Abstract
Objectives
To assess the impact of exposure to evidence-based medication following hospital discharge for Medicare beneficiaries with acute myocardial infarction (AMI).
Design
A discrete-time hazard model was used to estimate time-to-outcome associated with exposure to four drug classes (angiotensin-converting enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs), beta-blockers (BBs), statins, and clopidogrel) used for post-AMI secondary prevention of cardiovascular events and mortality.
Setting
Medicare administrative data for a 5% random sample of beneficiaries.
Participants
Medicare beneficiaries (n=9,538) hospitalized for an AMI between 4/1/2006 and 12/31/2007 who survived for at least 30 days after discharge. The cohort was followed until death or 12/31/2008.
Measurements
Time-varying exposure was measured as proportion of days covered (PDC) for each quarter during the follow-up period. PDC was classified into five categories (0–0.2; >0.2–0.4; >0.4–0.6; >0.6–0.8; >0.8–1.0). Outcomes were mortality and a composite outcome of death or post-AMI hospitalization.
Results
Over a median follow-up of 18 months, mean PDC rates ranged from 0.37 (clopidogrel) to 0.50 (statins). When comparing the highest versus lowest categories of exposure, the hazard of the composite outcome was significantly lower for all drug classes except BBs [statins, adjusted hazard ratio (aHR) = 0.71, ACEIs/ARBs, aHR = 0.81, clopidogrel, aHR = 0.85, BBs, aHR = 0.93]. All four drug classes were significantly associated with reductions in mortality; the magnitude of effect for the mortality outcome was largest for statins and smallest for BBs. Age modified the effect of statins on mortality.
Conclusion
Use of evidence-based medications for secondary prevention post-AMI is suboptimal in the Medicare population and low exposure rates are associated with significantly higher risk for subsequent hospitalization and death.
Keywords: Myocardial infarction, Medicare, Pharmacotherapy, Medicare Part D, Secondary prevention
INTRODUCTION
Coronary artery disease is a major cause of morbidity and the leading cause of death in older adults. In the United States, approximately 800,000 adults over the age of 65 years suffer an acute myocardial infarction (AMI) or fatal coronary heart disease each year.1 Over the past several decades, advances in the medical treatment of coronary heart disease have resulted in a significant decline in hospital and short-term mortality.2–4 Numerous clinical trials have demonstrated the efficacy of HMG-CoA reductase inhibitors (statins), beta-blockers (BBs), angiotensin-converting enzyme inhibitors (ACEIs), angiotensin-II receptor blockers (ARBs), and antiplatelet agents such as clopidogrel for secondary prevention in patients who have suffered an AMI.5–10 Use of these evidence-based medications is now a cornerstone of long-term medical therapy in this patient population.11–14
Despite encouraging decreases in population death rates from coronary heart disease and hospital mortality after an AMI in the US, older adults remain at increased risk for adverse outcomes after hospitalization for AMI. Pooled data from the Framingham Heart Study, the Atherosclerosis Risk in Communities study, and the Cardiovascular Health Study of the National Heart, Lung, and Blood Institute demonstrate that patients over the age of 65 who survive an AMI have a substantial risk of recurrent AMI, sudden death, chronic heart failure (CHF), or stroke. Specifically, these studies show that within five years of a first AMI, 22% of patients over 65 years old will have another infarction; 28–54% will die; 20–23% will develop CHF; and 5–8% will suffer a stroke.1
Use of evidence-based pharmacotherapy for secondary prevention is associated with improvements in post-AMI outcomes.15–18 Unfortunately, evidence suggests these medications are neither consistently prescribed when appropriate, nor consistently adhered to by patients.19–24 Studies evaluating secondary prevention commonly focus on a single medication class, and outcomes such as mortality are investigated only up to one year post-AMI.9,15,17,25–29 Thus, there are limited data documenting the long-term impact on post-AMI outcomes when patients do not receive or adhere to evidence-based treatment regimens.30,31 The purpose of this study was to examine the effect of patient exposure to four key evidence-based medication classes (statins, BBs, ACEIs/ARBs, and clopidogrel) on a composite outcome of post-AMI hospitalization or all-cause death as well as on mortality alone within the time period of up to 33 months after hospital discharge for first AMI. We also examined whether the relationship between use of these four drug classes and outcomes varied by patient age.
METHODS
Study Population
The study cohort was selected from a 5% simple random sample of Medicare beneficiaries with a discharge diagnosis of AMI (ICD-9 410.xx) in the first or second position on an inpatient claim between April 1, 2006 and December 31, 2007 (the ‘index AMI’) who survived at least 30 days after discharge. To assure complete data capture, we required all subjects to have continuous coverage for Medicare Parts A, B, and D during the study period. Individuals with an AMI diagnosis on a Medicare claim prior to April 2006 or a missing value for discharge date were excluded. We also excluded beneficiaries enrolled in capitated Medicare Advantage plans (Part C) as these plans do not submit claims data to the Centers for Medicare & Medicaid Services. As a result, all analyses were restricted to beneficiaries enrolled in stand-alone, fee-for-service Medicare and prescription drug plans. Subjects were tracked from 3 months prior to their index AMI hospitalization until death or the end of the study period, defined as December 31, 2008.
Measures
The two endpoints of interest were: (a) a composite outcome of post-AMI hospitalization for recurrent AMI, stroke, transient ischemic attack (TIA), CHF, or all-cause death; and (b) a separate measure of mortality alone. Nonfatal endpoints were identified in the first position on inpatient hospital claims. To minimize bias introduced by competing risks, the first post-AMI event was identified as a composite endpoint that represented the earliest of the outcomes described above. Time-to-first-endpoint was measured in months.
The primary independent variable was a measure of drug exposure defined as the monthly proportion of days covered (PDC) for ACEIs/ARBs, BBs, statins, and clopidogrel from January 2006 through death or the end of the study period. The monthly PDC measures for each drug class were based upon refill records and were calculated by totaling the number of days during the month when patients had a medication from the drug class available and dividing by the total number of days within the month. Thus, PDC ranged from 0 to 1, with 0 representing no drug use and 1 representing drug use on every day within the month. Since drug exposure may change over time and to capture the most relevant medication utilization pattern, time-varying PDCs were calculated for each month that captured the average PDC of the prior quarter (three months). Drugs were identified by their National Drug Code in the Part D Prescription Drug Event File using the First DataBank drug dictionary (Hearst Corporation, San Francisco, CA).
