disease occurs, such as asthma, arthritis, psoriasis, and cancer [14–19]. Increased expression and activity of 5-LOX were observed in prostate tumor tissues compared to adjacent non-tumor tissues [20]. Recently, it was observed that though 5-LOX is heavily expressed in prostate tumor tissues, its expression in normal prostate glands is undetectable (Sarveswaran et al.; Manuscript in preparation). This finding, together with a critical role of 5-LOX in the survival of prostate cancer cells, leads to the concept that 5-LOX may play an important role in the development and progression of prostate cancer. Thus, the 5-LOX pathway is emerging as a promising target for therapeutic development against prostate cancer. However, downstream signaling mechanisms mediating the survival-promoting effects of 5-LOX metabolites in prostate cancer cells are yet to be characterized.
To gain an insight into the mechanisms underlying regulation of prostate cancer cell survival by 5-LOX activity, we systematically addressed the involvement of, (1) the phosphatidylinositol 3′-kinase-Akt/protein kinase B (PI3K-Akt), (2) the mitogen-activated protein kinase kinase-extracellular signal regulated kinase (MEK-ERK), and (3) the protein kinase C-epsilon (PKCε) pathway as potential mediator(s), because these pathways are known to promote growth and survival of a variety of cells including cancer cells. The PI3K-Akt axis plays an important role in the cellular signaling network regulating various cell functions including proliferation, apoptosis, cell growth, and metabolism [21–25]. This pathway is over-activated in many types of cancer cells and is well known to contribute to cell survival through defined apoptosis-preventing mechanisms [18–20]. Because of its role in prevention of apoptosis via multiple mechanisms, and its frequent activation in cancer cells, the PI3K-Akt pathway is now targeted for anticancer drug development [24,25]. Similarly, the MEK-ERK pathway is also known to promote growth and survival of a variety of cells including cancer cells [26–28]. Interestingly, we observed no reduction in the phosphorylation of Akt at Ser473 or the enzymatic activity of Akt when prostate cancer cells are treated with MK591 to undergo apoptosis [29]. MK591 is a widely used specific inhibitor of 5-LOX activity, and it does not inhibit cyclooxygenase, epoxygenase or 12-lipoxygenase activities [30,31]. We also observed that treatment of prostate cancer cells with MK591 does not inhibit the phosphorylation/activation of ERK while triggering apoptosis in prostate cancer cells [29]. Altogether, these findings indicated that the 5-LOX inhibition-induced apoptosis in prostate cancer cell occurs without inhibition of Akt or ERK, and suggested the existence of an Akt- and ERK-independent survival mechanism in prostate cancer cells regulated by 5-LOX activity.
The initial findings of 5-LOX inhibition-induced apoptosis in prostate cancer cells without inhibition of Akt or ERK invoked us to examine the role of PKCε as a potential mediator of the survival-promoting effects of 5-LOX. The PKC-family of serine-threonine protein kinases includes about ten isoforms and are known to regulate various cell functions, such as cell proliferation, apoptosis, angiogenesis, carcinogenesis, metabolism, and cell motility [32–35]. Based on structural similarities and co-factor dependence the PKC isoforms have been classified into three subfamilies [35–38]. The classical PKCs (α, ßI, ßII, and γ) are Ca2+-dependent and are activated by diacylglycerol (DAG) and phosphatidylserine (PS). The novel PKCs (δ, ε, η, and θ) are Ca2+-independent and require DAG/PS for full activation. The atypical PKCs (λ and ζ) require only PS for activation. Among the PKC isoforms PKCε is known to be oncogenic which promotes tumor growth and recurrence by increasing cell proliferation as well as by decreasing apoptosis [32–35,39]. Thus, we wanted to examine whether PKCε plays a role as a downstream mediator in the survival-promoting effects of 5-LOX in prostate cancer cells. We observed that when prostate cancer cells are treated with MK591 to induce apoptosis, there is a rapid and significant loss of PKCε activity in these cells, and both the reduction of PKCε activity and induction of apoptosis are prevented by 5-oxoETE, a metabolite of 5-LOX. Moreover, we observed that 5-LOX inhibition-induced apoptosis is prevented by chemical or peptide activators of PKCε. These novel findings suggest that 5-LOX inhibition-induced apoptosis in prostate cancer cells is mediated via down-regulation of PKCε. Since, prostate cancer cells readily generate 5-HETE-series of eicosanoids from arachidonic acid, our findings suggest that prostate cancer cells gain survival advantage from arachidonic acid-rich Western diets [9,10,12], by generating 5-LOX metabolites followed by downstream signaling through the serine/threonine kinase, PKCε.
2. Materials and methods
2.1. Cell culture and reagents
LNCaP human prostate cancer cells were purchased from American Type Culture Collection (Manassas, VA). Cells were grown in RPMI medium 1640 (Invitrogen, Carlsbad, CA) supplemented with 10% FBS and antibiotics. Polyclonal antibodies against PKCε and PARP were from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal anti-5-Lox was purchased from BD Biosciences (Lexington, KY). Anti-beta-actin antibody and ibuprofen were purchased from Sigma (St. Louis, MO). 5-OxoETE was purchased from Cayman Chemical (Ann Arbor, MI). Lentiviral particles generating 5-LOX shRNA (small hairpin-RNA) were bought from Santa Cruz Biotech (Santa Cruz, CA). ELISA kit to measure PKCε activity was purchased from Cell Signaling Technology (Danvers, MA). MK591 was obtained as a generous gift from Dr. Robert N. Young (Merck-Frosst Centre for Therapeutic Research, Quebec, Canada).
2.2. Cell viability assay
LNCaP prostate cancer cells (~5000 per well) were plated overnight in 96 well plates in complete growth medium (RPMI or DMEM plus 10% FBS) and treated with varying doses of MK591. Plates were incubated further for 72 h at 37 °C in the CO2 incubator. Cell viability was measured by One Solution MTS/PES Cell Titer assay from Promega (Madison, WI) as described before [9,12].
2.3. Eicosanoid measurement
LNCaP cells (1 × 106 per plate) were plated in 100 mm diameter tissue culture plates in phenol red-free RPMI medium supplemented with 10% FBS and allowed to grow for 48 h. The old medium was then replaced with 5 ml fresh RPMI medium and treated with MK591 for 24 h. Cell culture media were harvested, centrifuged to clear cells and debris, and then lipids were extracted using Hypersep C18 solid-phase extraction columns (Thermo Fisher). Samples were dried with gentle nitrogen flush and re-suspended in 40 μl methanol. Metabolites of 5-LOX were measured by liquid chromatography/ESI mass-spectrometry (LC/MS/MS) with a Micromass-Waters QuattroLC mass spectrometer (Milford, MA) using 5(S)-HETE-d8 and 5-oxoETE-d7 as internal standards (Cayman Chemicals, Ann Arbor, MI) following methods published recently by Maddipati et al. [40].
