Abstract
The mechanisms by which DNA-incorporated radionuclides impart lethal damage to mammalian cells were investigated by examining the capacity of dimethyl sulfoxide (DMSO) to protect against lethal damage to Chinese hamster V79 cells caused by unbound tritium (3H2O), DNA-incorporated 125I-and 131I-iododeoxyuridine (125IdU, 131IdU), and cytoplasmically localized 210Po citrate. The radionuclides 3H and 131I emit low-and medium-energy β particles, respectively, 125I is a prolific Auger electron emitter, and 210Po emits 5.3 MeV α particles. Cells were radiolabeled and maintained at 10.5°C for 72 h in the presence of different concentrations of DMSO (5–12.5% v/v), and the surviving fraction compared to that of unlabeled controls was determined. DMSO afforded no protection against the lethal effects of the high-LET α particles emitted by 210Po. Protection against lethal damage caused by unbound 3H, 131IdU and 125IdU depended on the concentration of DMSO in the culture medium. Ten percent DMSO provided maximum protection in all cases. The dose modification factors obtained at 10% DMSO for 3H2O, 131IdU, 125IdU and 210Po citrate were 2.9 ± 0.01, 2.3 ± 0.5, 2.6 ± 0.2 and 0.95 ± 0.07, respectively. These results indicate that the toxicity of Auger electron and β-particle emitters incorporated into the DNA of mammalian cells is largely radical-mediated and is therefore indirect in nature. This is also the case for the low-energy β particles emitted by 3H2O. In contrast, α particles impart lethal damage largely by direct effects. Finally, calculations of cellular absorbed doses indicate that β-particle emitters are substantially more toxic when incorporated into the DNA of mammalian cells than when they are localized extracellularly.
INTRODUCTION
The toxicity of radionuclides that emit Auger electrons depends on their subcellular distribution. When Auger electron emitters are localized in the cytoplasm, their toxicity is akin to that of low-LET radiations (1, 2). However, when they decay in the immediate vicinity of DNA, they are as toxic as high-LET α particles (3, 4). Thus the response of mammalian cells to DNA-incorporated Auger electron emitters has been termed “high-LET-type”. The mechanism by which DNA-incorporated Auger electron emitters elicit high-LET-type responses in biological systems has been of considerable interest and sustained debate. Because of the similarity in the dose–response curves for these radionuclides and those for high-LET α particles, and the nature of the distribution of strand breaks observed in 125I-labeled oligonucleotides (5), it was believed at first that the mechanism was largely direct deposition of energy in the immediate vicinity of the decay site (6). This direct deposition of energy could, in principle, have two sources: (1) direct irradiation of radiosensitive targets by the low-energy Auger electrons (direct effects) or (2) localized energy deposition due to charge neutralization of the residual tellurium daughter atom (6). However, this premise was challenged when it was shown that chemical radioprotectors were able to mitigate the biological effects of Auger electron emitters in vivo (2). This unexpected finding provoked studies to further elucidate the mechanism of the action of irradiation by Auger electron emitters.
A variety of radioprotectors were studied by Rao and colleagues in the mouse testis model, including cysteamine (MEA) (2, 7), vitamin C (8), S-(2-aminoethyl)isothiouronium bromide hydrobromide (AET) (9), vitamin A and soybean oil (10), and DMSO (11). The data for DMSO are presented in ref. (11) together with a summary of the data for the studies with the other protectors. For each radioprotector, several different radiochemicals were investigated, including cytoplasmically localized N,N,N′-trimethyl-N′-(2-hydroxyl-3-methyl-5-iodobenzyl)-1,3-propane-diamine (H125IPDM), DNA-incorporated iododeoxyuridine (125IdU), and the α-particle emitter 210Po citrate. The hydroxyl radical scavengers DMSO and vitamin C afforded considerable protection in vivo against low-LET-type damage caused by cytoplasmically localized 125I and high-LET-type damage caused by DNA-incorporated 125IdU. However, these radical scavengers provided no protection against effects caused by the 5.3 MeV α particles emitted by 210Po. These data, along with those for the other radioprotectors mentioned above, provided considerable evidence that the mechanism by which Auger electron emitters impart high-LET-type damage in vivo is largely radical-mediated and is therefore indirect in nature.
In contrast to the indirect mechanisms proposed by Rao and colleagues (2, 7–12), Hofer and Bao (13) reported results of experiments suggesting that direct mechanisms were dominant. They synchronized Chinese hamster ovary (CHO) cells, labeled them with 125IdU, and froze the cells at −196°C in cryoprotective medium (with or without 25 mM MEA) at different times after radiolabeling. A dose–response curve with a shoulder, characteristic of low-LET-type radiations, was observed for cells frozen 30 min after labeling. The shape of the survival curve and the capacity of MEA to afford some protection against these effects [dose modification factor (DMF) = 1.5] led the authors to conclude that these were indirect effects. In contrast, the high-LET-type response observed for cells frozen 5 h after labeling and the inability of MEA to afford protection (DMF = 1.0) suggested that direct effects were responsible. Based on these data and on the fact that MEA protects in part by scavenging free radicals, the authors concluded that direct effects are the primary mechanism for the high-LET-type lethality of Auger electron emitters incorporated into the DNA of mammalian cells (13).
