Abstract
Discourse cohesion and coherence give communication its continuity providing the grammatical and lexical links that hold an utterance or text together and give it meaning. Researchers often link cohesion and coherence deficits to the frontal lobes by drawing attention frontal lobe dysfunction in populations where discourse cohesion and coherence deficits are reported and through attribution of these deficits to underlying cognitive impairments putatively associated with the frontal lobes. We examined the distinct contribution of a region of the frontal lobes, the ventromedial prefrontal cortex (vmPFC), to discourse cohesion and coherence across a range of discourse tasks. We found that bilateral vmPFC damage does not impair cohesion and coherence in spoken discourse. This study provides insights into contribution of the major anatomical subdivisions of the frontal lobes to language use and furthers our understanding of the neural and cognitive underpinnings of discourse cohesion and coherence.
Keywords: cohesion, coherence, referential processing, ventromedial prefrontal cortex, discourse
Discourse cohesion and coherence give communication its continuity providing the grammatical and lexical links that hold an utterance or text together and give it meaning. Discourse cohesion and coherence are also among the most widely studied macrolingusitic measures of discourse production. Cohesive ties are defined as surface level (word and sentence) indicators of the relations within and between sentences that allow interlocutors to make connections within and across utterances, speakers, topics, and time (Halliday & Hasan, 1976). Cohesive ties can take multiple linguistic forms including anaphora (e.g., John and Mary are walking to school. She has been leading the way.) and conjunctions (e.g., John was walking to work. But he had to walk fast to beat the rain.)(e.g., Liles & Coelho, 1998). Whereas cohesion refers to the continuity within and between sentences, coherence refers to continuity in the overall meaning. Analyses of spoken discourse typically divide coherence further into local (the interrelatedness, or topic maintenance, across adjacent utterances) and global (the interrelatedness, or topic maintenance, across larger stretches of discourse such as an entire conversation or narrative) coherence (Glosser & Deser, 1991).
Deficits in discourse cohesion and coherence have been reported across a number of clinical populations including Alzheimer’s disease (AD) (e.g., Almor, Kemplier, MacDonald, Andersen, & Tyler, 1999; Dijkstra, Bourgeois, Allen, & Burgio, 2004; Ripich, Carpenter & Ziol, 2000), aphasia (e.g., Coelho & Flewellyn, 2003; Rogalski & Edmonds, 2008), traumatic brain injury (TBI) (e.g., Coelho, Grela, Corso, Gamble, & Feinn, 2005; Davis & Coelho, 2004; Hartley & Jensen, 1991; Youse & Coelho, 2005), right hemisphere syndrome (Davis, O’Neil-Pirozzi, & Coon, 1997) and schizophrenia (e.g., Docherty, DeRosa, & Andreasen, 1996; Rochester & Martin, 1979). Researchers have linked these deficits to the frontal lobes by drawing attention to the presence of frontal lobe damage or dysfunction in clinical populations where discourse cohesion and coherence deficits are reported (e.g., AD, TBI, schizophrenia) and through attribution of these deficits to underlying cognitive impairments putatively associated with the frontal lobes (e.g., executive function; theory of mind; working memory) (e.g., Coelho, 2002; Dijkstra et al., 2004; Glosser & Deser, 1991; Hartley & Jensen, 1991;Youse & Coelho, 2005). There have been calls to elucidate the relationship between the site of frontal lobe lesion and abnormal discourse cohesion (e.g., Levin, Goldstein, Williams, & Eisenberg, 1991). However, linking these deficits to specific cognitive domains or neural substrates is challenging because of insufficient lesion information in previous reports and the diffuse neural pathology and cognitive disruption in some populations studied to date (e.g., TBI, AD, schizophrenia).
In a previous study examining the contribution of hippocampal declarative memory to discourse cohesion and coherence, we circumvented these challenges by taking advantage of a rare patient group with a well-characterized neuropsychological and neuroanatomical profile of severe and selective declarative memory impairments secondary to bilateral hippocampal lesions (Kurczek & Duff, 2011). We argued that the role of the hippocampal declarative memory system in the creation of on-line representations for successive events including information about the co-occurrences of people, places, and things, and the ability to link the spatial, temporal and interactional relations among them across time (see Duff & Brown-Schmidt, 2012; Eichenbaum & Cohen, 2001) made it an attractive candidate system for contributing to the demands of discourse cohesion and coherence. Furthermore, hippocampal damage/dysfunction and declarative memory impairment are hallmark in populations where deficits in discourse cohesion and coherence have been reported (e.g., TBI, AD, schizophrenia) (Bourgeois & Hickey, 2009; Hanlon, Houck, Pyeatt, Lundy, Euler, Weisend, Thoma, et al., 2011; Murray, Ramage, & Hopper, 2001). In our study, we found that patients with hippocampal amnesia did have discourse cohesion and coherence deficits; they produced fewer cohesive ties, the adequacy of their ties were more often judged to be incomplete, and ratings of their local coherence were consistently lower than comparison participants (Kurczek & Duff, 2011). While this result was the first to link declarative memory to discourse cohesion and coherence, the finding alone does not preclude frontal lobe contributions to discourse cohesion and coherence. Indeed, successful communication is likely the result of a network of neural structures and the orchestration of multiple cognitive systems. However, the approach of working with patients with well characterized lesions to the hippocampus and selective and severe declarative memory deficits provided a rich and unparalleled opportunity to test our hypothesis regarding the role of particular neural and cognitive substrates to discourse cohesion and coherence.