Covariates included baseline characteristics in 2006 such as age, sex, race, region, low-income subsidy (LIS) status, selected chronic and cardiovascular conditions likely to impact use of the four drug classes under review; AMI severity measures such as length of index AMI hospital stay and history of any hospitalization during the 3-month period before the index AMI; treatment characteristics such as evidence of transfer to another hospital within one day of the index AMI admission or percutaneous coronary interventions during index AMI hospital stay; and pre-index AMI measures including any use of medications in the four drug classes and number of unique chronic medications taken in the 3-month period prior to index AMI.
Statistical analysis
We estimated separate sets of maximum partial likelihood discrete-time hazard models for the composite (i.e. time-to-first-endpoint) and mortality outcomes. Models 1 through 4 analyzed the effect of exposure to each drug class individually, while Model 5 included all four drug classes in the analysis. The rationale for estimating Model 5 is that any unobserved behavior common to drug exposure across classes is removed (covaried out) from the estimated drug effects. A common problem in observational studies that evaluate drug effects on clinical outcomes is that individuals who are more likely to use and be adherent with the drug may have better outcomes independent of the drug effect if exposure is associated with unobserved healthy behaviors. Including several drugs as explanatory variables in the same model is one way to control for such healthy behavior bias.
For all models, we included timing-varying PDCs for the prior three months for each drug class of interest as the primary independent variables, together with baseline characteristics for adjusted models. As the association of increasing exposure with the outcomes was found to be non-linear, patients were stratified into one of five categories of exposure for each medication class (Category 1 PDC = 0.0–0.2; Category 2 PDC = >0.2–0.4; Category 3 PDC = >0.4–0.6; Category 4 PDC = >0.6–0.8; Category 5 PDC = >0.8–1.0), with Category 1 used as the reference category. To test for the effect modification of age on the relationship between drug use and outcomes, we included age-by-exposure interaction terms in the adjusted models. A likelihood ratio (LR) test was used to estimate the significance of the interaction terms; the null hypothesis is that there was no interaction. A nonsignificant LR test would mean that the interaction terms can be removed from the model (that there was no modifying effect of age on drug exposure and outcomes).
Several features were considered with these models. First, discrete approximations were used for handling datasets with outcomes that occur at the same time (tied data). In the context that all PDC values and outcomes were measured at monthly intervals, all time variables can only take on integer values and discrete-time Cox models naturally become most suitable for the analyses. Second, since exposure changes over time, time-dependent covariates included in the models enabled the estimation of the effect of most recent medication utilization pattern on the outcome. Third, models estimating the composite outcome minimized the bias possibly caused by competing risks, that is, an outcome (e.g., death) that occurs before the outcome of interest (e.g., recurrent AMI). Finally, models estimating the mortality outcome can quantify the effect of drug exposure on the most clinically severe prognosis, therefore serving as a sensitivity analysis for the composite outcome models. All regression models were estimated using SAS 9.2 (SAS Institute, Inc., Cary, NC). The study design was approved by the University of Maryland, Baltimore Institutional Review Board.
RESULTS
Characteristics of the study sample
Between 2006 and 2008, 9,583 Medicare beneficiaries met the sample inclusion criteria. Characteristics of these beneficiaries are shown in Table 1. The sample was predominantly white (83.8%) and female (64.7%); more than half (56.7%) were over 74 years of age, with a high concentration residing in the South (41.8%). More than half of the sample beneficiaries (51.8%) received the Part D low-income subsidy (LIS), primarily through dual (Medicare/Medicaid) eligibility. Hypertension was the most prevalent comorbid condition (86.9%), followed by ischemic heart disease (78.9%), hyperlipidemia (66.6%), CHF (55.3%), and diabetes mellitus (47.4%).
Table 1.
Characteristics of the study sample (n=9,583)
| Characteristics | N | Percent |
|---|---|---|
| Sample Size | 9,583 | 100 |
| Age (%) | ||
| <65 | 1,239 | 12.9 |
| 65–74 | 2,910 | 30.4 |
| 75–84 | 3,418 | 35.7 |
| 85+ | 2,016 | 21.0 |
| Sex (%) | ||
| Female | 6,200 | 64.7 |
| Male | 3,383 | 35.3 |
| Race (%) | ||
| White | 8,029 | 83.8 |
| Black | 1,002 | 10.5 |
| Hispanic | 245 | 2.6 |
| Asian | 147 | 1.5 |
| Other | 160 | 1.7 |
| Region (%) | ||
| Northeast | 1,707 | 17.8 |
| North Central | 2,566 | 26.8 |
| South | 4,005 | 41.8 |
| West | 1,283 | 13.4 |
| Other | 22 | 0.2 |
| Low-income subsidy (LIS) status (%) | ||
| No LIS | 4,619 | 48.2 |
| LIS Dual Medicare-Medicaid | 4,395 | 45.9 |
| LIS non-Dual | 569 | 5.9 |
| Comorbidities (%) | ||
| Atrial fibrillation | 1,996 | 20.8 |
| Alzheimer’s or related dementia | 2,082 | 21.7 |
| Chronic heart failure | 5,297 | 55.3 |
| Chronic kidney disease | 3,114 | 32.5 |
| COPD | 3,416 | 35.6 |
| Diabetes | 4,539 | 47.4 |
| Ischemic heart disease | 7,562 | 78.9 |
| Valvular heart disease | 2,810 | 29.3 |
| Stroke/TIA | 2,211 | 23.1 |
| Idiopathic cardiomyopathy | 1,164 | 12.1 |
| Hypertension | 8,327 | 86.9 |
| Hyperlipidemia | 6,378 | 66.6 |
| Peripheral vascular disease | 3,078 | 32.1 |
| Disease severity (%) | ||
| Any use of drug/non-drug eluting stent in index AMI | 2,783 | 29.0 |
| NSTEMI | 6,543 | 68.3 |
| Transfer or readmission within 1 day of the index AMI admission | 1,121 | 11.7 |
| Evidence of any hospitalization within 3 months before index AMI | 1,756 | 18.3 |
| Any use of ACE-inhibitors/ARBs within 3 months before index AMI | 4,526 | 47.2 |
| Any use of Beta-blockers within 3 months before index AMI | 2,868 | 29.9 |
| Any use of statins within 3 months before index AMI | 3,262 | 34.0 |
| Any use of clopidogrel within 3 months before index AMI | 1,353 | 14.1 |
Abbreviations: COPD, chronic obstructive pulmonary disease; TIA, transient ischemic attack; AMI, acute myocardial infarction; ACE-inhibitors, angiotensin-converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; NSTEMI, non-ST elevation myocardial infarction.