In addition to the mammalian cell experiments described above, other studies have been carried out with plasmids and oligonucleotides in an effort to elucidate the mechanisms by which Auger electron emitters impart biological damage in DNA (14–18). These studies have uniformly implicated direct effects as the primary mechanism involved in the damage suffered by naked DNA.
Seemingly supporting these findings were the recent results of Howell et al. (19), who used cultured Chinese hamster V79 cells. It was shown that although a nontoxic concentration of DMSO (5% v/v, 0.64 M) provided substantial protection against lethal effects caused by chronic exposure (72 h) of V79 cells to unbound 32P and tritiated water (3H2O) at 10.5°C (also nontoxic), no significant protection was observed against the lethal effects of chronic exposure to DNA-incorporated 131IdU, 125IdU and tritiated thymidine ([3H]dThd). These results appeared to contradict those of the studies in vivo showing that DMSO provides substantial protection against cell killing by DNA-incorporated 125IdU and cytoplasmically localized H125IPDM (11). Therefore, the authors concluded that 5% DMSO was not capable of protecting mammalian cells in vitro against lethal damage caused by DNA-incorporated radionuclides.
At about the same time, Walicka et al. (20, 21) demonstrated that higher concentrations of DMSO (10%, 1.28 M) provide marked protection against DNA double-strand breaks and death of V79 cells caused by 125IdU at 0.3°C. Under their experimental conditions, where 125I decays were accumulated over a period of 6 to 48 h, the temperature of 0.3°C was cryotoxic, although the use of 10% DMSO mitigated the cryotoxicity to some extent. The observed toxicity was factored out by using similarly treated controls to assess the effects of the 125I decays. The high DMF of 5.4 for the survival end point led the authors to conclude that indirect effects were principally responsible for the high-LET-type effects of DNA-incorporated 125I. To explain their findings, Walicka et al. (21) proposed a model consisting of densely packed nucleosomes that would facilitate cross irradiation by the low-energy Auger electrons whereby indirect effects may be expected to predominate. Notably, Terrissol’s (22) track structure calculations in a nucleosome model suggest that 75% of double-strand breaks produced by DNA-incorporated 125I are due to indirect interactions.
In an attempt to sort out the inconsistencies between the various experimental data sets, the present work examines the concentration dependence of the protective action of DMSO against lethal damage caused by incorporated radionuclides. In addition, studies have been carried out to determine the maximum protection provided against several different radiochemicals. As in our previous communication (19), cultured Chinese hamster V79 cells are used as the experimental model with cell survival serving as the biological end point. The radiochemicals studied include IdU labeled with either the prolific Auger electron emitter 125I or the β-particle emitter 131I, 3H2O or 210Po citrate.
MATERIALS AND METHODS
Radiochemicals
Tritiated water (3H2O, 0.37 GBq/ml), Na131I in 0.1 N NaOH (36 GBq/ml), and Na125I in 0.1 N NaOH (13.6 GBq/ml) were obtained from NEN Life Science Products (Boston, MA). The radionuclide 210Po (3700 kBq/ml) was obtained as PoCl4 in 2 M HCl from Isotope Products Laboratories (Burbank, CA). Radiolabeled iododeoxyuridine (131IdU and 125IdU) was synthesized and purified by HPLC in our laboratory according to procedures reported previously (10). Polonium-210 citrate was prepared by mixing the stock 210Po solution with 1 M sodium citrate (pH 7.0) in the ratio of 1:9 as described previously (4). All radiochemical solutions were sterile and carrier-free. The radiochemicals 131IdU, 125IdU and 210Po citrate are incorporated into the V79 cells, whereas 3H2O diffuses freely in the cells (19, 23).
Quantification of Radioactivity
The 3H and 210Po activities were determined with a Beckman LS3800 automatic liquid scintillation counter (Fullerton, CA) by transferring aliquots of radioactive culture medium into 6 ml of Aquasol® liquid scintillation cocktail (NEN Research Products, Boston, MA). The detection efficiencies for the 3H 5.7 keV β particles and 210Po 5.3 MeV α particles were 0.65 and 1.0, respectively. The 131I activity was quantified with a Canberra HpGe (Meriden, CT) well detector (364.5 keV photopeak, efficiency = 0.175, yield = 0.812). The 125I activity was quantified with a Beckman 5500 automatic γ-ray counter equipped with a 3-inch sodium iodide crystal (K-shell X rays + 35.5 keV γ rays, efficiency = 0.48, yield = 1.47).