Here, we apply this same approach to examining the distinct contribution of a region of the frontal lobes, the ventromedial prefrontal cortex (vmPFC), to cohesion and coherence in spoken discourse. We targeted the vmPFC for several reasons. First, the vmPFC is vulnerable in clinical populations such as TBI and AD (e.g., Adams, Doyle, Graham, Lawrence, McLellan, Gennarelli, et al. 1985; Chalmers, Wilcock, & Love, 2005; Mattson & Levin, 1990) and deficits in cohesion and coherence in these populations have been linked to frontal lobe dysfunction. Second, the critical role of the vmPFC in a variety of social and emotional processes is well established including in social judgments and decision making (e.g., Bechara, Damasio, Damasio, & Anderson, 1994; Beer, Heerey, Keltner, Scabini, & Knight, 2003; Croft, Duff, Kovack, Adolphs, & Tranel, 2010; Stone, Baron-Cohen, & Knight, 1998), and theory of mind (e.g., Shamay-Tsoory, Tomer, Berger, & Aharon-Peretz, 2003; Stone et al., 1998; Stuss, Gallup, & Alexander, 2001). While deficits in these aspects of social and emotional processing following vmPFC damage have been linked to impairments in communication such as understanding humor, sarcasm and faux pas (e.g., Shamay-Tsoory, Tomer, & Aharon-Peretz, 2005; Shammi & Stuss, 1999), whether or not these same social and emotional processing deficits impact discourse cohesion and coherence is unknown. Finally, a recent study of discourse cohesion in patients with DLPFC lesions found no impairment (Le et al., 2009). The current study examining the contribution of the vmPFC to discourse cohesion is an important next step in characterizing the relationship between the anatomical subdivisions of the frontal lobes and language.
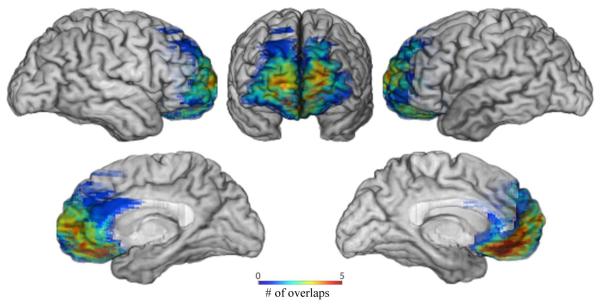
Replicating the procedures of Kurczek and Duff (2011), we analyzed cohesion and coherence across a variety of discourse genres (narrative, procedural, picture description, story re-telling) in six participants (3 females) with bilateral vmPFC damage (see Figure 1) and six healthy comparison participants matched pairwise to the patients on age (t (8) = 0.15, p = 0.88), education (t (8) = 0.98, p = 0.35), and sex. Data were analyzed using a two-tailed t-tests and Bonferroni correction for multiple comparisons (alpha of 0.01). Although vmPFC participants have well documented post-morbid changes in social and emotional processing, their neuropsychological profiles are relatively intact (i.e., normal intelligence, memory, speech, language, visual discrimination (see Table 1).
Figure 1.
Lesion overlap map of five vmPFC participants
Table 1.
Demographic and neuropsychological profiles of vmPFC participants.
| Sub | Age | Ed | Chron | Etiology | FSIQ | WMS GMI |
WMS WMI |
DS | Arith | Sent Rept |
BN | Token | COWA | WCS # Cat. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| 318 | 71 | 14 | 36 | Meningioma Resection |
143 | 109 | 124 | 12 | 15 | 14 | 60 | 44 | 54 | 6 |
| 2352 | 62 | 14 | 13 | SaH; ACoA | 106 | 109 | 124 | 13 | 10 | N/A | 54 | 44 | 34 | 6 |
| 2391 | 65 | 12 | 12 | Meningioma Resection |
109 | 132 | 102 | 9 | 13 | 11 | 57 | 43 | 59 | 6 |
| 2577 | 71 | 11 | 13 | SaH; ACoA | 84 | 96 | 88 | 7 | 9 | 15 | 55 | 44 | 44 | 0 |
| 3349 | 68 | 12 | 6 | Meningioma Resection |
101 | 103 | 104 | 11 | 11 | 11 | 53 | 44 | 40 | 5 |
| 3350 | 59 | 18 | 8 | Meningioma Resection |
118 | 108 | 118 | 11 | 11 | 14 | 52 | N/A | 40 | 6 |
|
| ||||||||||||||
|
(SD) |
66 (4.9) |
13.5 (2.5) |
14.7 (10.8) |
110.2 (19.6) |
109.5 (12.1) |
110.0 (14.4) |
10.5 (2.2) |
11.5 (2.2) |
13.0 (1.9) |
55.2 (2.9) |
43.8 (0.4) |
45.2 (9.5) |
4.8 (2.4) |
|
Note: Sub = Subject; Ed = Education; SaH = Subarachnoid hemorrhage; ACoA = Anterior communicating artery; Chron = Chronicity, time since injury; FSIQ = Full Scale IQ from the Wechsler Adult Intelligence Scale IV; WMS GMI = Wechsler Memory Scale General Memory Index; WMS WMI = Wechsler Memory Scale Working Memory Index; WMS-III, Wechsler memory scale-III; DS = digit span subtest; WAIS-III, age-corrected scaled score, Arith = arithmetic subtest, WAIS-III, age-corrected scaled score; Sent Rept = sentence repetition subtest, WAIS-III, age-corrected scaled score; BN = Boston Naming Test; COWA = Controlled Oral Word Association Test; WCS # Cat. = Wisconsin Card Sort Test, number of categories achieved.