2.4. Annexin-V binding
Cells (~3×105) were plated in RPMI medium and allowed to grow for 48 h. The spent culture medium was replaced with fresh 2 ml RPMI medium and the cells were treated with MK591 or ibuprofen for 24 h at 37 °C. Then the cells in the plate were treated with FITC-labeled annexin-V and propidium-iodide (PI) for 15 min in the dark using Annexin V-Binding Detection Kit following a protocol supplied by the manufacturer (BD Biosciences). After washing, cells were photographed with a Nikon digital camera attached to a LEICA fluorescence microscope at 20×. Image acquisition and data processing were done with a Dell computer attached to the microscope using SPOT-Advanced software.
2.4.1. Flow cytometry
Cells were treated with MK591 for 24 h at 37 °C, harvested by trypsinization, washed in PBS, and finally suspended in Annexin-V binding buffer. Cells were stained with Annexin V and PI using Annexin-V-Binding Detection assay kit from BD Biosciences and apoptosis was quantitatively measured by flow cytometry (Accuris, Ann Arbor, MI).
2.5. Western blot
Cells (~3×105) were plated and allowed to grow for 48 h. The old medium was then replaced with 2 ml fresh RPMI medium and the cells were treated with inhibitors. After treatment, cells were harvested, washed, and lysed in lysis buffer (50 mM HEPES, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM orthovanadate, 10 mM sodium pyrophosphate, 10 mM sodium fluoride, 1% NP-40, and a cocktail of protease inhibitors). Proteins were separated by 12% SDS-PAGE and transferred to nitrocellulose membranes. Membranes were blocked with 5% nonfat-milk solution and then blotted with appropriate primary antibody followed by horseradish peroxidase-labeled secondary antibody. Bands were visualized by enhanced chemiluminescence detection kit from Pierce Biotech (Rockford, IL) and analyzed with a densitometer using Kodak imaging software. Unless otherwise mentioned, blots of proteins of interest were analyzed in three separate experiments.
2.6. Cell fractionation
Cells were plated in 100 mm diameter tissue culture plates and allowed to grow for 48 h. Then the cells were treated with MK591 for varying periods of time. At the end of incubation, cells were washed with PBS and the plasma membrane fractions were isolated using a kit following manufacturer's protocol (Biovision, Mountain View, CA). Membrane fractions were stored at –80 °C and solubilized in lysis buffer for analysis by Western blot.
2.7. Measurement of PKCε activity by immunoprecipitation-kinase (IP-kinase) assay
Prostate cancer cells (~1×106) were plated in 100 mm diameter plates and allowed to grow for 48 h. The old culture medium was then replaced with fresh 5 ml RPMI medium and the cells were treated with inhibitors or solvent vehicle (0.2% DMSO) for varying periods of time up to 6 h. Then the cells were lysed in lysis buffer (50 mM HEPES, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM orthovanadate, 10 mM sodium pyrophosphate, 10 mM sodium fluoride) containing 0.4% Triton-X100, 10% glycerol, and a cocktail of protease inhibitors. Enzymatic activity of PKCε was measured by an ELISA method using biotin-labeled peptide substrate (Cell Signaling Technology, Danvers, MA). Briefly, cell lysates were cleared by centrifugation at 12,000 ×g for 10 min at 4 °C and the supernatants (~500 μg proteins) were used for immunoprecipitation of PKCε using 4 μg anti-PKCε antibody. The tubes were rotated overnight at 4 °C and the immune complexes were precipitated using anti-rabbit IgG-coated magnetic beads (Invitrogen) for 2 h at 4 °C. Then the beads were washed five times with lysis buffer containing 0.1% Triton-X100 and finally suspended in 25 μl of 1× kinase assay buffer. Enzymatic reactions in 50 μl were carried out for 15 min at room temperature (RT) using 10 μl of IP-slurry with beads, and stopped with 50 μl of 50 mM EDTA. Aliquots of reaction mixtures (25 μl) were placed into streptavidin-coated 8-well strips and incubated for 60 min at RT. Wells were washed and phosphorylation of biotinylated-peptide substrate (cAMP response element-binding protein or CREB at Ser-133) was detected by specific anti-phosphoserine primary antibody followed by secondary HRP-labeled anti-rabbit antibody. After washing, color was developed using ABTS (2,2′-azino-di (3-ethylbenzthiazoline-6-sulfonate) as substrate for 15 min at RT. Absorbance was measured at 405 nm in a digital plate reader (Bio-Tek Instruments).
2.8. Measurement of DNA degradation
Apoptosis was quantitatively measured by detecting degradation of nuclear DNA to nucleosomal fragments by sandwich-ELISA. LNCaP cells (~3×105) were plated in 60 mm dishes and allowed to grow for 48 h. Cells were then treated either with the experimental agents or the solvent vehicle for varying periods of time up to 24 h. At the end of incubation periods, cells were lysed and the degradation of chromatin-DNA to nucleosomal fragments was measured by Cell Death Detection ELISAplus kit from Roche (Indianapolis, IN) as described before [12,13].
2.9. Statistical analysis
Significance of the difference between values in treated and untreated groups were calculated by two-tailed student's t-test using GraphPad Instat Software. A p value of <0.05 was considered to be statistically significant.
3. Results
3.1. Inhibition of 5-LOX decreases viability of prostate cancer cells
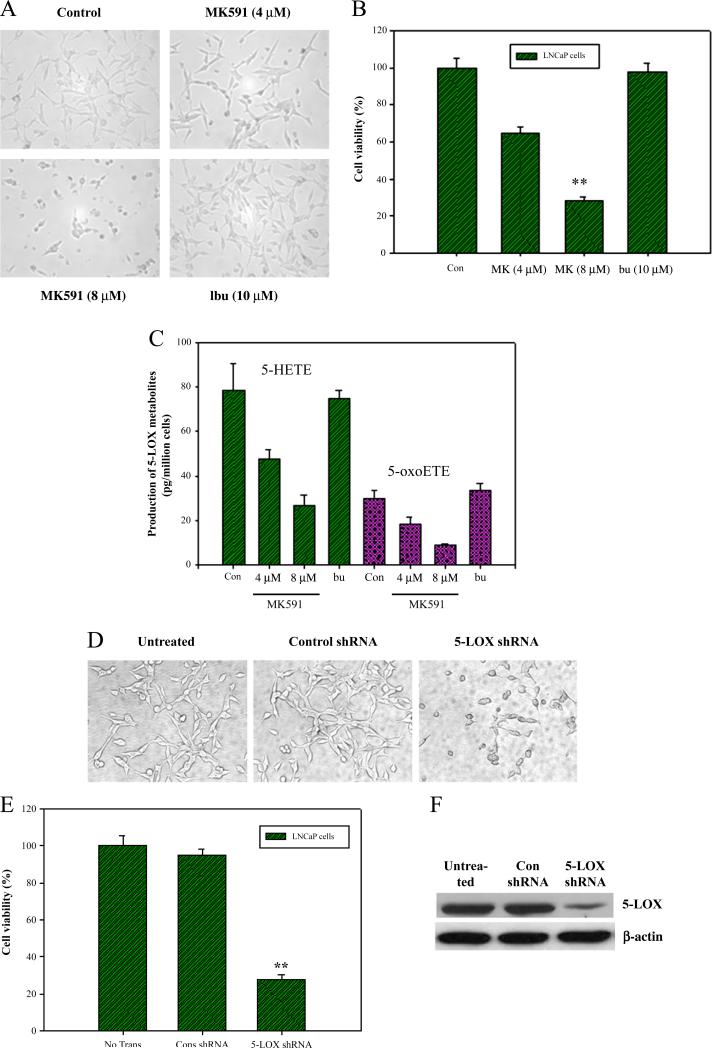
We observed that MK591, a specific inhibitor of 5-LOX activity [30,31], decreases viability of prostate cancer cells, which is accompanied by a significant inhibition in the production of 5-LOX metabolites (Fig. 1A–C). Ibuprofen (an inhibitor of cyclooxygenase) was used as negative control which did not affect viability of cells or the production of 5-LOX metabolites. Decreased cell viability was also observed when the cells were treated with lentiviral 5-LOX shRNA to decrease level of 5-LOX expression, confirming a role of 5-LOX in prostate cancer cell survival (Fig. 1D–F). These experiments were performed with LNCaP human prostate cancer cell line which represents the most authentic prostate cancer cell line available [41]. However, other human (PC3, DU145) as well as mouse (TRAMP-C1, Myc-Cap) prostate cancer cell lines are also similarly affected when treated with MK591 (Sarveswaran et al.; Manuscripts in preparation).