Cell Culture and Rationale for Protocol Design
Chinese hamster V79 lung fibroblasts (kindly provided by A. I. Kassis, Harvard Medical School, Boston, MA) were used in these studies with clonogenic survival as the biological end point. Culturing conditions, culture media (MEMA, MEMB and wash MEMA), and the rationale for the design of the protocol are provided in ref. (19). This design is followed in the present work with some modifications of the DMSO concentrations.
Toxicity of DMSO at 10.5°C
Preliminary studies were carried out to determine the toxicity of the chemical protector DMSO at 10.5°C as a function of the concentration of DMSO in the medium. To 1 ml of conditioned cells (4 × 105 cells/ml), as defined in ref. (19), was added an additional 1 ml of MEMB, and all tubes were returned to the rocker-roller. After a 12-h incubation period, the cells were washed three times with wash MEMA and finally suspended in 2 ml ice-cold MEMA containing 5–12.5% DMSO (Sigma Chemical Co., St. Louis, MO). The tubes were capped tightly and immediately placed in ice and then transferred onto the rocker-roller at 10.5°C. The tubes were maintained there for 72 h; then the cells were washed three times with cold wash MEMA. After they were resuspended in 2 ml MEMA, the cells were serially diluted, seeded into tissue culture dishes, and incubated under standard conditions (37°C, 95% air and 5% CO2) for 1 week; the resulting colonies stained and scored. The surviving fraction compared to parallel control cell cultures (0% DMSO) was determined. No toxicity was observed when the V79 cells were maintained at 10.5°C for 72 h in culture medium containing 5% DMSO. However, some concentration-dependent toxicity resulted at higher DMSO concentrations. Therefore, in all subsequent experiments involving the radioprotective capacity of DMSO, parallel controls for each DMSO concentration were strictly maintained to correct for the toxicity.
Survival after Exposure to 3H2O in the Presence of Different Concentrations of DMSO
Two sets of culture tubes (five per set) containing 1 ml of conditioned cells were prepared. Then 0.75 ml of MEMB containing 55.5 MBq of 3H2O was added to the first set of five tubes and 0.75 ml of MEMB alone was added to the second set of five tubes, and the tubes were placed on a rocker-roller under standard conditions. After 12 h, an additional 0.25 ml of MEMB containing different amounts of DMSO was added to each set of tubes while vortexing to achieve final DMSO concentrations of 0, 5, 7.5, 10 and 12.5%, and the tubes were transferred to a rocker-roller at 10.5°C. Thus, for each concentration of DMSO, two tubes were prepared with final 3H2O concentrations of 0 and 27.8 MBq/ml, respectively. After 72 h, the tubes were centrifuged at 2000 rpm, 4°C for 10 min. Aliquots of the supernatant were used to check the radioactivity added. The were cells washed three times with wash MEMA, and cell survival was determined. To correct for chemotoxicity, the cell surviving fraction, SF, was calculated according to Eq. (1):
| (1) |
where N is the number of colonies corresponding to the treatment regimen described in the parentheses. As indicated in the Results section, the highest surviving fraction for equivalent levels of 3H was observed for 10% DMSO.
Survival after Exposure to 3H2O in the Presence of 10% DMSO
In separate experiments, 0.8 ml of MEMB containing different activities of 3H2O (0–92.5 MBq) was transferred to two sets of culture tubes (five tubes per set) containing 1 ml of conditioned V79 cells. The tubes were returned to the rocker-roller (standard conditions). After 12 h, an additional 0.2 ml of DMSO (first set of tubes) or MEMA (second set) was added while vortexing and the tubes were transferred to a rocker-roller at 10.5°C. Thus, for each concentration of 3H2O, two tubes were made with final DMSO concentrations of 0 and 10%, respectively. After 72 h, the cells were processed as described above. The surviving fractions were again calculated according to Eq. (1). More specifically, these can be expressed as indicated below in Eqs. (2) and (3).
| (2) |
| (3) |
Survival after Exposure to 210Po, 131IdU and 125IdU in the Presence of Different Concentrations of DMSO
The protocols for the intracellularly bound radiochemicals were similar to those described for 3H2O. After a 30-min (210Po) or 12-h (131IdU, 125IdU) exposure to a single concentration of the radiochemical in MEMB, the cells were washed three times with wash MEMA and resuspended in 2 ml MEMA containing different concentrations of DMSO (0–12.5%). The tubes were transferred to the rocker-roller at 10.5°C. Parallel DMSO controls were strictly maintained for each experiment. After 72 h at 10.5°C, the cells were processed for determination of cell survival. Aliquots were taken from each tube before serial dilution, and the mean radioactivity per cell was determined (4, 19). The surviving fraction compared to that of parallel controls was determined using Eq. (1).