Results
Frequency of cohesive ties
Across the entire data set and all participants, 3133 cohesive ties were coded with 1414 and 1719 ties coded in the discourse of the vmPFC and comparison participants, respectively, a difference that was not significant, t(46) = −0.882, p = 0.38. There was also no significant group differences in the number of ties across individual tasks: story generation = t(10) = 0.31, p = 0.76; story retelling = t (10) = 0.04, p = 0.97; narrative = t(10) = −1.19, p = 0.26; procedural = t(10) = −1.04, p = 0.32. The number of cohesive ties per T-unit across the entire data set for vmPFC and comparison participants was 2.66 (SD = 0.83) and 2.70 (SD = 0.76) (t(46) = −0.145, p = 0.89). Row one in Table 2 presents the average number of cohesive ties per T-unit for each type of tie by discourse type and group. vmPFC participants consistently produced as many markers per T-unit as comparison participants across all discourse types as there was no difference across discourse types: story generation, t(10) = −1.22, p = 0.25; story retelling, t(10) = −0.31, p = 0.76; narrative, t(10) = 0.58, p = 0.58; procedural, t(10) = 0.95, p = 0.37.
Table 2.
Cohesion and Coherence Ratings by Discourse Type and Group
| Story Generation |
Story Retelling | Narrative | Procedural | Average across all discourse types |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| VM | CP | VM | CP | VM | CP | VM | CP | VM | CP | ||
| Discourse | Words | 113.83 (83.89) |
122.67 (88.70) |
188.33 (123.01) |
260.67 (149.00) |
576.33 (267.36) |
803.17 (476.97) |
491.50 (271.86) |
501.67 (116.36) |
342.50 (276.26) |
422.04 (358.69) |
| T-Units | 9.17 (6.34) |
7.00 (2.83) |
16.00 (9.72) |
16.17 (5.04) |
2.64 (0.58) |
2.65 (0.69) |
2.85 (0.93) |
2.44 (0.43) |
21.13 (13.01) |
27.29 (25.79) |
|
| Ties | 23.17 (19.77) |
20.17 (13.23) |
53.67 (36.01) |
53.00 (24.40) |
92.67 (31.82) |
140.33 (93.15) |
66.17 (25.36) |
73.00 (23.49) |
58.92 (37.13) |
71.63 (64.71) |
|
| Cohesion |
Ties/T-
Units |
2.24 (0.66) |
2.80 (0.93) |
3.00 (1.03) |
3.17 (0.86) |
2.64 (0.58) |
2.65 (0.69) |
2.85 (0.93) |
2.44 (0.43) |
2.66 (0.84) |
2.84 (0.79) |
|
%
Complete |
0.89 (0.23) |
0.95 (0.05) |
0.96 (0.04) |
0.97 (0.03) |
0.99 (0.01) |
0.98 (0.02) |
0.96 (0.05) |
1.00 (0.01) |
0.95 (0.12) |
0.97 (0.03) |
|
| Coherence | Global | 3.94 (0.37) |
4.29 (0.48) |
4.90 (0.16) |
4.59 (0.24) |
4.47 (0.33) |
4.34 (0.28) |
4.68 (0.46) |
4.49 (0.45) |
4.50 (0.49) |
4.43 (0.37) |
| Local | 4.32 (0.38) |
4.32 (0.38) |
4.96 (0.07) |
4.70 (0.23) |
4.53 (0.33) |
4.65 (0.26) |
4.65 (0.33) |
4.57 (0.34) |
4.59 (0.41) |
4.56 (0.32) |
|
Note: Data are presented as Mean (SD). Ties/T-unit is the mean number of cohesive ties per T-unit across all coding categories. Referential, lexical, and conjunctive ties are presented as the mean number of ties per T-unit. Coherence ratings are presented as the mean rating on the 5-point scale.