Nearly one-fifth (18.3%) of the cohort had a hospitalization in the 3 months prior to the index AMI. The mean count of chronic medication use was 5.4 within 3 months before the index AMI. During the index AMI hospitalization, 29.0% received a coronary artery stent, and 11.7% were transferred to another hospital within a day of the index AMI admission. The average length of stay for the index AMI hospitalization, including transfers to other hospitals, was 8.0 days. The median follow-up time after the index AMI hospital discharge was 18.0 months (range 0.07 to 33.0 months). One-third (33.0%) of the sample died and 52.2 % experienced at least one of the outcomes of interest during the follow-up period.
Patterns of exposure to study medications over time
Within one month after hospital discharge for the index AMI, more than half of the subjects filled prescriptions for ACEIs/ARBs (59%), BBs (51%), and statins (54%), while less than half (46%) filled prescriptions for clopidogrel (Figure 1). During the follow-up period, the prevalence of use of ACEIs/ARBs, BBs, and statins remained relatively stable with less than 7% change over time. By contrast, the prevalence of clopidogrel use peaked in the second month after discharge but then showed a slow and steady decline.
Figure 1.
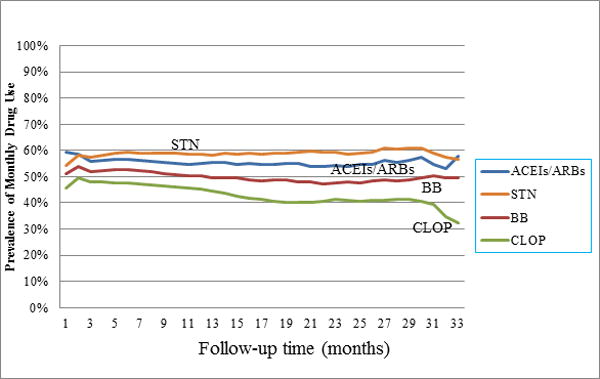
Trend of prevalence of use of study medications during follow-up
Abbreviations: ACE, angiotensin-converting enzyme inhibitor; ARB, antiotensin-II receptor blocker; BB, beta-blockers; STN, statins; CLOP, clopidogrel.
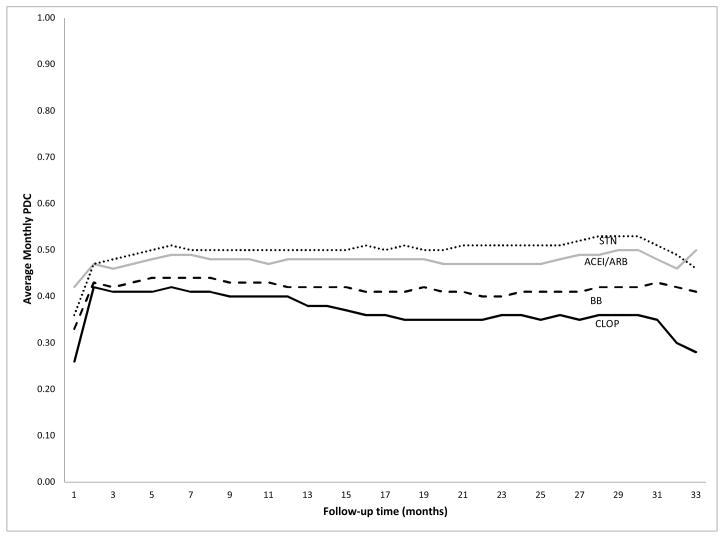
The PDCs of the four study medication classes showed similar patterns of change during the follow-up period (Figure 2). Exposure to statins was highest among the four study medication classes, followed by ACEIs/ARBs. Clopidogrel had the lowest exposure, as measured by PDC. The average quarterly PDCs during follow-up for statins, ACEIs/ARBs, BBs, and clopidogrel were 0.50, 0.48, 0.42, and 0.37, respectively. The trends reflect stable use over the entire follow-up period with similar numbers of subjects initiating and discontinuing drug use each month.
Figure 2.
Trend of monthly PDC of study medications during follow-up
Abbreviations: PDC, proportion of days covered; ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor blockers; BB, beta-blockers; STN, statins; CLOP, clopidogrel.
Multivariable analysis
Composite outcome
When the four study medication classes were analyzed individually (Table 2, Models 1 through 4), for each class the hazard of the composite outcome was significantly lower in subjects exhibiting the greatest exposure (PDC Category 5) compared to subjects in the lowest exposure category (PDC Category 1), with adjusted hazard ratios ranging from 0.64 (statins) to 0.83 (beta-blockers). Statins showed the most protective effect, and the effect increased as exposure increased. Beta-blockers had the smallest effect on reducing the hazard of the composite outcome compared to the other drug classes.
Table 2.