Survival after Exposure to 210Po, 131IdU and 125IdU in the Presence of 10% DMSO
For the experiments with 10% DMSO, conditioned V79 cells were radiolabeled as described above using different concentrations of radioactivity in MEMB. After the cells were washed free of extracellular radioactivity, they were resuspended in MEMA containing 10% DMSO and kept at 10.5°C for 72 h. Then the cells were processed for colony formation. The surviving fractions for the radiolabeled cells exposed to 0 and 10% DMSO were calculated using Eqs. (2) and (3).
RESULTS
Toxicity of DMSO at 10.5°C
The chemotoxicity of DMSO as a function of its concentration in the culture medium was determined by averaging the data for DMSO alone (no radiochemical) from all experiments. The surviving fraction for cells treated with 5–12.5% DMSO at 10.5°C compared to that for untreated controls was 1.04 ± 0.08, 0.84 ± 0.07, 0.74 ± 0.07 and 0.65 ± 0.06 for 5, 7.5, 10 and 12.5% DMSO, respectively. There was no toxicity associated with maintaining the cells at 10.5°C, 0% DMSO (19).
Survival after Exposure to 3H2O, 131IdU and 125IdU in the Presence of Different Concentrations of DMSO
Figure 1 shows the profile of the dependence of the dose modification factor (DMF) on the concentration of DMSO in the culture medium. These data are from two independent experiments in which cells exposed to a single concentration of radioactivity in the culture medium were subsequently treated with different concentrations of DMSO (0–12.5%) at 10.5°C for 72 h. The mean lethal concentration, C0 (3H2O), and cellular uptake, A0, of radioactivity (125IdU, 131IdU) corresponding to each concentration of DMSO was estimated by assuming that the surviving fraction is exponentially dependent on these variables as per Eqs. (4) and (5).
FIG. 1.
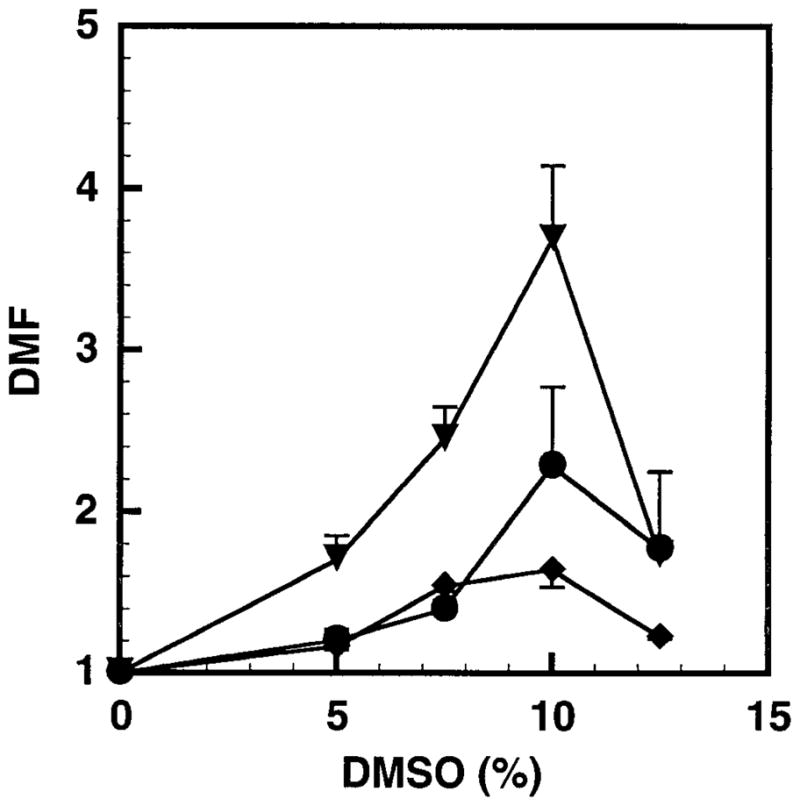
Dose modification factor (DMF) as a function of DMSO concentration. V79 cells were exposed to 3H2O (▼), 131IdU (◆) or 125IdU (●) at 37°C for 12 h and subsequently treated with different concentrations of DMSO at 10.5°C for 72 h. Each point indicates the mean ± SD for two individual experiments.
| (4) |
| (5) |
Since the absorbed dose received by the cells is directly proportional to the concentration of 3H2O in the culture medium or the mean cellular uptake of 131IdU or 125IdU, the DMF, or the degree of protection provided by DMSO against the lethal effects of radionuclides, is given by the ratio of the C0’s or A0’s in the presence and absence of DMSO:
| (6) |
The results in Fig. 1 illustrate that DMSO protects V79 cells from damage caused by DNA-bound 131IdU and 125IdU and unbound 3H2O in a concentration-dependent manner. Little protection was afforded by 5% DMSO against damage caused by DNA-bound 131IdU or 125IdU; however, more substantial protection was observed in the case of 3H2O. DMSO-mediated protection was optimal at 10% for all three radiochemicals. A marked decline in the DMF was observed for all three radiochemicals at 12.5% DMSO.