Completeness of cohesive ties
Across the entire data set, 3025 ties were coded as complete, with 1336 and 1689 complete ties coded in the discourse of the vmPFC and comparison participants, respectively. The scoring of cohesive ties as complete across discourse types was slightly lower for vmPFC participants than comparison participants, however, this difference was not statistically significant, t(46) = −1.109, p = 0.27. An investigation of each discourse task reveals no difference in the percent of complete ties: story generation = t(10) = −0.633, p = 0.54; story retelling = t(10) = −0.88, p = 0.40; narrative = t(10) = 0.36, p = 0.72; procedural = t(10) = −1.86, p = 0.09.
Coherence
Across the entire data set (all discourse tasks collapsed), there was no difference in scores of global coherence (t(46) = 0.58, p = 0.57). An investigation of each discourse task reveals no difference in the global coherence scores: story generation (t(10) = −1.40, p = 0.19), story retelling (t(10) = 2.70, p = 0.02), narrative (t(10) = 0.74, p = 0.48), or procedural discourse (t(10) = 0.71, p = 0.49) .For local coherence, again, across all the discourse tasks, the scores were comparable between groups as there were no significant differences: story generation (t (10) = −0.410, p = 0.69), story retelling (t(10) = 2.63, p = 0.03), narrative (t(10) = −0.71, p = 0.50), and procedural (t(10) = 0.43, p = 0.68). It is worth noting that the Bonferroni correction increases the risk of Type 2 errors and that statistically significant group differences would have been observed for global and local coherence in story retelling.
Discussion
Identifying the neural and cognitive underpinnings of discourse cohesion and coherence has been challenging because of the diffuse nature of pathology and cognitive impairments in populations where discourse cohesion and coherence deficits occur (e.g., TBI, AD). In this study, we sought to examine the role of the vmPFC, an area of the brain frequently damaged in patients with cohesion and coherence deficits. We found that bilateral vmPFC damage does not impair cohesion and coherence in spoken discourse.
Much of the work linking language and discourse abilities to the frontal lobes has focused on the dorsolateral prefrontal cortex (DLPFC) (e.g., Alexander et al., 1989; Frattali & Grafman, 2005; Le, Mozeiko, Coelho, Krueger, & Grafman, 2009; Sirigu et al., 1998). The DLPFC plays a role in working memory and working memory accounts of language processing have garnered much attention due to its purported on-line maintenance capacity and the ease of correlating performance on working memory tasks with language ability. Given that the vmPFC participants here do not have deficits on standardized neuropsychological measures of working memory (see Table 1) we cannot address a connection to discourse cohesion and coherence. However, the DLPFC does not seem to be involved in discourse cohesion, despite its putative role in working memory. Coelho and colleagues examined discourse cohesion and coherence of six individuals with left (L) DLPFC damage and nine individuals with right (R) DLPFC from penetrating head injuries received during the Vietnam War (Le et al., 2009). They found that, compared to a group of healthy participants, damage to neither the L nor R DLPFC produced deficits in discourse cohesion. These authors also reported that while neither L nor R DLPFC damage produced a deficit in local coherence, the patients with L DLPFC damage had significantly lower scores on global coherence. Follow-up testing revealed that the L DLPFC group had significantly lower scores on a measure of working memory (Spatial Span-Backward) than the R DLPFC and comparison groups. These findings suggest a potential relationship between L DLPFC, working memory, and global coherence. Taken together with the findings of the current study, it appears that neither DLPFC nor vmPFC make significant contributions to discourse cohesion.
Given the strong connection in the literature between the frontal lobes and discourse cohesion and coherence, this set of results is surprising. They also may give more prominence to our previous work reporting disruptions in discourse cohesion in patients with bilateral hippocampal damage and severe and selective declarative memory impairment (Kurczek & Duff, 2011). Outside of acquiring and supporting semantic knowledge (i.e., lexical access) before neocortical consolidation, the hippocampal declarative memory system has received little attention as a candidate mechanism for supporting language use. This is likely due to the long-held assumption that this form of memory contributes only to long-term memory representations and not to those that are available quickly enough to guide information processing in the moment. Recent evidence has challenged this view suggesting that hippocampal declarative memory is critical even over very short delays, or no delay at all, over a timescale typically considered working memory (e.g., Barense, Gaffan, & Graham, 2007; Hannula, Tranel, & Cohen, 2006). This work has significant implications for theories of language processing in general and for referential processing in particular. In addition to the ability to hold information on-line, discourse cohesion also requires the linking, integration, and tracking of various linguistic elements; abilities which are hallmark of the hippocampal declarative memory system (Duff & Brown-Schmidt, 2012; Eichenbaum & Cohen, 2001). In our original study we also found that the amnesic patients had lower scores of local coherence than global coherence. That is, the amnesic patients had difficulty linking each utterance to the next, in terms of the theme or content, but they are able to stay on topic in general (see Ogden & Corkin, 1991 for a similar observation in the patient HM). Researchers have suggested that local and global coherence may rely on distinct cognitive and neural substrates (e.g., Arbuckle & Gold, 1993; Rogalski, Altmann, Plummer-D’Amato, Behrman, & Marsiske, 2010). While speculative at this point, one possibility is that the relational binding of hippocampal declarative memory system supports local coherence (and cohesion) and that DLPFC supports global coherence. Additional research is needed to corroborate such a proposal. However, we think it is possible that some of the observed deficits in cohesion and coherence attributed to the frontal lobes in previous work may be attributable to hippocampal declarative memory system, particularly in populations with diffuse neural damage (e.g., TBI and AD) where declarative memory impairments are also common. The contribution of hippocampal declarative memory would likely be more transparent in the contexts of everyday language use where cohesion and coherence are maintained across speakers (two or more people), time (days, weeks), and communicative resources (gesture) (see Kurczek & Duff, 2011).