Multivariable analysis of the association of PDC with composite outcome estimated by discrete-time Cox proportional hazards regression
| Model† | Most Recent 3 Month PDCa | Unadjusted HR | 95% C.I. | Adjusted HRb | 95% C.I. |
|---|---|---|---|---|---|
| 1 | Beta-Blockers | ||||
| PDC Category 2 | 1.10 | 0.99–1.23 | 1.02 | 0.92–1.14 | |
| PDC Category 3 | 1.14 | 1.01–1.28 | 0.99 | 0.87–1.11 | |
| PDC Category 4 | 1.05 | 0.95–1.16 | 0.90 | 0.81–1.01 | |
| PDC Category 5 | 0.94 | 0.88–1.01 | 0.83 | 0.76–0.90 | |
| 2 | ACEIs/ARBs | ||||
| PDC Category 2 | 1.09 | 0.98–1.21 | 1.04 | 0.93–1.15 | |
| PDC Category 3 | 1.04 | 0.92–1.17 | 0.94 | 0.83–1.07 | |
| PDC Category 4 | 1.01 | 0.91–1.11 | 0.90 | 0.81–1.00 | |
| PDC Category 5 | 0.78 | 0.72–0.83 | 0.74 | 0.68–0.80 | |
| 3 | Statins | ||||
| PDC Category 2 | 0.74 | 0.66–0.82 | 0.86 | 0.77–0.97 | |
| PDC Category 3 | 0.61 | 0.53–0.70 | 0.68 | 0.59–0.78 | |
| PDC Category 4 | 0.69 | 0.62–0.76 | 0.73 | 0.65–0.82 | |
| PDC Category 5 | 0.60 | 0.55–0.64 | 0.64 | 0.59–0.70 | |
| 4 | Clopidogrel | ||||
| PDC Category 2 | 0.73 | 0.64–0.83 | 0.88 | 0.77–1.01 | |
| PDC Category 3 | 0.70 | 0.59–0.81 | 0.81 | 0.68–0.95 | |
| PDC Category 4 | 0.80 | 0.71–0.90 | 0.87 | 0.76–0.99 | |
| PDC Category 5 | 0.64 | 0.59–0.69 | 0.75 | 0.68–0.82 | |
| 5 | Beta-Blockers | ||||
| PDC Category 2 | 1.14 | 1.02–1.28 | 1.03 | 0.92–1.15 | |
| PDC Category 3 | 1.21 | 1.08–1.37 | 1.04 | 0.91–1.17 | |
| PDC Category 4 | 1.11 | 1.00–1.24 | 0.96 | 0.86–1.07 | |
| PDC Category 5 | 1.08 | 1.00–1.16 | 0.93 | 0.85–1.01 | |
| ACEIs/ARBs | |||||
| PDC Category 2 | 1.16 | 1.04–1.29 | 1.06 | 0.94–1.18 | |
| PDC Category 3 | 1.13 | 1.00–1.27 | 0.99 | 0.87–1.12 | |
| PDC Category 4 | 1.09 | 0.98–1.20 | 0.95 | 0.85–1.06 | |
| PDC Category 5 | 0.88 | 0.82–0.94 | 0.81 | 0.75–0.88 | |
| Statins | |||||
| PDC Category 2 | 0.75 | 0.66–0.84 | 0.86 | 0.76–0.97 | |
| PDC Category 3 | 0.62 | 0.54–0.71 | 0.70 | 0.60–0.80 | |
| PDC Category 4 | 0.70 | 0.63–0.78 | 0.76 | 0.68–0.86 | |
| PDC Category 5 | 0.66 | 0.61–0.71 | 0.71 | 0.65–0.77 | |
| Clopidogrel | |||||
| PDC Category 2 | 0.75 | 0.65–0.86 | 0.89 | 0.77–1.02 | |
| PDC Category 3 | 0.74 | 0.63–0.87 | 0.86 | 0.73–1.01 | |
| PDC Category 4 | 0.85 | 0.75–0.96 | 0.93 | 0.81–1.06 | |
| PDC Category 5 | 0.74 | 0.68–0.80 | 0.85 | 0.77–0.94 |
Abbreviations: PDC, proportion of days covered; HR, hazard ratio; C.I., confidence interval; ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers. The composite outcome was the first occurrence of either death or hospitalization for one of the following events: recurrent AMI, stroke or transient ischemic attack; chronic heart failure.
PDC Category 1 = PDC 0.0–0.2 (reference); PDC Category 2 = >0.2–0.4; PDC Category 3 = >0.4–0.6; PDC Category 4 => 0.6–0.8; PDC Category 5 => 0.8–1.0
Models 1–5 were adjusted for age, sex, race, region, low-income subsidy status, comorbidities, and disease severity variables listed in Table 1. PDC was treated as a time-varying variable.
When all four study medication classes were included in the same model (Table 2, Model 5), the hazard of the composite outcome remained significantly lower in subjects exhibiting the greatest exposure compared to those in the reference category for all classes except beta-blockers. Exposure to statins was associated with the largest reduction in the adjusted hazard ratio when compared to the other three classes. There was no effect modification of age on exposure and outcomes in any of the composite outcomes models.
Mortality
The impact of drug exposure on post-AMI mortality was analyzed in the same manner as the composite outcome. Similar findings were observed and are noted in Table 3. As with the composite outcome, it was found that when each class was studied individually, the highest exposure category (PDC Category 5) for all four study medication classes was associated with a significant reduction in the hazard of death; in addition, the magnitude of the reduction in mortality was greater than what was observed for the composite outcome (Table 3, Models 1–4). Also of note, while exposure to beta-blockers lost significance when all four classes were analyzed simultaneously for the composite outcome, this was not the case for the mortality outcome, as high exposure (PDC Category 5) to beta-blockers was associated with a significant reduction in the hazard of death (Table 3, Model 5). Exposure to statins was associated with the largest reduction in the hazard of death, with the magnitude of the reduction increasing as exposure improved, similar to what was seen for the composite outcome. Additionally, results of the LR test indicated that the interaction between age and statin exposure was significant (Table 3, Model 3: LR test p<0.03, Model 5: LR test p<0.02), meaning that age significantly modified the survival benefit of statin exposure. Therefore, for Models 3 and 5, age-specific HRs are reported for statins.
Table 3.