Survival after Exposure to 3H2O, 131IdU, 125IdU and 210Po in the Presence of 10% DMSO
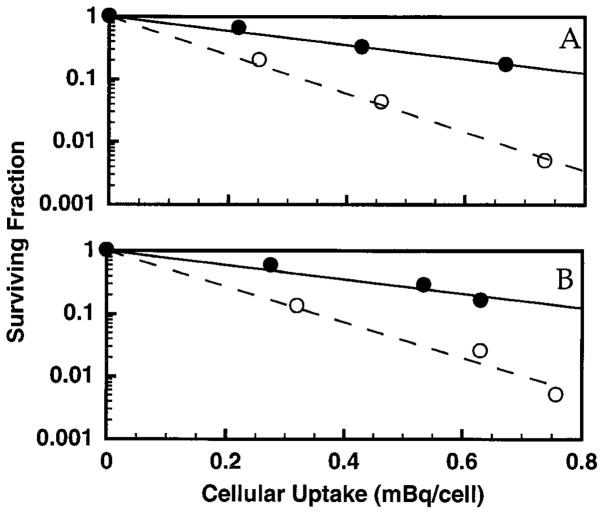
The radioprotective efficacy of 10% DMSO was investigated further by exposing V79 cells to different concentrations of unbound 3H2O and different cellular uptakes of DNA-incorporated 131IdU and 125IdU. In addition, the capacity of 10% DMSO to protect against the direct effects of 210Po α particles was investigated. Figure 2 shows the survival of V79 cells after a 72-h exposure at 10.5°C to various concentrations (0–46.3 MBq/ml) of 3H2O in the absence and presence of 10% DMSO. The data were fitted by least squares to Eq. (4) to obtain the mean lethal concentration, C0, for 3H2O. The DMF values for each experiment as presented in Table 1 were calculated using Eq. (6). The mean DMF for the two experiments was 2.9 ± 0.01.
FIG. 2.
Survival of V79 cells after a 12-h exposure at 37°C followed by a 72-h exposure at 10.5°C to various concentrations of 3H2O in the culture medium in the absence (▽) and presence (▼) of 10% DMSO. The data for two independent experiments are shown in panels A and B. Least-squares fits of the data to a monoexponential function are shown. Standard deviations for individual data points are of the order of the dimensions of the symbols.
TABLE 1.
Dose Modification Factors in the Presence of 10% DMSO
| 10% DMSO
|
0% DMSO
|
DMF | ||||
|---|---|---|---|---|---|---|
| Radiochemical | Experiment no. | n | C0 (MBq/ml) | n | C0 (MBq/ml) | |
| 3 H2O | 1 | 1 | 21.6 | 1 | 7.26 | 2.96 |
| 2 | 1 | 36.0 | 1 | 12.2 | 2.94
|
|
| 2.9 ± 0.01 | ||||||
| Radiochemical | Experiment no. | 10% DMSO
|
0% DMSO
|
DMF | ||
|---|---|---|---|---|---|---|
| n | A0 (mBq/cell) | n | A0 (mBq/cell) | |||
| 210Po citrate | 1 | 1 | 0.0218 | 1 | 0.0243 | 0.90 |
| 2 | 1 | 0.0193 | 1 | 0.0191 | 1.01
|
|
| 0.95 ± 0.07 | ||||||
| 131IdU | 1 | 2.10 | 7.85 | 3.25 | 4.03 | 1.94 |
| 2 | 1.78 | 8.40 | 3.87 | 3.13 | 2.68
|
|
| 2.3 ± 0.5 | ||||||
| 125IdU | 1 | 1 | 0.383 | 1 | 0.141 | 2.72 |
| 2 | 1 | 0.382 | 1 | 0.153 | 2.50
|
|
| 2.6 ± 0.2 | ||||||
Figures 3–5 show the survival of V79 cells after treatment with 131IdU, 125IdU or 210Po. The surviving fraction as a function of mean activity per cell was plotted for each experiment, and the data were fitted to the relationship
FIG. 3.