Fig. 1.
Effect of 5-LOX inhibition on the viability of prostate cancer cells. In (A–C), LNCaP prostate cancer cells (~25,000 per well) were plated in 24 well tissue culture plates in complete growth medium with 10% FBS and treated with varying doses of MK591. Plates were incubated for 24 h at 37 °C in the CO2 incubator and photographs were taken at 400× (A). Cell viability was measured by MTS/PES Cell Titer assay from Promega (B). Data are presented as mean value of each data point±standard error (*p<0.05, n=4). Production of 5-LOX metabolites was measured by LC/MS/MS as described in the “Methods” section (C). Results are shown as mean value of each data point±standard error (*p<0.05, n=3). In (d–f), LNCaP cells (~50,000 per well) were plated in 12 well tissue culture plates in complete growth medium and treated with control or 5-LOX shRNA lentiviral particles (cell to virus ratio=1:20). After 96 h, photographs were taken at 400× (D), and cell viability was measured by MTS/PES Cell Titer assay (*p<0.05, n=4) (E). Expression of 5-LOX was analyzed by Western blot (F).
3.2. Inhibition of 5-LOX induces membrane lipid-asymmetry, PARP-cleavage, and DNA-degradation in prostate cancer cells
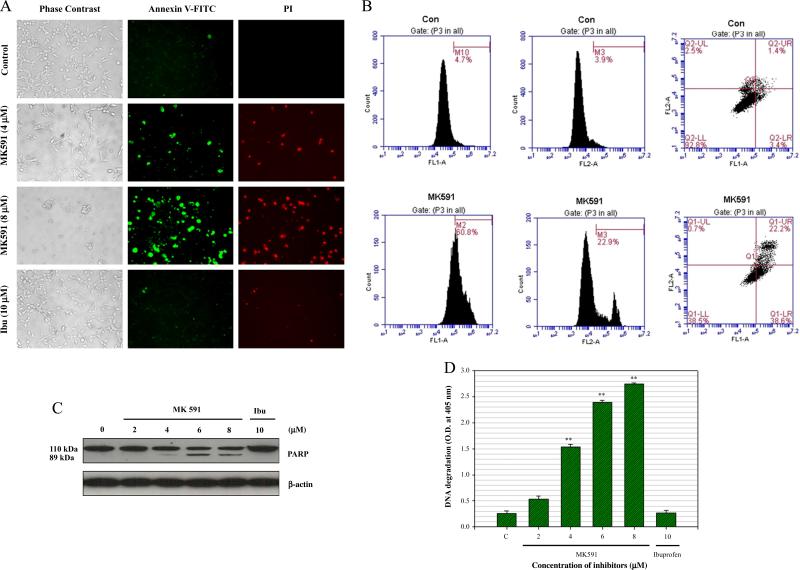
Next we examined whether prostate cancer cells externalize phosphatidylserine (a hall-mark of apoptotic cell death) when treated with MK591. We observed that LNCaP cells show distinctly positive binding with annexin-V when treated with MK591, suggesting externalization of phosphatidylserine to the cell surface (Fig. 2A). Annexin binding was also quantitatively measured by flow cytometry (Fig. 2B). Characteristic cleavage of PARP (poly-ADP ribose polymerase), an indicator of caspase-mediated apoptosis, was observed to occur in a dose-dependent manner (Fig. 2C). Further analysis revealed degradation of nuclear-DNA to nucleosomal fragments which is an indicator of advanced stage of apoptosis (Fig. 2D). Cells treated with ibuprofen in parallel experiments, did not show any signs of apoptotic features, suggesting that the effect of 5-LOX inhibition to induce apoptosis in prostate cancer cells is selective.
Fig. 2.
Induction of apoptosis in prostate cancer cells by MK591. LNCaP cells (3×105) were plated in 60 mm diameter plates and allowed to grow for 48 h. Then the old medium was replaced with 2 ml fresh RPMI medium and the cells were treated either with MK591 (8 μM) or ibuprofen (10 μM) at 37 °C for 24 h. Control cells were treated with vehicle only (0.2% DMSO). At the end of incubation period, cells in binding buffer were treated with FITC-labeled annexin-V and propidium iodide (PI), and were observed under microscope at 200× (A). In (B), cells were treated with 8 μM MK591 for 24 h and apoptosis was quantitatively measured detecting annexin-V binding by flow cytometry. In (C), at the end of incubation, cells were lysed and cleavage of PARP was detected by Western blot. In (D), degradation of DNA was detected by ELISA. Results are shown as mean values of each data point±standard error (**p<0.005, n=4).
3.3. Inhibition of 5-LOX decreases expression and membrane localization of PKCε in prostate cancer cells
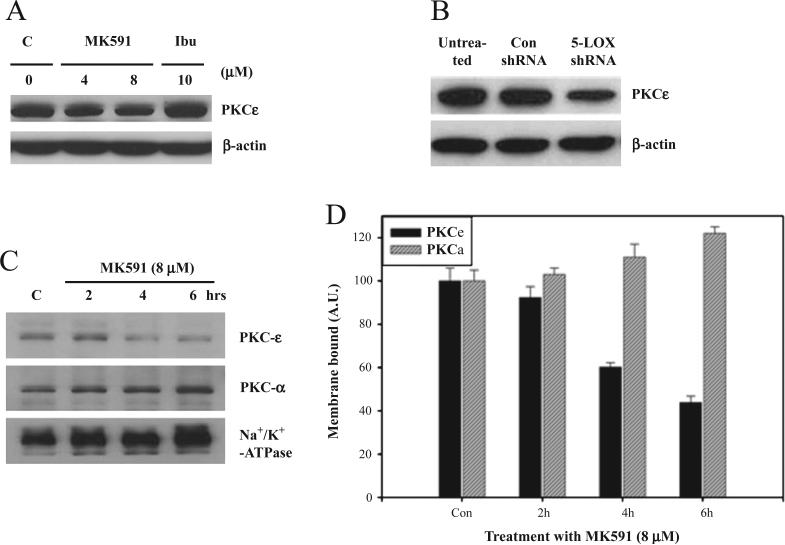
The mechanism how 5-LOX activity regulates prostate cancer cell survival is an intriguing but unanswered question. Recently, we reported that the 5-LOX inhibition-induced apoptosis in prostate cancer cells occurs without inhibition of Akt, or ERK [29]. Thus, next we examined the involvement of PKCε which is reported to decrease apoptosis in a variety of cell types [32,38,39]. To investigate the potential role of PKCε as a downstream mediator of the survival-promoting effects of 5-LOX, we observed that treatment with MK591 or 5-LOX shRNA decreases expression of PKCε in prostate cancer cells (Fig. 3A,B). We also observed that prostate cancer cells treated with MK591 showed a rapid decrease in the membrane localization of PKCε in a time-dependent manner (Fig. 3C,D). A minor decrease was noticed in the level of cytosolic PKCε in short-term (4–6 h) treatment (not shown).