Multivariable analysis of the association of PDC with mortality estimated by discrete-time Cox proportional hazards regression
| Model† | Most Recent 3 Month PDCa | Unadjusted HR | 95% C.I. | Adjusted HRb | 95% C.I. |
|---|---|---|---|---|---|
| 1 | Beta-Blockers | ||||
| PDC Category 2 | 1.06 | 0.93–1.21 | 1.04 | 0.91–1.19 | |
| PDC Category 3 | 1.06 | 0.91–1.21 | 0.99 | 0.85–1.14 | |
| PDC Category 4 | 1.03 | 0.91–1.16 | 0.95 | 0.84–1.08 | |
| PDC Category 5 | 0.70 | 0.64–0.77 | 0.66 | 0.60–0.73 | |
| 2 | ACEIs/ARBs | ||||
| PDC Category 2 | 0.99 | 0.87–1.13 | 1.03 | 0.90–1.17 | |
| PDC Category 3 | 0.93 | 0.80–1.07 | 0.91 | 0.78–1.05 | |
| PDC Category 4 | 0.82 | 0.73–0.92 | 0.81 | 0.71–0.92 | |
| PDC Category 5 | 0.52 | 0.48–0.57 | 0.56 | 0.51–0.62 | |
| 3 | Statins | ||||
| Age <65 | |||||
| PDC Category 2 | 1.07 | 0.72–1.54 | 1.22 | 0.82–1.77 | |
| PDC Category 3 | 0.63 | 0.35–1.04 | 0.68 | 0.38–1.13 | |
| PDC Category 4 | 0.70 | 0.46–1.03 | 0.74 | 0.48–1.09 | |
| PDC Category 5 | 0.62 | 0.46–0.84 | 0.69 | 0.51–0.93 | |
| Age 65–74 | |||||
| PDC Category 2 | 0.60 | 0.44–0.81 | 0.68 | 0.49–0.92 | |
| PDC Category 3 | 0.77 | 0.56–1.04 | 0.86 | 0.62–1.15 | |
| PDC Category 4 | 0.69 | 0.53–0.89 | 0.71 | 0.55–0.92 | |
| PDC Category 5 | 0.35 | 0.28–0.43 | 0.38 | 0.30–0.47 | |
| Age 75–84 | |||||
| PDC Category 2 | 0.92 | 0.75–1.13 | 1.01 | 0.81–1.24 | |
| PDC Category 3 | 0.59 | 0.45–0.77 | 0.65 | 0.49–0.84 | |
| PDC Category 4 | 0.66 | 0.53–0.80 | 0.69 | 0.56–0.84 | |
| PDC Category 5 | 0.42 | 0.36–0.49 | 0.47 | 0.40–0.55 | |
| Age >85 | |||||
| PDC Category 2 | 0.64 | 0.48–0.85 | 0.71 | 0.52–0.93 | |
| PDC Category 3 | 0.77 | 0.57–1.02 | 0.81 | 0.59–1.08 | |
| PDC Category 4 | 0.71 | 0.56–0.90 | 0.77 | 0.60–0.97 | |
| PDC Category 5 | 0.46 | 0.39–0.54 | 0.50 | 0.41–0.59 | |
| 4 | Clopidogrel | ||||
| PDC Category 2 | 0.75 | 0.64–0.87 | 1.02 | 0.87–1.20 | |
| PDC Category 3 | 0.54 | 0.44–0.66 | 0.74 | 0.59–0.91 | |
| PDC Category 4 | 0.68 | 0.59–0.79 | 0.90 | 0.76–1.04 | |
| PDC Category 5 | 0.42 | 0.38–0.47 | 0.60 | 0.54–0.68 | |
| 5 | Beta-Blockers | ||||
| PDC Category 2 | 1.09 | 0.95–1.24 | 1.02 | 0.88–1.16 | |
| PDC Category 3 | 1.15 | 0.99–1.33 | 1.04 | 0.89–1.20 | |
| PDC Category 4 | 1.15 | 1.01–1.30 | 1.03 | 0.90–1.17 | |
| PDC Category 5 | 0.93 | 0.84–1.02 | 0.81 | 0.73–0.90 | |
| ACEIs/ARBs | |||||
| PDC Category 2 | 1.05 | 0.92–1.20 | 1.02 | 0.89–1.16 | |
| PDC Category 3 | 1.02 | 0.88–1.18 | 0.93 | 0.80–1.08 | |
| PDC Category 4 | 0.91 | 0.80–1.03 | 0.84 | 0.74–0.95 | |
| PDC Category 5 | 0.68 | 0.61–0.74 | 0.66 | 0.60–0.73 | |
| Statins | |||||
| Age <65 | |||||
| PDC Category 2 | 1.09 | 0.73–1.59 | 1.21 | 0.81–1.75 | |
| PDC Category 3 | 0.71 | 0.40–1.18 | 0.74 | 0.42–1.23 | |
| PDC Category 4 | 0.80 | 0.52–1.17 | 0.81 | 0.53–1.20 | |
| PDC Category 5 | 0.89 | 0.65–1.19 | 0.89 | 0.65–1.21 | |
| Age 65–74 | |||||
| PDC Category 2 | 0.62 | 0.45–0.83 | 0.67 | 0.48–0.91 | |
| PDC Category 3 | 0.85 | 0.61–1.14 | 0.89 | 0.65–1.21 | |
| PDC Category 4 | 0.78 | 0.60–1.00 | 0.77 | 0.59–1.00 | |
| PDC Category 5 | 0.45 | 0.37–0.56 | 0.47 | 0.37–0.58 | |
| Age 75–84 | |||||
| PDC Category 2 | 0.93 | 0.75–1.15 | 0.99 | 0.80–1.22 | |
| PDC Category 3 | 0.63 | 0.47–0.81 | 0.67 | 0.50–0.87 | |
| PDC Category 4 | 0.70 | 0.57–0.86 | 0.73 | 0.59–0.90 | |
| PDC Category 5 | 0.52 | 0.45–0.61 | 0.55 | 0.47–0.65 | |
| Age >85 | |||||
| PDC Category 2 | 0.65 | 0.48–0.86 | 0.69 | 0.51–0.91 | |
| PDC Category 3 | 0.80 | 0.59–1.06 | 0.83 | 0.61–1.11 | |
| PDC Category 4 | 0.76 | 0.59–0.96 | 0.81 | 0.63–1.03 | |
| PDC Category 5 | 0.57 | 0.48–0.67 | 0.60 | 0.49–0.71 | |
| Clopidogrel | |||||
| PDC Category 2 | 0.77 | 0.65–0.90 | 1.00 | 0.85–1.18 | |
| PDC Category 3 | 0.57 | 0.46–0.70 | 0.75 | 0.60–0.92 | |
| PDC Category 4 | 0.74 | 0.63–0.86 | 0.95 | 0.81–1.11 | |
| PDC Category 5 | 0.56 | 0.50–0.63 | 0.75 | 0.67–0.85 |
Abbreviations: PDC, proportion of days covered; HR, hazard ratio; ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin II receptor blockers.
PDC Category 1 = PDC 0.0–0.2 (reference); PDC Category 2 = >0.2–0.4; PDC Category 3 = >0.4–0.6; PDC Category 4 => 0.6–0.8; PDC Category 5 => 0.8–1.0
Models 1–5 were adjusted for age, sex, race, region, low-income subsidy status, comorbidities, and disease severity variables listed in Table 1. PDC was treated as a time-varying variable.