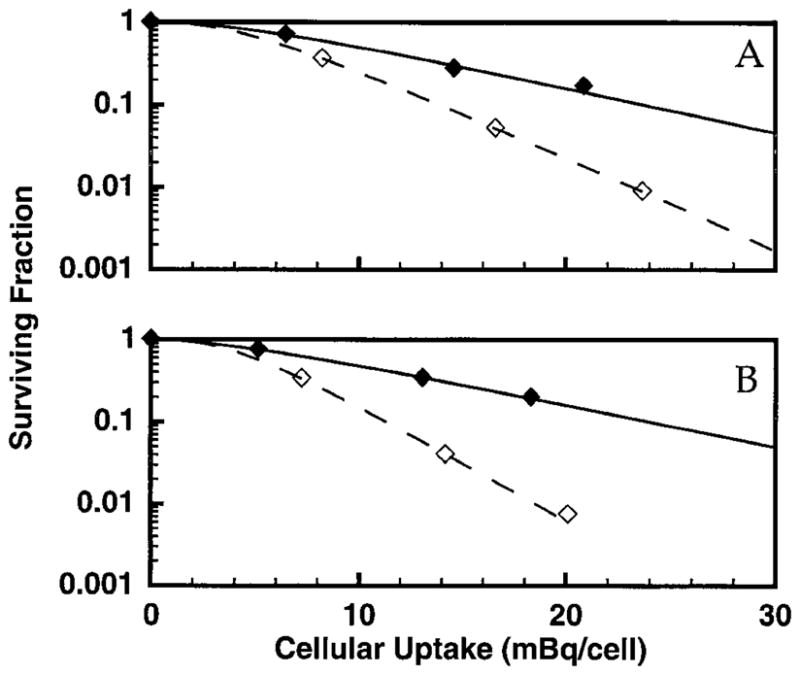
Survival of V79 cells as a function of intracellular activity of 131IdU in the absence (⋄) and presence (◆) of 10% DMSO in the culture medium. The data for two independent experiments are shown in panels A and B along with the corresponding least-squares fits to the single-hit multi-target model. Standard deviations for individual data points are of the order of the dimensions of the symbols.
FIG. 5.
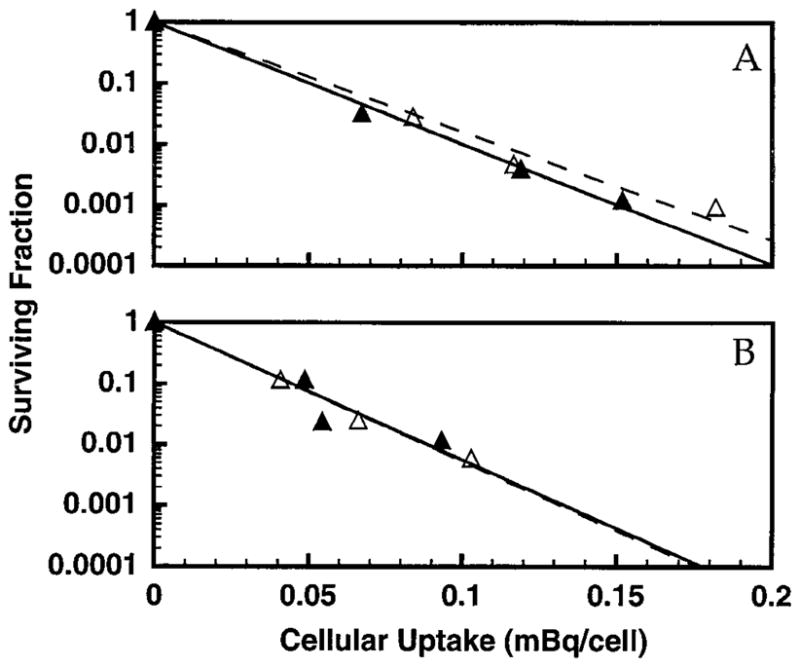
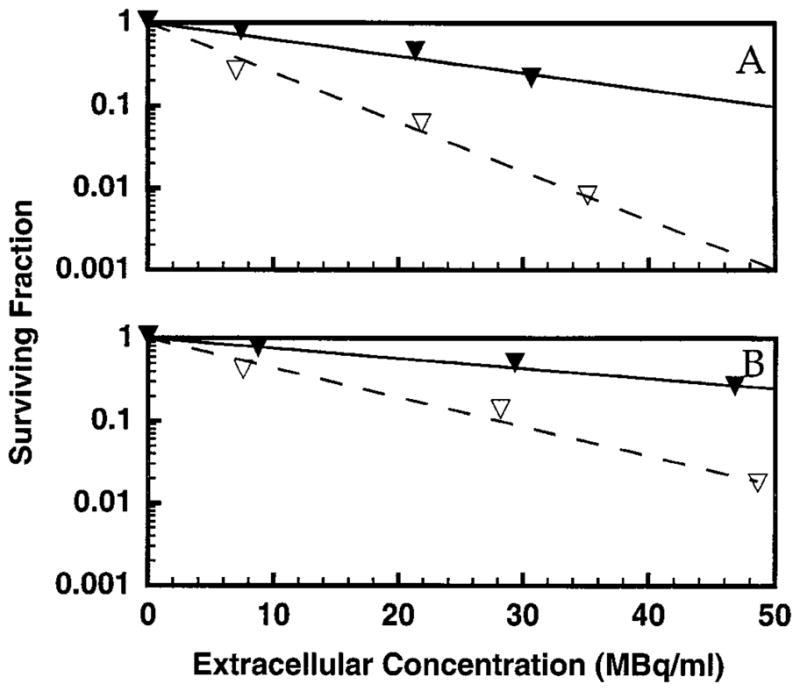
Survival of V79 cells as a function of intracellular activity of 210Po in the absence (△) and presence (▲) of 10% DMSO. The data for two independent experiments are shown in panels A and B. Least-squares fits of the data to a monoexponential function are shown. Standard deviations for individual data points are of the order of the dimensions of the symbols.