Fig. 3.
Expression and membrane localization of PKCε in prostate cancer cells. LNCaP cells (3×105) were plated in 60 mm diameter plates and allowed to grow for 48 h. Then the cells were treated as indicated for 24 h, washed and lysed in lysis buffer. In (A), cell lysate proteins (100 μg per lane) were separated in 12% SDS-PAGE and expression of PKCε was detected by Western blot. In (B), cells were plated and treated with 5-LOX shRNA lentiviral particles for 96 h as described in Fig. 1D–F above, and cell lysates were analyzed by Western blot. In (D), LNCaP cells (1×106 per plate) were plated and allowed to grow for 48 h. Then the cells were treated with MK591 for times as indicated. Control cells were treated with vehicle only (0.2% DMSO) for 6 h. In (C), at the end of treatment, membrane fractions were isolated, lysed and analyzed for PKCε by Western blot. (D) Represents mean densitometric values of two experiments±standard deviation normalized to beta-actin (AU. = arbitrary units).
3.4. Inhibition of 5-LOX down-regulates the enzymatic activity of PKCε in prostate cancer cells
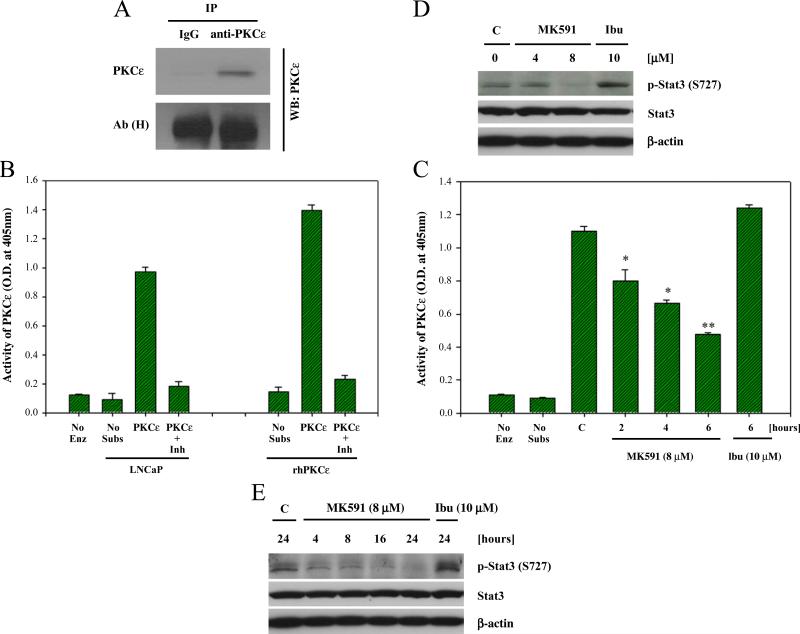
To explore the state of activity of PKCε in prostate cancer cells, we observed that prostate cancer cells maintain catalytically active state of PKCε under normal culture condition without any exogenous treatment. We analyzed the enzymatic activity of PKCε after immunoprecipitation, and further confirmed PKCε activity using specific peptide inhibitor of PKCε (KIE1-1) (Fig. 4A,B). Interestingly, it was observed that treatment of cells with the 5-LOX inhibitor, MK591, decreases the enzymatic activity of PKCε in a time-dependent manner, suggesting that PKCε is regulated by 5-LOX activity in prostate cancer cells (Fig. 4C). We also observed that phosphorylation of the transcription factor Stat3 at serine-727, which has been characterized to be a PKCε phosphorylation site [42], is decreased in a dose and time-dependent manner (Fig. 4D,E).
Fig. 4.
Inhibition of PKCε activity in prostate cancer cells by MK591 treatment. LNCaP cells (1×106 per plate) were plated in 100 mm plates and allowed to grow for 48 h. Then the cells were treated with drugs for times as indicated. Control cells were treated with vehicle only (0.2% DMSO) for 6 h. PKCε was immunoprecipitated from 500 μg of cell lysate proteins and detected by Western blot (A). In (B), kinase activity of PKCε was measured by IP-kinase assay as described in the “Methods” section. No Enz (=no enzyme added) and No peptide (=no peptide substrate added) were used as negative controls, and recombinant human PKCε (20 ng) was used in parallel as positive control. Activity of PKCε was confirmed using 50 μM of the specific peptide inhibitor KIE1-1 [44]. Results are shown as mean values±standard error (n=4). In (C), LNCaP cells were treated as indicated and the kinase activity of PKCε was measured by IP-kinase assay (*p<0.05, **p<0.005, n=4). In (D and E), LNCaP cells (3×105) were plated in 60 mm diameter plates as in Fig. 3A above, and treated either with MK591 or ibuprofen as indicated for 24 h. Control cells were treated with vehicle only (0.2% DMSO). Cell lysate proteins (100 μg per lane) were separated in 12% SDS-PAGE and phosphorylation of Stat3 at serine-727 was detected by Western blot. A representative of two experiments with similar results is shown here.
3.5. 5-OxoETE, a metabolite of 5-LOX, prevents MK591-induced inhibition of PKCε and induction of apoptosis
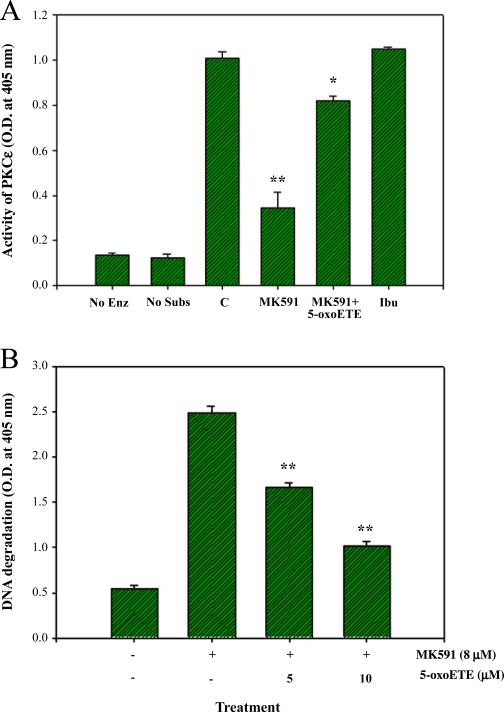
Inhibition of PKCε activity in prostate cancer cells treated with MK591 suggested that 5-LOX activity regulates PKCε. However, if 5-LOX activity regulates PKCε, then it is expected that metabolic products of 5-LOX should reverse the inhibitory effects of MK591 treatment. Thus, we treated prostate cancer cells with 5-oxoETE, a metabolic product of 5-LOX, before treating them with MK591 and observed that 5-oxoETE effectively prevents MK591-induced inhibition of PKCε activity (Fig. 5A). In a separate set of experiments, we observed that MK591 treatment-induced apoptosis in prostate cancer cells is prevented by 5-oxoETE, confirming that PKCε is under regulation of 5-LOX activity in prostate cancer cells (Fig. 5B).