DISCUSSION
Overall, we found low exposure to the four study medication classes, as measured by PDC. While use of these medication classes paralleled previous studies, the magnitude of exposure to these medications was generally lower than in previous studies. 9,20,23,28,29,32–34 For example, we observed lower clopidogrel use than previously reported. 28,35 In our study, clopidogrel exposure peaked after two months post-AMI and declined steadily thereafter. This was not unexpected, as guidelines generally suggest use of clopidogrel for two weeks to at least 12 months in patients with acute myocardial infarction, depending on the type of infarction and coronary stent used. 11–14 While the magnitude of exposure to clopidogrel observed in our study was markedly lower than that observed by Zhu et al, it is important to note that the study populations differed substantially. Specifically, Zhu et al only included patients who had undergone percutaneous coronary intervention (PCI), a patient characteristic which has been shown to be associated with higher rates of clopidogrel exposure. 28,29,35
In addition, use of beta-blockers in our study was similar to previous reports, though exposure to beta-blockers never surpassed a mean PDC of 44%. 32,34 Our finding is lower than the magnitude of exposure to beta-blockers observed in a recent international study; 29 however, it is important to note that study participants were younger in that study than in our study, and access to medications may differ outside of the United States. Notably, studies have shown that with increasing age, adherence to medication decreases.17–19,28
While we found statistically significant mortality and composite outcome risk reduction benefits for all medication classes in the individual drug models (Models 1–4), when all four medication classes were included in the same model (Model 5) the benefit of beta-blockers was only observed for the mortality outcome. The smaller estimated impact of each drug class in Model 5 is consistent with the presence of a healthy behavior effect, which is controlled for in this model specification. The magnitude of the observed mortality risk reduction was largest for statins and smallest for beta-blockers. These findings are similar to those of Rasmussen et al, who found that while both statins and beta-blockers had survival benefits as adherence to each class improved, the magnitude of benefit was larger for statins than for beta-blockers.18 The mortality benefit from beta-blockers seen in patients with the greatest level of exposure (PDC Category 5) was independent of the other three study medication classes. Our findings are consistent with those of multiple large clinical studies, which found that treatment with beta-blockers post-AMI improved survival,36–38 and that beta-blockers afforded an additional decrease in cardiovascular mortality independent of the effect of ACEIs. 39 Our findings support the importance of beta-blocker therapy in elderly post-AMI patients, even with the advent of newer therapies such as ACEIs and clopidogrel.
Except for statins, there were no differential effects of drug exposure by age group. However, statin exposure, as assessed by PDC, consistently showed a greater survival benefit among patients age 65–74 and 75–84, compared to those in the youngest (age<65) and oldest (age >85) age groups.
Our study has some limitations. Specifically, because this analysis was based on administrative data, measurement and ascertainment were limited by information available in such data. We were unable to account for use of aspirin due to its availability over-the-counter. Another limitation was the inability to determine specific reasons for nonuse of, or low exposure to, study medication classes. Unfortunately, we could not distinguish between physician behavior (failing to prescribe the drug) or patient behavior (failure to fill or refill prescriptions). This is a particularly important limitation as it provides little guidance for how best to implement interventions designed to increase medication utilization. Additionally, drug exposure was determined by refill patterns; information on actual ingestion of the medication or actual dose taken was not available. However, exposure as ascertained by prescription refill patterns has been shown to be a valid measure of patient adherence. 40–42 Moreover, we were unable to identify individual patients for whom a study medication may not have been indicated and who were therefore appropriately not receiving a medication. As with any observational study, there is the potential for residual confounding. Although we believe Model 5 addresses potential confounding due to healthy behavior, there are other ways in which patients with higher PDC may be systematically different than patients with lower PDC. For example, it is possible that patients who are responding well to the medications are more likely to continue their therapy (and thus have higher exposure) while patients who are not responding well to the medications are more likely to either stop their medications or have their medications discontinued by their physicians. Finally, although our study sample was drawn from a random sample of Medicare beneficiaries with AMI, our findings may not be generalizable to all Medicare patients with AMI due to study exclusion criteria. Additionally, we found a mortality risk reduction benefit of medication exposure post-AMI across the spectrum of AMI events; this benefit may not translate into a mortality risk reduction benefit for specific types of AMI events, such as non-ST segment elevation or ST-segment elevation AMIs.
Particular strengths of our study include a relatively long post-AMI follow-up period as well as the use of time-varying measures for PDC to account for variations in exposure to cardiovascular medications over time. Another strength of the study is our control for healthy behavior bias. Additionally, our study was conducted using a large nationally representative sample of older adults at proven risk for cardiovascular events given their history of AMI. Finally, the study sample comprised Medicare beneficiaries with drug coverage under Part D, and there is both clinical and policy interest in the impact of Part D on beneficiaries’ health.
Our study adds to the literature by demonstrating that in an older population in a “real-world” setting, increased exposure to evidence-based pharmacotherapy after AMI resulted in beneficial outcomes, specifically reduced mortality risk. Thus, it is reasonable to conclude that efforts to increase patient exposure to indicated pharmacotherapy may in turn have a long-term beneficial effect on patients’ outcomes post-AMI. In fact, a recent study examined the effect of eliminating copayments for cardiac medications (statins, beta-blockers, and ACEIs/ARBs) on adherence and outcomes in patients with AMI and found that among those who had copayments waived, adherence to each medication class increased significantly and the rates of vascular events or revascularization decreased significantly.43 In another study, clopidogrel discontinuation patterns were found to be sensitive to costs, with decreasing clopidogrel copayments resulting in improved adherence to clopidogrel and fewer post-AMI hospitalizations.44 Based on these findings, future interventional studies of post-AMI outcomes designed to eliminate barriers to medication use across the full spectrum of evidence-based cardiac medications appear warranted.
Acknowledgments
We acknowledge Ms. Loreen Walker, Ms. Christine Franey and Mr. James Gardner of Pharmaceutical Research Computing for managing the Medicare database. Funding for this study was provided by GlaxoSmithKline.
Funding sources: Funding for this study was provided by GlaxoSmithKline. Dr. Rattinger was supported by NIH Institutional Career Development Grant K12 HD043489.
Linda Simoni-Wastila, Stephen Gottlieb, and Ilene Zuckerman are co-investigators on a contract with GlaxoSmithKline, which funded this project.
Bruce Stuart is the principal investigator on a contract with GlaxoSmithKline, which funded this project.