| (7) |
where A is the mean activity per cell at the end of the uptake period and A0 and n are analogous to the D0 and n in the single-hit multi-target model (24), respectively. The DMF is given by the ratio of the values of A0 in the presence and absence of DMSO. A value of n = 1 was used for the fit to the data for 125IdU and 210Po. As given in Table 1, the mean DMFs for protection by 10% DMSO against the lethal effects of 131IdU, 125IdU and 210Po are 2.3 ± 0.5, 2.6 ± 0.2 and 0.95 ± 0.07, respectively.
DISCUSSION
Mean Lethal Number of Decays, Mean Lethal Dose, and Relative Biological Effectiveness
Cellular dosimetry is used to calculate the mean lethal number of intracellular decays and mean absorbed dose to the cell nucleus for each of the intracellularly incorporated radiochemicals examined in the present study. Using the methods outlined in the Appendix, the mean lethal number of decays, N37, that are required to achieve 37% survival in the absence of DMSO is 7.1, 2240 and 49 for 210Po citrate, 131IdU and 125IdU, respectively (Table A1). The values for 210Po citrate and 125IdU are in good agreement but are slightly lower than the values of 9 and 66 obtained in our earlier work carried out at 37°C (4). The mean lethal absorbed dose, D37, to the cell nucleus (calculated as described in the Appendix) in the absence of DMSO is 0.68, 2.4 and 0.33 Gy, respectively. The 0.68 Gy obtained for 210Po citrate corresponds to about two complete α-particle traversals across the cell nucleus (4). Also calculated in the Appendix is the D37 for 3H2O. This radiochemical yields a D37 of 2.7 Gy. These data are presented in Table 2 and are compared with the D37 of 7.1 Gy for unbound 32P, an emitter of energetic β particles (see ref. 19 and Appendix). This radionuclide emits energetic β particles which are clearly low-LET in nature and are the same type of particle emitted by 3H, 125I and 131I (i.e. electron); it also delivers the exposure in a chronic fashion. Thus, in keeping with the definition of RBE (24), it serves as a good reference radiation. In view of the historical use of external γ rays as the reference radiation, it would also be useful to study the effects of acute 137Cs or chronic 99mTc γ rays. However, the geometry of the present experiment precluded their use. The RBE of 3H2O, 210Po citrate, 131IdU and 125IdU compared to extracellular 32P (D37 of 7.1 Gy) at the D37 are 2.6, 10, 3.0 and 22, respectively (Table 2). The high RBE values for 210Po citrate and 125IdU are expected and are similar to those obtained by Howell et al. (4) when chronic 99mTc γ rays were used as the reference radiation and the V79 cells were maintained at 37°C. While the RBE of 2.6 for 3H2O is not surprising in view of the LET (3.3 keV/μm) of the 5.7 keV β particles emitted by 3H (25), it also falls within the range of RBE values observed for 3H2O for a variety of end points (23). The RBE of 3.0 for 131IdU is perhaps surprising given the low-LET nature of the β particles (mean energy = 191 keV) emitted by 131I. Possible explanations for this relatively high RBE are discussed at the end of the Discussion section.
TABLE A1.
Calculation of D37 to Cell Nucleus from Radiochemicals
| Radiochemical | A0 (mBq/cell) | ÃI (Bq s) | ÃM (Bq s) | ÃCF (Bq s) | Ã0 (Bq s) a | D0 (Gy) | D37 (Gy) | N37 (decays)a |
|---|---|---|---|---|---|---|---|---|
| 210Po citrate | 0.0217 ± 0.0037 | 1.95 × 10−2 | 5.57 × 100 | 1.53 × 100 | 7.1 ± 1.3 | 0.68 ± 0.12 | 0.68 ± 0.12 | 7.1 ± 1.3 |
| 131IdU | 3.58 ± 0.64 | 7.73 × 101 | 8.15 × 102 | 1.71 × 102 | 1060 ± 190 | 1.14 ± 0.20 | 2.4 ± 0.4 | 2240 ± 370 |
| 125IdU | 0.147 ± 0.0085 | 3.18 × 100 | 3.74 × 101 | 8.83 × 100 | 49.0 ± 3.0 | 0.33 ± 0.02 | 0.33 ± 0.02 | 49.0 ± 3.0 |
The cumulated activity, Ã, is equivalent to the number of decays. Hence the number of decays required to achieve 37% survival, N37, is equivalent to Ã37.