Fig. 5.
Prevention of MK591-induced inhibition of PKCε and induction of apoptosis by 5-oxoETE. In (A), LNCaP cells were plated as in Fig. 4A and treated with 8 μM MK591 with or without 10 μM 5-oxoETE for 6 h. Ibuprofen (10 μM) was used in parallel as negative control. Control cells were treated with vehicle only (0.2% DMSO) for 6 h. Then the cells were lysed and the enzymatic activity of PKCε was determined by IP-kinase assay. Results represent mean value of each data point±standard error (*p<0.05, **p<0.005, n=3). In (B), cells were plated and treated with MK591 as in Fig. 2D with or without the addition of 5-oxoETE for 24 h. At the end of incubation period, apoptosis was measured by ELISA. Results represent mean values of each data point±standard error (**p<0.005, n=3).
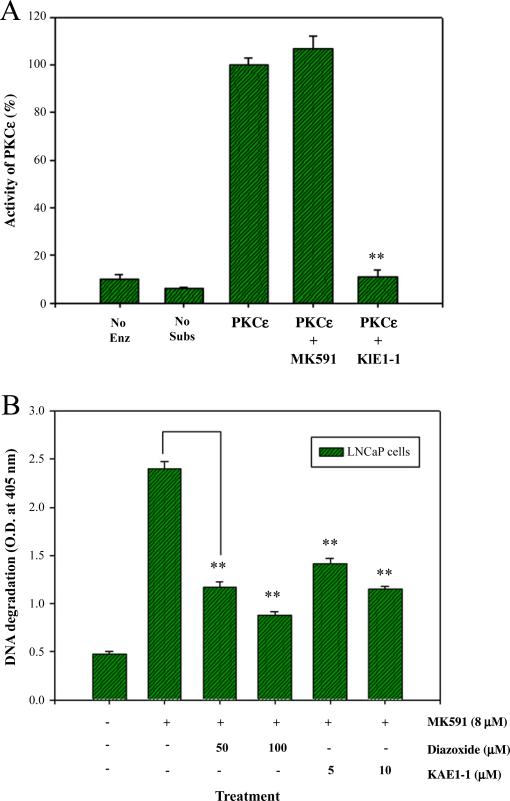
3.6. MK591 is not a direct inhibitor of PKCε, and MK591-induced apoptosis in prostate cancer cells is prevented by activators of PKCε
We wanted to address the question whether MK591 directly inhibits the enzymatic activity of pure PKCε through an off-target effect in addition to its authentic inhibitory effects on 5-LOX activity. We observed that MK591 does not inhibit PKCε activity when directly added to the assay mixture containing immunoprecipitated PKCε from LNCaP cells (Fig. 6A). Next we asked the question whether activation of PKCε by other means can prevent 5-LOX inhibition-induced apoptosis in prostate cancer cells, because if inhibition of 5-LOX induces apoptosis via inactivation of PKCε, then it is expected that activators of PKCε should overcome the apoptosis-inducing effects of 5-LOX inhibition. We addressed this question by treating prostate cancer cells with diazoxide, a chemical activator of PKCε [43], before treating them with 5-LOX inhibitor and observed that diazoxide effectively prevents 5-LOX inhibition-induced apoptosis (Fig. 6B). We also observed that pretreatment of cells with KAE1-1, a TAT-labeled specific octa-peptide activator of PKCε [44] is effective in preventing MK591-induced apoptosis. Altogether, these findings suggest that inhibition of 5-LOX induces apoptosis in prostate cancer cells via inactivation of PKCε, and that PKCε is a mediator of survival signals downstream of 5-LOX metabolites.
Fig. 6.
Direct effect of MK591 on PKCε, and prevention of 5-Lox inhibition-induced apoptosis in prostate cancer cells by activators of PKCε. In (A), PKCε was immunoprecipitated from 2×106 exponentially growing LNCaP cells without treatment. Then, equal aliquots of the isolated PKCε enzymes were treated with the agents (MK591=10 μM; KIE1-1=50 μM) for 10 min at RT before measuring the kinase activity. Results are shown as mean values of each data point±standard error (**p<0.005, n=3). In (B), LNCaP cells (3×105) were plated as in Fig. 2D and the cells were treated with MK591 (8 μM) with or without the activators of PKCε as indicated for 24 h. Control cells were treated with solvent only (0.2% DMSO). At the end of incubation period, apoptosis was measured by Cell Death ELISA. Data presented as mean values±standard error (**p<0.005, n=4).
4. Discussion
Our observation of a dramatic reduction in the viability of prostate cancer cells by MK591, or by 5-LOX-specific shRNA, demonstrates that prostate cancer cells depend on 5-LOX activity for survival (Fig. 1). We also observed that MK591 treatment-induced cell death is accompanied by standard apoptotic features such as externalization of phosphatidylserine, cleavage of PARP, and degradation of chromatin-DNA to nucleosomal fragments (Fig. 2). It is interesting to note that under normal health conditions most normal parenchyma body cells do not express 5-LOX. However, over-expression of 5-LOX has been implicated in inflammatory diseases, such as asthma, arthritis, psoriasis, and a variety of cancers such as cancer of the prostate, lung, pancreas, and brain [14–19]. Thus, agents that specifically block the activity of 5-LOX may turn out to be attractive tools to treat these diseases. MK591 is a specific synthetic inhibitor of 5-LOX and is currently under development for the treatment of asthma [30,31]. It blocks synthesis of leukotrienes by inhibiting 5-LOX activity via binding with its activating protein, FLAP, but it does not inhibit cyclooxygenase, epoxygenase, or 12-lipoxygenase activities. Our observation of the prevention of MK591-induced apoptosis in prostate cancer cells by exogenous metabolites of 5-Lox confirms that induction of apoptosis by MK591 occurs via inhibition of 5-LOX activity. Recently, we observed that MK591 strongly inhibits the growth of prostate tumors in nude mice xenografts (Sarveswaran et al., Manuscript in preparation). Thus, MK591 is emerging as a novel, targeted agent for prostate cancer therapy.