Rahul Shenolikar is an employee of GlaxoSmithKline, which funded this project.
Rahul Shenolikar owns GSK stock.
Stephen Gottlieb is a consultant for Merck.
Sponsor’s Role: One of the authors, Rahul Shenolikar, is an employee of the sponsor, GlaxoSmithKline. Dr. Shenolikar was involved in study concept and design and interpretation of data. GlaxoSmithKline played no role in the study concept or design, acquisition, analyses or manuscript preparation. Dr. Shenolikar is an employee of GSK and had input in study concept and interpretation of data as a coauthor.
Footnotes
Presentations: Portions of this paper were presented at the AcademyHealth 2012 Annual Research Meeting.
Author Contributions
Ilene Zuckerman and Xianghua Yin were involved in the study concept and design, analysis and interpretation of data, and preparation of the manuscript. Gail Rattinger was involved in analysis, interpretation of data and preparation of the manuscript. Sarah Pierce was involved in interpretation of data and preparation of the manuscript. Linda Simoni-Wastila, Stephen Gottlieb, Ting-Ying Huang and Rahul Shenolikar were involved in study concept and design and interpretation of data. Bruce Stuart was involved in study concept and design, and acquisition and interpretation of data. All authors were involved with revision of the manuscript and approved the final version.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2011 update: A report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 2007;356:2388–2398. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 3.Heidenreich PA, McClellan M. Trends in treatment and outcomes for acute myocardial infarction: 1975–1995. Am J Med. 2001;110:165–174. doi: 10.1016/s0002-9343(00)00712-9. [DOI] [PubMed] [Google Scholar]
- 4.Eagle KA, Montoye CK, Riba AL, et al. Guideline-based standardized care is associated with substantially lower mortality in medicare patients with acute myocardial infarction: The American College of Cardiology’s Guidelines Applied in Practice (GAP) Projects in Michigan. J Am Coll Cardiol. 2005;46:1242–1248. doi: 10.1016/j.jacc.2004.12.083. [DOI] [PubMed] [Google Scholar]
- 5.Hansen JF, Hagerup L, Sigurd B, et al. Cardiac event rates after acute myocardial infarction in patients treated with verapamil and trandolapril versus trandolapril alone. Danish Verapamil Infarction Trial (DAVIT) Study Group. Am J Cardiol. 1997;79:738–741. doi: 10.1016/s0002-9149(96)00860-0. [DOI] [PubMed] [Google Scholar]
- 6.Baigent C, Collins R, Appleby P, et al. ISIS-2: 10 year survival among patients with suspected acute myocardial infarction in randomised comparison of intravenous streptokinase, oral aspirin, both, or neither. The ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. BMJ. 1998;316:1337–1343. doi: 10.1136/bmj.316.7141.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dickstein K, Kjekshus J. Effects of losartan and captopril on mortality and morbidity in high-risk patients after acute myocardial infarction: The OPTIMAAL randomised trial. Optimal Trial in Myocardial Infarction with Angiotensin II Antagonist Losartan. Lancet. 2002;360:752–760. doi: 10.1016/s0140-6736(02)09895-1. [DOI] [PubMed] [Google Scholar]
- 8.Freemantle N, Cleland J, Young P, et al. Beta Blockade after myocardial infarction: systematic review and meta regression analysis. BMJ. 1999;318:1730–1737. doi: 10.1136/bmj.318.7200.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tay EL, Chan M, Tan V, et al. Impact of combination evidence-based medical therapy on mortality following myocardial infarction in elderly patients. Am J Geriatr Cardiol. 2008;17:21–26. doi: 10.1111/j.1076-7460.2007.07242.x. [DOI] [PubMed] [Google Scholar]
- 10.Setoguchi S, Glynn RJ, Avorn J, et al. Improvements in long-term mortality after myocardial infarction and increased use of cardiovascular drugs after discharge: A 10-year trend analysis. J Am Coll Cardiol. 2008;51:1247–1254. doi: 10.1016/j.jacc.2007.10.063. [DOI] [PubMed] [Google Scholar]
- 11.Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50:e1–e157. doi: 10.1016/j.jacc.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction; A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of patients with acute myocardial infarction) J Am Coll Cardiol. 2004;44:E1–E211. doi: 10.1016/j.jacc.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 13.Antman EM, Hand M, Armstrong PW, et al. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2008;51:210–247. doi: 10.1016/j.jacc.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Braunwald E, Antman EM, Beasley JW, et al. ACC/AHA 2002 guideline update for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction--summary article: A report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee on the Management of Patients With Unstable Angina) J Am Coll Cardiol. 2002;40:1366–1374. doi: 10.1016/s0735-1097(02)02336-7. [DOI] [PubMed] [Google Scholar]
- 15.Rogers AM, Ramanath VS, Grzybowski M, et al. The association between guideline-based treatment instructions at the point of discharge and lower 1-year mortality in Medicare patients after acute myocardial infarction: The American College of Cardiology’s Guidelines Applied in Practice (GAP) initiative in Michigan. Am Heart J. 2007;154:461–469. doi: 10.1016/j.ahj.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Gouya G, Reichardt B, Ohrenberger G, et al. Survival of patients discharged after acute myocardial infarction and evidence-based drug therapy. Eur J Epidemiol. 2007;22:145–149. doi: 10.1007/s10654-006-9087-9. [DOI] [PubMed] [Google Scholar]
- 17.Ho PM, Spertus JA, Masoudi FA, et al. Impact of medication therapy discontinuation on mortality after myocardial infarction. Arch Intern Med. 2006;166:1842–1847. doi: 10.1001/archinte.166.17.1842. [DOI] [PubMed] [Google Scholar]
- 18.Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA. 2007;297:177–186. doi: 10.1001/jama.297.2.177. [DOI] [PubMed] [Google Scholar]
- 19.Benner JS, Glynn RJ, Mogun H, et al. Long-term persistence in use of statin therapy in elderly patients. JAMA. 2002;288:455–461. doi: 10.1001/jama.288.4.455. [DOI] [PubMed] [Google Scholar]