How do the current findings integrate with the role of the frontal lobes in language and communication more broadly? Stuss and Levin (2002) propose that the DLPFC subserves functions that are more cognitive in nature while the vmPFC is associated with abilities that are more affective. Our work, and others, examining the contribution of the vmPFC to various aspects of communication provides support to this distinction. For example, here we have shown that the vmPFC is not critical for linking and signaling the relations within and between sentences through the use of cohesive ties. Classifying discourse cohesion and coherence as a cognitive rather than affective ability, and thus an ability that is intact following vmPFC damage, would seem to make sense. Additionally, in previous work, we have shown that the acquisition and use common ground (i.e., knowledge of what information is shared between conversational partners that is used to tailor specific utterances) is also independent of the vmPFC (Gupta, Tranel, & Duff, 2012); a finding consistent with a dissociation in the cognitive and affective components of theory of mind where cognitive theory of mind is intact following vmPFC damage (e.g., Shamay-Tsoory & Aharon-Peretz, 2007; Shamay-Tsoory, Tibi-Elhanany, & Aharon-Peretz, 2006). Other aspects of communication, however, place higher demands on affective processing and appear to be disrupted in patients with vmPFC damage. Deficits in humor, sarcasm, and faux pas have been observed in patients with vmPFC lesions and these impairments have been attributed to diruptions in the ability of the vmPFC to integrate cognitive and affective information (e.g., Shamay-Tsoory, Tomer, & Aharon-Peretz, 2005; Shammi & Stuss, 1999). Furthermore, we found impairments in the same patients studied here in the ability to converge, or become more similar or in synch, with a communication partner on linguistic variables such length of an utterance across a conversation (Gupta, Duff, & Tranel, 2011). Discourse convergence is thought to be an affective component of communication as it is an indication of reciprocity and rapport in social interactions (Cappella, 1981; Giles, Coupland, & Coupland, 1991). Thus, the distinction between affective and cognitive functions appears fruitful in delineating the unique contributions of the major anatomical subdivisions of the frontal lobes to various aspects of language use. Of course, language use and communication rely on a broader neural network and set of cognitive processes than those of the frontal lobes. Consideration of contributions from within and outside the frontal lobes as well as the products of the complex interactions of the frontal lobes with other neural systems (e.g., medial temporal lobe) promises to offer a more complete understanding of the neurobiology of language.
Methods
Participants
The participants with vmPFC damage have been well characterized neuropsychologically and neuroanatomically in our lab (e.g., Bechara et al., 1994; Croft et al., 2010; Gupta, Tranel, & Duff, 2012; Koenigs & Tranel, 2008) and all present with characteristic post-morbid changes in emotion processing, decision-making, and personality and/or social and interpersonal functioning. Performance on standardized tests of intelligence, memory, language, and visual discrimination are within normal limits (see Table 1). All participants are community dwelling and free of aphasia and dysarthria as determined by a certified speech-language pathologist. Bilateral vmPFC lesions are due to meningioma resection or subarachnoid hemorrhage. The vmPFC is defined as the region encompassing the medial orbital and lower medial sector of the prefrontal lobe, Broadmann areas, 25, 24, 32, 10, 11, 12 (Barrash, Tranel, & Anderson, 2000). An overlap map depicting the lesion overlap for five of the six vmPFC patients is provided in Figure 1. Subject 3350 was not included because of difficulty transferring the lesion to a common space.
Procedures
Procedures replicated Kurczek and Duff (2011). Cohesion and coherence were analyzed across three narrative and three procedural discourse samples using the Mediated Discourse Elicitation Protocol (MDEP; Hengst & Duff, 2007) and one picture description (Norman Rockswell’s The Runaway) and one story-retelling sample (Paula Winter’s The Bear and the Fly; e.g., Youse & Coelho, 2005). Cohesive ties were identified and each tie was judged for adequacy (Coelho, 2002; Liles, 1985). Ties were coded as complete (referent easily located in previous text), incomplete (referent difficult to ascertain in context) or erroneous (more than one referent or no referent indentified in discourse) and are reported as percent correct (Liles, 1985). Following Glosser and Deser (1991), coherence was rated on a 5-point Likert scale (5 is highest) for both global (related to overall topic) and local (connected to preceding thoughts) coherence.