Though 5-LOX activity plays an essential role in the survival of prostate cancer cells, downstream signaling mechanisms underlying regulation of prostate cancer cell survival by 5-LOX are not characterized yet. We addressed this question by examining the effects of 5-LOX inhibition on major signaling pathways known to play important roles in cell-viability. The PI3K-Akt axis is a well known pro-survival mechanism and plays an important role in the viability of many types of cancer cells [21–25]. Thus, this pathway has emerged as a valid target for anti-cancer drug development, and a series of compounds have been identified/generated for mechanistic studies as well as for clinical testing. The ERK pathway is also known to promote growth and survival of a variety of cell types including cancer cells [26–28]. However, we recently reported that MK591-induced apoptosis in prostate cancer cells occurs without inhibition of Akt, and an increase rather than decrease in the activation of ERK1/2 [29]. These findings indicated that the 5-LOX activity feeds a mechanism which is independent of Akt and ERK, and suggested that prostate cancer cells are equipped with additional survival mechanisms which may help them to bypass chemotherapies that are directed against Akt and ERK, two well-characterized anti-cancer molecular targets. Existence of an Akt-independent survival mechanism in LNCaP prostate cancer cells was reported previously using a panel of survival/growth factors in the presence or absence of inhibitors of PI3K-Akt [45]. However, details about the identity of possible kinase(s) behind the Akt-independent mechanism of prostate cancer cell survival were not characterized. Thus, we wanted to investigate the involvement of PKCε as a potential mechanism of prostate cancer cell survival regulated by 5-LOX activity, because this kinase is also well known to promote survival and growth of a variety of cells including cancer cells [32,33].
A characteristic feature of prostate cancer cells appears to be maintenance of a continuous activation of PKCε. This feature was previously demonstrated in prostate tumor tissues by immunohistochemistry showing membrane localization of PKCε, and also by its colocalization, phosphorylation and activation of the transcription factor Stat3 [42]. In this study, we observed that the level of PKCε is down-regulated when prostate cancer cells are treated with MK591 or shRNA against 5-LOX (Fig. 3A,B). We also found that under normal culture condition a substantial amount of PKCε protein in prostate cancer cells is membrane-localized which corresponds to its active state, and that treatment with MK591 decreases membrane localization of PKCε in a time-dependent manner (Fig. 3C,D). Only a minor decrease was noticed in the level of cytosolic PKCε, suggesting that MK591 mainly induces a decrease in membrane-localization rather than degradation of PKCε in short-term treatment (not shown). Moreover, the enzymatic (IP-kinase) assay confirmed that prostate cancer cells maintain significant levels of PKCε activity under normal culture conditions, and MK591 treatment decreases the catalytic activity of PKCε in a time-dependent manner (Fig. 4C). Inhibition of the activity PKCε was also reflected by decreased phosphorylation of the transcription factor Stat3 at serne-727 which is a well known substrate of PKCε (Fig. 4D,E). It is interesting to note that both the MK591 treatment-induced inhibition of PKCε activity, and induction of apoptosis are inhibited by 5-LOX metabolites, which suggest that 5-LOX activity regulates survival of prostate cancer cells via PKCε (Fig. 5A,B). To rule out the possibility of any off-target effect of MK591 on PKCε activity, we tested direct effect of MK591 on isolated PKCε from LNCaP cells, which showed that MK591 does not affect the activity of pure PKCε enzyme by direct interaction (Fig. 6A). Finally, prevention of 5-LOX inhibition-induced apoptosis by chemical as well as specific peptide-activators of PKCε supports the concept that the survival-promoting effects of 5-LOX in prostate cancer cells are mediated, at least partially, via signaling through a PKCε-dependent mechanism (Fig. 6B).
Based on the report of Aziz et al. [42], and our current work, it appears that PKCε is continuously active in prostate tumor tissues and in prostate cancer cells in culture. However, how PKCε maintains continuous activity in prostate cancer cells is an intriguing but unanswered question. A generally accepted paradigm for the activation of PKCε involves two sequential steps. In the first step, PKCε gets phosphorylated at a threonine residue at the activation loop by phospholipid-dependent kinase1 (PDK1) which triggers autophosphorylation on a threonine residue in the turn motif and serine residues in the hydrophobic motif [37]. In un-stimulated cells, the mature triple-phosphorylated form of PKCε stays in the cytosol where the pseudo-substrate region blocks its active site and the enzyme remains in an auto-inhibited state. Subsequent activation of PKCε depends on binding with a lipid second messenger, such as diacylglycerol (DAG), in the membrane which allows PKCε to become active and phosphorylate target substrates. However, upstream signals that may feed PKCε for its continuous activity in prostate cancer cells are yet to be characterized. Earlier, we reported that prostate cancer cells continuously generate 5-LOX metabolites from arachidonic acid [9,12], which may ideally provide the signals needed for continuous action of PKCε. However, the mechanism how 5-LOX metabolites may activate PKCε in prostate cancer cells is still uncharacterized. It was observed that prostate cancer cells express both the mRNA and protein of OXER1 [[46], and Sarveswaran et al.; Unpublished observations], a G protein-coupled receptor (GPCR), for which the 5-LOX metabolites 5-oxoETE and to a lesser extent 5(S)-HETE serve as ligands [47,48]. These findings led us to hypothesize that 5-LOX metabolites may provide survival signaling to prostate cancer cells via the GPCR, OXER1, and consequent production of diacylglycerol (DAG) to activate PKCε. Further work is undergoing to test this hypothesis.
Inhibition of PKCε in prostate cancer cells by treatment with the 5-LOX inhibitor MK591, its reversal by 5-oxoETE, and prevention of MK591-induced apoptosis by chemical as well as peptide activators of PKCε indicate that 5-LOX inhibition-induced apoptosis in prostate cancer cells occur via inhibition of PKCε, and suggest that the survival-promoting effects of arachidonate 5-LOX in prostate cancer cells are mediated via PKCε. Thus, a fundamental mechanism of cell survival has been uncovered which is continuously active in prostate cancer cells. Regulation of PKCε activity in prostate cancer cells by inhibitors and metabolites of 5-LOX opens up a new avenue to explore activation mechanisms of PKCε in prostate and other types of cancer cells. PKCε is a transforming oncogene which is well characterized to promote cell survival by regulating Bcl-2 family members, such as Bcl-2, Bad and Bax, and to increase resistance to apoptosis-inducing agents [38,39,49–52]. Moreover, a recent gene-ablation study revealed that PKCε plays an important role in prostate cancer development and metastasis in transgenic mice [53]. Cancer-specific expression of 5-LOX in prostate epithelial cells together with a critical role of 5-LOX in the survival of prostate cancer cells suggests for a pivotal and possibly an indispensable role of 5-LOX in the development and progression of prostate cancer. Thus, 5-LOX is emerging as a promising, novel target for prostate cancer therapy. Since arachidonic acid is a common fatty acid in “Western-diets” (where prostate cancer is also more common), and 5-LOX is highly expressed in prostate cancer cells, our present findings suggest that metabolism of arachidonic acid via 5-LOX may greatly contribute to the pathobiology of prostate cancer by enhancing the protean effects of PKCε and its downstream oncogenic signaling.
Acknowledgements
This work was supported in part by the United States Department of Defense Prostate Cancer Research Programs, DAMD 17-02-1-0153 and W81XWH-05-1-0022, and a Henry Ford Health System internal research grant A-10203. We thank Dr. K.R. Maddipati (Wayne State University) for providing kind help with LC/MS/MS, and Dr. Steve Harrison (KAI Pharma, San Francisco, CA) for generously providing peptide activator (KAE1-1) and inhibitor (KIE1-1) of PKCε.