- 20.Choudhry NK, Setoguchi S, Levin R, et al. Trends in adherence to secondary prevention medications in elderly post-myocardial infarction patients. Pharmacoepidemiol Drug Saf. 2008;17:1189–1196. doi: 10.1002/pds.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akincigil A, Bowblis JR, Levin C, et al. Long-term adherence to evidence based secondary prevention therapies after acute myocardial infarction. J Gen Intern Med. 2008;23:115–121. doi: 10.1007/s11606-007-0351-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eagle KA, Kline-Rogers E, Goodman SG, et al. Adherence to evidence-based therapies after discharge for acute coronary syndromes: An ongoing prospective, observational study. Am J Med. 2004;117:73–81. doi: 10.1016/j.amjmed.2003.12.041. [DOI] [PubMed] [Google Scholar]
- 23.Chang TI, Desai M, Solomon DH, et al. Kidney function and long-term medication adherence after myocardial infarction in the elderly. Clin J Am Soc Nephrol. 2011;6:864–869. doi: 10.2215/CJN.07290810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee HY, Cooke CE, Robertson TA. Use of secondary prevention drug therapy in patients with acute coronary syndrome after hospital discharge. J Manag Care Pharm. 2008;14:271–280. doi: 10.18553/jmcp.2008.14.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackevicius CA, Li P, Tu JV. Prevalence, predictors, and outcomes of primary nonadherence after acute myocardial infarction. Circulation. 2008;117:1028–1036. doi: 10.1161/CIRCULATIONAHA.107.706820. [DOI] [PubMed] [Google Scholar]
- 26.Ho PM, Magid DJ, Masoudi FA, et al. Adherence to cardioprotective medications and mortality among patients with diabetes and ischemic heart disease. BMC Cardiovasc Disord. 2006;6:48. doi: 10.1186/1471-2261-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winkelmayer WC, Charytan DM, Levin R, et al. Poor short-term survival and low use of cardiovascular medications in elderly dialysis patients after acute myocardial infarction. Am J Kidney Dis. 2006;47:301–308. doi: 10.1053/j.ajkd.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Boggon R, van Staa TP, Timmis A, et al. Clopidogrel discontinuation after acute coronary syndromes: Frequency, predictors and associations with death and myocardial infarction--a hospital registry-primary care linked cohort (MINAP-GPRD) Eur Heart J. 2011;32:2376–2386. doi: 10.1093/eurheartj/ehr340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maio V, Marino M, Robeson M, et al. Beta-blocker initiation and adherence after hospitalization for acute myocardial infarction. Eur J Cardiovasc Prev Rehabil. 2011;18:438–445. doi: 10.1177/1741826710389401. [DOI] [PubMed] [Google Scholar]
- 30.Blackburn DF, Dobson RT, Blackburn JL, et al. Cardiovascular morbidity associated with nonadherence to statin therapy. Pharmacotherapy. 2005;25:1035–1043. doi: 10.1592/phco.2005.25.8.1035. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen HL, Saczynski JS, Gore JM, et al. Long-term trends in short-term outcomes in acute myocardial infarction. Am J Med. 2011;124:939–946. doi: 10.1016/j.amjmed.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krumholz HM, Radford MJ, Wang Y, et al. National use and effectiveness of beta-blockers for the treatment of elderly patients after acute myocardial infarction: National Cooperative Cardiovascular Project. JAMA. 1998;280:623–629. doi: 10.1001/jama.280.7.623. [DOI] [PubMed] [Google Scholar]
- 33.Lai EJ, Grubisic M, Palepu A, et al. Cardiac medication prescribing and adherence after acute myocardial infarction in Chinese and South Asian Canadian patients. BMC Cardiovasc Disord. 2011;11:56. doi: 10.1186/1471-2261-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rathore SS, Mehta RH, Wang Y, et al. Effects of age on the quality of care provided to older patients with acute myocardial infarction. Am J Med. 2003;114:307–315. doi: 10.1016/s0002-9343(02)01531-0. [DOI] [PubMed] [Google Scholar]
- 35.Zhu B, Zhao Z, McCollam P, et al. Factors associated with clopidogrel use, adherence, and persistence in patients with acute coronary syndromes undergoing percutaneous coronary intervention. Curr Med Res Opin. 2011;27:633–641. doi: 10.1185/03007995.2010.551657. [DOI] [PubMed] [Google Scholar]
- 36.The Norwegian Multicenter Study Group. Timolol-induced reduction in mortality and reinfarction in patients surviving acute myocardial infarction. N Engl J Med. 1981;304:801–807. doi: 10.1056/NEJM198104023041401. [DOI] [PubMed] [Google Scholar]
- 37.Zelis R. Calcium-blocker therapy for unstable angina pectoris. N Engl J Med. 1982;306:926–928. doi: 10.1056/NEJM198204153061508. [DOI] [PubMed] [Google Scholar]
- 38.Spargias KS, Hall AS, Greenwood DC, et al. beta blocker treatment and other prognostic variables in patients with clinical evidence of heart failure after acute myocardial infarction: evidence from the AIRE study. Heart. 1999;81:25–32. doi: 10.1136/hrt.81.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vantrimpont P, Rouleau JL, Wun CC, et al. Additive beneficial effects of beta-blockers to angiotensin-converting enzyme inhibitors in the Survival and Ventricular Enlargement (SAVE) Study. SAVE Investigators. J Am Coll Cardiol. 1997;29:229–236. doi: 10.1016/s0735-1097(96)00489-5. [DOI] [PubMed] [Google Scholar]
- 40.Andrade SE, Kahler KH, Frech F, et al. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15:565–74. doi: 10.1002/pds.1230. discussion 75–77. [DOI] [PubMed] [Google Scholar]
- 41.Hess LM, Raebel MA, Conner DA, et al. Measurement of adherence in pharmacy administrative databases: A proposal for standard definitions and preferred measures. Ann Pharmacother. 2006;40:1280–1288. doi: 10.1345/aph.1H018. [DOI] [PubMed] [Google Scholar]
- 42.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50:105–116. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 43.Choudhry NK, Avorn J, Glynn RJ, et al. Full coverage for preventive medications after myocardial infarction. N Engl J Med. 2011;365:2088–2097. doi: 10.1056/NEJMsa1107913. [DOI] [PubMed] [Google Scholar]
- 44.Philipson TJ, Mozaffari E, Maclean JR. Pharmacy cost sharing, antiplatelet therapy utilization, and health outcomes for patients with acute coronary syndrome. Am J Manage Care. 2010;16:290–297. [PubMed] [Google Scholar]