Data Analysis
All discourse was audio and video taped and transcribed using a consensus transcription process (see Duff, Hengst, Tranel, & Cohen, 2008). Transcripts were coded for the number of words. Utterances were distributed into T-units. Consistent with our previous work, words were broadly defined and fillers (e.g., uh = 1 word), contractions (e.g., don’t = 1 word), and each word in a false start (e.g., and then put and then you should put = 8 words) were included in the total word counts. Although comparison participants (CP) produced more words overall across discourse elicitations (10,129; M = 389.58, SD = 362.66) than vmPFC participants (8220; M = 316.15, SD = 280.85), this difference was not significantly different (t(46) = −1.33, p = 0.19). Additionally, no individual discourse type revealed a significant group difference: story generation = t(10) = −0.18, p = 0.86; story retelling = t(10) = −0.92, p = 0.38; narratives = t(10) = −1.02, p = 0.33; procedure = t(10) = −0.64, p = 0.54.
T-units were defined as an independent clause and any associated subordinate clauses (Hunt, 1970). While comparison participants produced more T-units across discourse samples (655; M = 25.2, SD = 25.8) than vmPFC participants (507, M = 19.5, SD = 13.7), this difference was not significant (t(46) = −1.22, p = 0.23). Additionally, no individual discourse type revealed a significant group difference: story generation t (10) = 0.77, p = 0.46; story retelling t(12) = −0.04, p = 0.97; narrative t(10) = −1.35, p = 0.21; procedural t(10) = −1.31, p = 0.22.
Reliability
Point by point inter- and intra-rater reliability for T-units, cohesive ties, cohesion adequacy, and coherence coding was calculated on 33% of the data. Intra-rater reliability for T-units, cohesive tie, cohesion adequacy, local and global coherence was 99.7%, 98.5%, 97.6%, 99.1%, and 98.7%, respectively and inter-rater reliability was 95.1%, 90.2%, 96.4%, 89.0%, and 94.7%, respectively.
Highlights.
We examined the role of the vmPFC in discourse cohesion and coherence.
Bilateral vmPFC damage does not impair cohesion and coherence in spoken discourse.
Findings elucidate contributions of the major anatomical subdivisions of the frontal lobes to language.
ACKNOWLEDGEMENTS
We thank M. Miller for assistance in data coding and analysis, Joel Bruss preparing Figure 1 and the Duff Communication and Memory Laboratory for helpful comments on earlier versions of this manuscript. Support from ASHFoundation, NINDS P50 NS19632, and NIDCD RO1 DC011755.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams JH, Doyle D, Graham DI, Lawrence E, McLellan DR, Gennarelli TA, et al. The contusion index: A reappraisal in human and experimental non-missile head injury. Neuropathology and Applied Neurobiology. 1985;11:299–308. doi: 10.1111/j.1365-2990.1985.tb00027.x. [DOI] [PubMed] [Google Scholar]
- Alexander M, Benson F, Stuss D. Frontal lobes and language. Brain and Language. 1989;37:656–691. doi: 10.1016/0093-934x(89)90118-1. [DOI] [PubMed] [Google Scholar]
- Almor A, Kemplier D, MacDonald MC, Andersen ES, Tyler LK. Why do Alzheimer patients have difficulty with pronouns? Working memory, semantics, and reference in comprehension and production in Alzheimer’s disease. Brain and Language. 1999;67(3):202–27. doi: 10.1006/brln.1999.2055. [DOI] [PubMed] [Google Scholar]
- Barense MD, Gaffan D, Graham KS. The human medial temporal lobe processes online representations of complex objects. Neuropsychologia. 2007;45:2963–297. doi: 10.1016/j.neuropsychologia.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Barrash J, Tranel D, Anderson SW. Acquired personality disturbances associated with bilateral damage to the ventromedial prefrontal region. Developmental Neuropsychology. 2000;18:355–381. doi: 10.1207/S1532694205Barrash. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to futuure consequences following damage to human prefrontal cortex. Cognition. 1994;50(1-3):7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Beer JS, Heerey EH, Keltner D, Scabini D, Knight RT. The regulatory function of self-conscious emotion: Insights from patients with orbitofrontal damage. Journal of Personality and Social Psychology. 2003;85:594–604. doi: 10.1037/0022-3514.85.4.594. [DOI] [PubMed] [Google Scholar]
- Bourgeois M, Hickey E. Dementia: From diagnosis to management—A functional approach. Taylor & Francis; New York: 2009. [Google Scholar]
- Cappella J. Mutual influence in expressive behavior: Adult-adult and infant-adult dyadic interaction. Psychological Bulletin. 1981;89:101–132. [PubMed] [Google Scholar]