Abbreviations
- 5-LOX
5-Lipoxygenase
- 5-oxoETE
5-oxoeicosatetraenoic acid
- PKCε
Protein Kinase C-epsilon
- PARP
poly-ADP ribose polymerase
- ELISA
enzyme-linked immunosorbent assay
Footnotes
Publisher's Disclaimer: This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier's archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J. Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Crawford ED, Eisenberger MA, McLeod DG, Spaulding JT, Benson R, Dorr FA, Blumenstein BA, Davis MA, Goodman PJ. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N. Engl. J. Med. 1989;321:419–424. doi: 10.1056/NEJM198908173210702. [DOI] [PubMed] [Google Scholar]
- 3.Fleshner N, Bagnell PS, Klotz L, Venkateswaran V. Dietary fat and prostate cancer. J. Urol. 2004;171:19–24. doi: 10.1097/01.ju.0000107838.33623.19. [DOI] [PubMed] [Google Scholar]
- 4.Kolonel LN, Nomura AN, Cooney RV. Dietary fat and prostate cancer: current status. J. Natl. Cancer Inst. 1999;91:414–428. doi: 10.1093/jnci/91.5.414. [DOI] [PubMed] [Google Scholar]
- 5.West DW, Slattery ML, Robison LM, French TK, Mahoney AW. Adult dietary intake and prostate cancer risk in Utah: a case control study with special emphasis on aggressive tumors. Cancer Causes Control. 1991;2:85–94. doi: 10.1007/BF00053126. [DOI] [PubMed] [Google Scholar]
- 6.Giovannucci EL, Rimm EB, Colditz GA, Stampfer MJ, Ascherio A, Chute C, Willett WC. A prospective study of dietary fat and risk of prostate cancer. J. Natl. Cancer Inst. 1993;85:1571–1579. doi: 10.1093/jnci/85.19.1571. [DOI] [PubMed] [Google Scholar]
- 7.Gann PH, Hennekens CH, Sacks FM, Grodstein F, Giovannucci EL, Stampfer MJ. Prospective study of plasma fatty acids and risk of prostate cancer. J. Natl. Cancer Inst. 1994;86:281–286. doi: 10.1093/jnci/86.4.281. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Corr JG, Thaler HT, Tao Y, Fair WR, Heston WD. Decreased growth of established prostate LNCaP tumors in nude mice fed a low-fat diet. J. Natl. Cancer Inst. 1995;87:1456–1462. doi: 10.1093/jnci/87.19.1456. [DOI] [PubMed] [Google Scholar]
- 9.Ghosh J, Myers CE. Arachidonic acid stimulates prostate cancer cell growth: critical role of 5-lipoxygenase. Biochem. Biophys. Res. Commun. 1997;235:418–423. doi: 10.1006/bbrc.1997.6799. [DOI] [PubMed] [Google Scholar]
- 10.Ghosh J, Myers CE. Central role of arachidonate 5-lipoxygenase in the regulation of cell growth and apoptosis in human prostate cancer cells. Adv. Exp. Med. Biol. 1999;469:577–582. doi: 10.1007/978-1-4615-4793-8_84. [DOI] [PubMed] [Google Scholar]
- 11.Anderson KM, Seed T, Vos M, Mulshine J, Meng J, Alrefai W, Ou D, Harris JE. 5-Lipoxygenase inhibitors reduce PC-3 cell proliferation and initiate nonnecrotic cell death. Prostate. 1998;37:161–173. doi: 10.1002/(sici)1097-0045(19981101)37:3<161::aid-pros5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 12.Ghosh J, Myers CE. Inhibition of arachidonate 5-lipoxygenase triggers massive apoptosis in human prostate cancer cells. Proc. Natl. Acad. Sci. U. S. A. 1998;95:13182–13187. doi: 10.1073/pnas.95.22.13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh J. Inhibition of arachidonate 5-lipoxygenase triggers prostate cancer cell death through rapid activation of c-Jun N-terminal kinase. Biochem. Biophys. Res. Commun. 2003;307:342–349. doi: 10.1016/s0006-291x(03)01201-4. [DOI] [PubMed] [Google Scholar]
- 14.Werz O, Steinhilber D. Therapeutic options for 5-lipoxygenase inhibitors. Pharmacol. Ther. 2006;112:701–718. doi: 10.1016/j.pharmthera.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Radmark O, Werz O, Steinhilber D, Samuelsson B. 5-Lipoxygenase: regulation of expression and enzyme activity. Trends Biochem. Sci. 2007;32:332–341. doi: 10.1016/j.tibs.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Furstenberger G, Krieg P, Muller-Decker K, Habenicht AJ. What are cyclooxygenases and lipoxygenases doing in the driver's seat of carcinogenesis? Int. J. Cancer. 2006;119:2247–2254. doi: 10.1002/ijc.22153. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh J. Targeting 5-lipoxygenase for prevention and treatment of cancer. Curr. Enzyme Inhib. 2008;4:18–28. [Google Scholar]
- 18.Wang D, DuBois RN. Eicosanoids and cancer. Nat. Rev. Cancer. 2010;10:181–193. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez-Periz A, Claria J. New approaches to the modulation of the cyclooxygenase-2 and 5-lipoxygenase pathways. Curr. Top. Med. Chem. 2007;7:297–309. doi: 10.2174/156802607779941378. [DOI] [PubMed] [Google Scholar]
- 20.Gupta S, Srivastava M, Ahmad N, Sakamoto K, Bostwick DG, Mukhtar H. Lipoxygenase-5 is overexpressed in prostate adenocarcinoma. Cancer. 2001;91:737–743. doi: 10.1002/1097-0142(20010215)91:4<737::aid-cncr1059>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 21.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 23.Marte BM, Downward J. PKB/Akt: connecting phosphoinositide3-kinase to cell survival and beyond. Trends Biochem. Sci. 1997;22:355–358. doi: 10.1016/s0968-0004(97)01097-9. [DOI] [PubMed] [Google Scholar]
- 24.Altomare DA, Testa JR. Perturbations of the Akt signaling pathway in human cancer. Oncogene. 2005;24:7455–7464. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- 25.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat. Rev. Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 26.Balmanno K, Cook SJ. Tumour cell survival signalling by the ERK1/2 pathway. Cell Death Differ. 2009;16:368–377. doi: 10.1038/cdd.2008.148. [DOI] [PubMed] [Google Scholar]
- 27.Boutros T, Chevet E, Metrakos P. Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: roles in cell growth, death, and cancer. Pharmacol. Rev. 2008;60:261–310. doi: 10.1124/pr.107.00106. [DOI] [PubMed] [Google Scholar]