- Chalmers K, Wilcock G, Love S. Contributors to white matter damage in the frontal lobe in Alzheimer’s disease. Neuropathology and Applied Neurobiology. 2005;31(6):623–631. doi: 10.1111/j.1365-2990.2005.00678.x. [DOI] [PubMed] [Google Scholar]
- Coelho CA. Story narrative of adults with closed head injury and non-brain-injured adults: influence of socioeconomic status, elicitation task, and executive functioning. Journal Speech Language Hearing Research. 2002;45(6):1232–48. doi: 10.1044/1092-4388(2002/099). [DOI] [PubMed] [Google Scholar]
- Coelho CA, Flewellyn L. Longitudinal assessment of coherence in an adult with fluent aphasia: A follow-up study. Aphasiology. 2003;17(2):173–182. [Google Scholar]
- Coelho CA, Grela B, Corso M, Gamble A, Feinn R. Microlinguistic deficits in the narrative discourse of adults with traumatic brain injury. Brain Injury. 2005;19(13):1139–1145. doi: 10.1080/02699050500110678. [DOI] [PubMed] [Google Scholar]
- Croft KE, Duff MC, Kovach CK, Anderson SW, Adolphs R, Tranel D. Detestable or Marvelous? – Neuroanatomical correlates of character judgments. Neuropsychologia. 2010;48:1789–1801. doi: 10.1016/j.neuropsychologia.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GA, Coelho CA. Referential cohesion and logical coherence of narration after closed head injury. Brain and Language. 2004;89(3):508–523. doi: 10.1016/j.bandl.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Davis GA, O’Neil-Pirozzi TM, Coon M. Referential cohesion and logical coherence of narration after right hemisphere stroke. Brain and Language. 1997;56(2):183–210. doi: 10.1006/brln.1997.1741. [DOI] [PubMed] [Google Scholar]
- Dijkstra K, Bourgeois MS, Allen RS, Burgio LD. Conversational coherence: discourse analysis of older adults with and without dementia. Journal of Neurolinguistics. 2004;17:263–283. [Google Scholar]
- Docherty NM, DeRosa M, Andreasen NC. Communication disturbances in schizophrenia and mania. Archives of General Psychiatry. 1996;53:358–364. doi: 10.1001/archpsyc.1996.01830040094014. [DOI] [PubMed] [Google Scholar]
- Duff MC, Brown-Schimdt S. The hippocampus and the flexible use and processing of language. Frontiers in Human Neuroscience. 2012;6:69. doi: 10.3389/fnhum.2012.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff MC, Hengst J, Tranel D, Cohen N. Collaborative discourse facilitates efficient communication and new learning in amnesia. Brain and Language. 2008;106:41–54. doi: 10.1016/j.bandl.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. From conditioning to conscious recollection: Memory systems of the brain. Oxford University Press; New York, NY: 2001. [Google Scholar]
- Frattali C, Grafman J. Language and Discourse Deficits Following Prefrontal Cortex Damage. Aphasia and related neurogenic language disorders. 3rd ed Thieme New York; New York, NY: 2005. [Google Scholar]
- Giles H, Coupland N, Coupland J. Accommodation theory: Communication, context, and consequence. In: Giles H, Coupland N, Coupland J, editors. Contexts of Accommodation. Cambridge University Press; Cambridge: 1991. pp. 1–68. [Google Scholar]
- Glosser G, Deser T. Patterns of discourse production among neurological patients with fluent language disorders. Brain and Language. 1991;40(1):67–88. doi: 10.1016/0093-934x(91)90117-j. [DOI] [PubMed] [Google Scholar]
- Gupta R, Tranel D, Duff MC. Ventromedial prefrontal cortex damage does not impair the development and use of common ground in social interaction: implications for cognitive theory of mind. Neuropsychologia. 2012;50:145–152. doi: 10.1016/j.neuropsychologia.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday M, Hasan R. Cohesion in English. Longman Group; London, UK: 1976. [Google Scholar]
- Hanlon FM, Houck JM, Pyeatt CJ, Lundy SL, Euler MJ, Weisend MP, Thoma RJ, et al. Bilateral hippicampal dysfunction in schizophrenia. Neuroimage. 2011;58(4):1158–1168. doi: 10.1016/j.neuroimage.2011.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula D, Tranel D, Cohen NJ. The long and the short of it: Relational memory impairments in amnesia, even at short lags. The Journal of Neuroscience. 2006;26(32):8352–8259. doi: 10.1523/JNEUROSCI.5222-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley LL, Jensen PJ. Narrative and procedural discourse after closed head injury. Brain Injury. 1991;5(3):267–85. doi: 10.3109/02699059109008097. [DOI] [PubMed] [Google Scholar]
- Hengst JA, Duff MC. Clinicians as communication partners. Topics in Language Disorders. 2007;27(1):37–49. [Google Scholar]
- Hunt K. Syntactic maturity in school children and adults. (Serial No. 134).Monographs of the Society for Research in Child Development. 1970;35 [PubMed] [Google Scholar]
- Kurczek J, Duff MC. Cohesion, coherence and declarative memory: Discourse patterns in patients with hippocampal amnesia. Aphasiology. 2011;25(6/7):700–712. doi: 10.1080/02687038.2010.537345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le K, Mozeiko J, Coelho C, Krueger F, Grafman J. Language impairment associated with lesions to dorsolateral prefrontal cortex: Dynamic aphasia?. Paper presented at the Clinical Aphasiology Conference; Keystone, CO. 2009. [Google Scholar]
- Levin HS, Goldstein FC, Williams DH, Eisenberg HM. The contribution of frontal lobe lesions to the neurobehavioral outcome in closed head injury. In: Levin HS, Eisenberg HM, Benton AI, editors. Frontal lobe function and dysfunction. Oxford University Press; New York: 1991. pp. 318–338. [Google Scholar]
- Liles BZ. Narrative ability in normal and language-disordered children. Journal of Speech and Hearing Research. 1985;23:123–133. doi: 10.1044/jshr.2801.123. [DOI] [PubMed] [Google Scholar]
- Liles BZ, Coelho CA. Cohesion analysis. In: Cherney LR, Shadden BB, Coelho CA, editors. Analyzing discourse in communicatively impaired adults. Aspen; Gaithersburg, MA: 1998. pp. 65–84. [Google Scholar]
- Mattson AJ, Levin HS. Frontal lobe dysfunction following closed head injury. A review of the literature. Journal of Nervous & Mental Disorders. 1990;178(5):282–291. doi: 10.1097/00005053-199005000-00002. [DOI] [PubMed] [Google Scholar]
- Murray LL, Ramage AE, Hopper T. Memory impairments in adults with neurogenic communication disorders. Seminars in Speech and Language. 2001;22(2):127–136. doi: 10.1055/s-2001-13937. [DOI] [PubMed] [Google Scholar]
- Ripich DN, Carpenter BD, Ziol EW. Conversational cohesion patterns in men and women with Alzheimer’s disease: a longitudinal study. International Journal of Language & Communication Disorders. 2000;35(1):45–64. doi: 10.1080/136828200247241. [DOI] [PubMed] [Google Scholar]
- Rochester S, Martin JR. Crazy talk: A study of the discourse of schizophrenic speakers. Plenum; New York: 1979. [Google Scholar]
- Rogalski Y, Edmonds LA. Attentive reading and constrained summarization (ARCS) treatment in primary progressive aphasia: A case study. Aphasiology. 2008;22(7-8):763–775. [Google Scholar]
- Shamay-Tsoory SG, Aharon-Peretz J. Dissociable prefrontal networks for cognitive and affective theory of mind: A lesion study. Neuropsychologia. 2007;45(13):3054–3067. doi: 10.1016/j.neuropsychologia.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Tibi-Elhanany Y, Aharon-Peretz J. The ventromedial prefrontal cortex is involved in understanding affective but not cognitive theory of mind stories. Social Neuroscience. 2006;1(3-4):149–66. doi: 10.1080/17470910600985589. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Tomer R, Aharon-Peretz J. The neuroanatomical basis of understanding sarcasm and its relationship to social cognition. Neuropsychology. 2005;19(3):288–300. doi: 10.1037/0894-4105.19.3.288. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Tomer R, Berger BD, Aharon-Peretz J. Characterization of empathy deficits following prefrontal brain damage: The role of the right ventromedial prefrontal cortex. Journal of Cognitive Neuroscience. 2003;15(3):324–337. doi: 10.1162/089892903321593063. [DOI] [PubMed] [Google Scholar]
- Shammi P, Stuss DT. Humour appreciation: A role of the right frontal lobe. Brain. 1999;122(4):657–666. doi: 10.1093/brain/122.4.657. [DOI] [PubMed] [Google Scholar]
- Sirigu A, Cohen L, Zalla T, Pradat-Diehl P, Van Eeckhout P, Grafman J, Agid Y. Distinct frontal regions for processing sentence syntax and story grammar. Cortex. 1998;34:771–778. doi: 10.1016/s0010-9452(08)70780-9. [DOI] [PubMed] [Google Scholar]
- Stone V, Baron-Cohen S, Knight R. Frontal lobe contributions to theory of mind. Journal of Cognitive Neuroscience. 1998;10(5):640–656. doi: 10.1162/089892998562942. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Gallup GG, Jr., Alexander MP. The frontal lobes are necessary for “theory of mind”. Brain. 2001;124:279–286. doi: 10.1093/brain/124.2.279. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Levine B. Adult clinical neuropsychology: Lessons from studies of the frontal lobes. Annual Reviews of Psychology. 2002;53:401–433. doi: 10.1146/annurev.psych.53.100901.135220. [DOI] [PubMed] [Google Scholar]
- Youse KM, Coelho CA. Working memory and discourse production abilities following closed-head injury. Brain Injury. 2005;19(12):1001–1009. doi: 10.1080/02699050500109951. [DOI] [PubMed] [Google Scholar]