- 28.Mebratu Y, Tesfaigzi Y. How ERK1/2 activation controls cell proliferation and cell death: is subcellular localization the answer? Cell Cycle. 2009;8:1168–1175. doi: 10.4161/cc.8.8.8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarveswaran S, Myers CE, Ghosh J. MK591, a leukotriene biosynthesis inhibitor, induces apoptosis in prostate cancer cells: synergistic action with LY294002, an inhibitor of phosphatidylinositol 3′-kinase. Cancer Lett. 2010;291:167–176. doi: 10.1016/j.canlet.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 30.Ferguson AD, McKeever BM, Xu S, Wisniewski D, Miller DK, Yamin TT, Spencer RH, Chu L, Ujjainwalla F, Cunningham BR, Evans JF, Becker JW. Crystal structure of inhibitor-bound human 5-lipoxygenase-activating protein. Science. 2007;317:510–512. doi: 10.1126/science.1144346. [DOI] [PubMed] [Google Scholar]
- 31.Evans JF, Ferguson AD, Mosley RT, Hutchinson JH. What's all the FLAP about?: 5-lipoxygenase-activating protein inhibitors for inflammatory diseases. Trends Pharmacol. Sci. 2008;29:72–78. doi: 10.1016/j.tips.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Basu A, Sivaprasad U. Protein kinase Cε makes the life and death decision. Cell. Signal. 2007;19:1633–1642. doi: 10.1016/j.cellsig.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sivaprasad U, Shankar E, Basu A. Downregulation of Bid is associated with PKCepsilon-mediated TRAIL resistance. Cell Death Differ. 2007;14:851–860. doi: 10.1038/sj.cdd.4402077. [DOI] [PubMed] [Google Scholar]
- 34.Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9:484–496. [PubMed] [Google Scholar]
- 35.Mellor H, Parker PJ. The extended protein kinase C superfamily. Biochem. J. 1998;332:281–292. doi: 10.1042/bj3320281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newton AC. Protein kinase C: structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem. Rev. 2001;101:2353–2364. doi: 10.1021/cr0002801. [DOI] [PubMed] [Google Scholar]
- 37.Griner EM, Kazanietz MG. Protein kinase C and other diacylglycerol effectors in cancer. Nat. Rev. Cancer. 2007;7:281–294. doi: 10.1038/nrc2110. [DOI] [PubMed] [Google Scholar]
- 38.Gavrielides MV, Frijhoff AF, Conti CJ, Kazanietz MG. Protein kinase C and prostate carcinogenesis: targeting the cell cycle and apoptotic mechanisms. Curr. Drug Targets. 2004;5:431–443. doi: 10.2174/1389450043345380. [DOI] [PubMed] [Google Scholar]
- 39.McJilton MA, Sikes CV, Wescott GG, Wu D, Foreman TL, Gregory CW, Weidner DA, Ford OH, Lasater AM, Mohler JL, Terrian DM. Protein kinase Cε interacts with Bax and promotes survival of human prostate cancer cells. Oncogene. 2003;22:7958–7968. doi: 10.1038/sj.onc.1206795. [DOI] [PubMed] [Google Scholar]
- 40.Maddipati KR, Zhou SL. Stability and analysis of eicosanoids and docosanoids in tissue culture media. Prostaglandins Other Lipid Mediat. 2011;94:59–72. doi: 10.1016/j.prostaglandins.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 41.Horoszewicz JS, Leong SS, Chu TM, Wajsman ZL, Friedman M, Papsidero L, Kim U, Chai LS, Kakati S, Arya SK, Sandberg AA. The LNCaP cell line—a new model for studies on human prostatic carcinoma. Prog. Clin. Biol. Res. 1980;37:115–132. [PubMed] [Google Scholar]
- 42.Aziz MH, Manoharan HT, Church DR, Dreckschmidt NE, Zhong W, Oberley TD, Wilding G, Verm AK. Protein kinase C-ε interacts with signal transducers and activators of transcription 3 (Stat3), phosphorylates Stat3-Ser727, and regulates its constitutive activation in prostate cancer. Cancer Res. 2007;67:8828–8838. doi: 10.1158/0008-5472.CAN-07-1604. [DOI] [PubMed] [Google Scholar]
- 43.Kim MY, Kim MJ, Yoon IS, Ahn JH, Lee SH, Baik EJ, Moon CH, Jung YS. Diazoxide acts more as a PKC-ε activator, and indirectly activates the mitochondrial KATP channel conferring cardioprotection against hypoxic injury. Br. J. Pharmacol. 2006;149:1059–1070. doi: 10.1038/sj.bjp.0706922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dorn GW, Souroujon MC, Liron T, Chen CH, Gray MO, Zhou HZ, Csukai M, Wu G, Lorenz JN, Mochly-Rosen D. Sustained in vivo cardiac protection by a rationally designed peptide that causes ε protein kinase C translocation. Proc. Natl. Acad. Sci. U. S. A. 1999;96:12798–12803. doi: 10.1073/pnas.96.22.12798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carson JP, Kulik G, Weber MJ. Antiapoptotic signaling in LNCaP prostate cancer cells: a survival signaling pathway independent of phosphatidylinositol 3′-kinase and Akt/Protein Kinase B. Cancer Res. 1999;59:1449–1453. [PubMed] [Google Scholar]
- 46.Sundaram S, Ghosh J. Expression of 5-oxoETE receptor in prostate cancer cells: critical role in survival. Biochem. Biophys. Res. Commun. 2006;339:93–98. doi: 10.1016/j.bbrc.2005.10.189. [DOI] [PubMed] [Google Scholar]
- 47.Hosoi T, Koguchi Y, Sugikawa E, Chikada A, Ogawa K, Tsuda N, Suto N, Tsunoda S, Taniguchi T, Ohnuki T. Identification of a novel human eicosanoid receptor coupled to G(i/o) J. Biol. Chem. 2002;277:31459–31465. doi: 10.1074/jbc.M203194200. [DOI] [PubMed] [Google Scholar]
- 48.Jones CE, Holden S, Tenaillon L, Bhatia U, Seuwen K, Tranter P, Turner J, Kettle R, Bouhelal R, Charlton S, Nirmala NR, Jarai G, Finan P. Expression and characterization of a 5-oxo-6E,8Z,11Z,14Z-eicosatetraenoic acid receptor highly expressed on human eosinophils and neutrophils. Mol. Pharmacol. 2003;63:471–477. doi: 10.1124/mol.63.3.471. [DOI] [PubMed] [Google Scholar]
- 49.Felber M, Sonnemann J, Beck JF. Inhibition of novel protein kinase C-epsilon augments TRAIL-induced cell death in A549 lung cancer cells. Pathol. Oncol. Res. 2007;13:295–301. doi: 10.1007/BF02940308. [DOI] [PubMed] [Google Scholar]
- 50.Flescher E, Rotem R. Protein kinase Cε mediates the induction of P-glycoprotein in LNCaP prostate carcinoma cells. Cell. Signal. 2002;14:37–43. doi: 10.1016/s0898-6568(01)00215-7. [DOI] [PubMed] [Google Scholar]
- 51.Gorin MA, Pan Q. Protein kinase Cε: an oncogene and emerging tumor biomarker. Mol. Cancer. 2009;8:9. doi: 10.1186/1476-4598-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu D, Foreman TL, Gregory CW. Protein kinase Cε has the potential to advance the recurrence of human prostate cancer. Cancer Res. 2002;62:2423–2429. [PubMed] [Google Scholar]
- 53.Hafeez BB, Zhong W, Weichert J, Dreckschmidt NE, Jamal MS, Verma AK. Genetic ablation of PKC epsilon inhibits prostate cancer development and metastasis in transgenic mouse model of prostate adenocarcinoma. Cancer Res. 2011;71:2318–2327. doi: 10.1158/0008-5472.CAN-10-4170. [DOI] [PMC free article] [PubMed] [Google Scholar]