TABLE 2.
Relative Biological Effectiveness
| Radiochemical | D37 (Gy) | RBE |
|---|---|---|
| Unbound | ||
| 32P | 7.1 ± 0.7 | 1.0 |
| 3H2O | 2.7 ± 1.0 | 2.6 ± 1.0 |
| Intracellular | ||
| 210Po citrate | 0.68 ± 0.12 | 10 ± 2 |
| 131IdU | 2.4 ± 0.4 | 3.0 ± 0.6 |
| 125IdU | 0.33 ± 0.02 | 22 ± 2 |
DMSO as a Radioprotector
Ionizing radiation is generally believed to impart damage to biological tissue by two primary mechanisms: direct and indirect. Direct effects are the result of energy deposited directly in critical molecular structures within the cell. Indirect effects are due to interactions between a variety of free radicals (OH•, H•, etc.) produced in the radiolysis of water with the critical molecules (26). The biological damage caused by high-LET radiations such as α particles has been attributed primarily to direct effects (27), whereas the biological effects of low-LET radiations (e.g. β particles, X rays) are caused primarily by indirect effects (26).
Dimethyl sulfoxide is a radioprotector whose protective action is due principally to scavenging of hydroxyl radicals (28–30). The capacity of DMSO to provide substantial protection against biological effects caused by low-LET radiations has been demonstrated using a variety of experimental end points (11, 28, 31–34). As a radical scavenger, the ability of DMSO to protect against the direct action of high-LET α particles is minimal (11, 34). Therefore, it is well suited for the study and comparison of the mechanisms of the action of different radiations emitted by tissue-incorporated radionuclides. However, typical cell survival experiments in vitro using DMSO as a radioprotector involve acute radiation exposures in the presence of DMSO concentrations as high as 15–20% (v/v), after which the cells are immediately washed free of the radioprotector to minimize the toxic effects of DMSO (31). When cells are irradiated by radionuclides, the dose is delivered chronically, and therefore the chemical agent should be present during the extended irradiation time. To minimize the toxicity associated with exposure of cultured cells to radioprotective concentrations of DMSO at 37°C, we have used our techniques reported previously (19) and have maintained the cells at 10.5°C throughout most of the exposure period. Under these conditions, the V79 cells were not proliferating, no cryotoxicity was observed, and only minimal toxicity resulted even at the highest DMSO concentration used (12.5%). This is in contrast to the studies of Walicka et al. (20), where a high degree of cryotoxicity was observed when the V79 cells were maintained at 0.3°C in the absence of DMSO. This is expected based on our previous studies (19) and therefore suggests that 10.5°C may be better suited for the present studies.
DMF as a Function of DMSO Concentration
The results presented in Fig. 1 show a marked dependence of the DMF on DMSO concentration in the culture medium. As indicated in the Materials and Methods section, these data were obtained using procedures that emphasized obtaining relative DMF values. The DMFs for the various radiochemicals rise rapidly as the DMSO concentration increases from 5% to 10% and then fall precipitously at 12.5% DMSO. This is in keeping with data reported by Vos and Kaalen (31), who found that when cells were acutely irradiated with 250 kVp X rays in the absence and presence of DMSO, the DMF increased linearly with DMSO concentration up to 30% v/v and then could not be measured due to extreme chemotoxicity. Therefore, for both chronic and acute irradiation conditions, there appears to be a window for optimum radioprotection with minimal undesired chemotoxic effects on the V79 cells. Similar observations were made by Roberts et al. (35) for the radioprotector MEA.
Contribution of Indirect Effects to Lethality of DNA-Incorporated Radionuclides
Figures 2–5 and Table 1 directly compare the radioprotection provided by 10% DMSO against lethal damage to V79 cells caused by 3H2O, 131IdU, 125IdU and 210Po citrate. The highest DMF was obtained for 3H2O: 2.9 ± 0.01. This radiochemical freely diffuses into the cells and does not bind to DNA. Similar DMF values were obtained for DNA-incorporated 131IdU (2.3 ± 0.5) and 125IdU (2.6 ± 0.2) despite their disparate radiation emissions and RBE values. Each decay of 131I emits low-LET β particles with a yield close to unity (25), whereas 125I emits about 25 low-energy Auger electrons per decay (36). In contrast to these results, a DMF of 0.95 ± 0.07 was obtained for intracellular 210Po, which emits a single 5.3 MeV α particle per decay. Therefore, 10% DMSO offers no protection against high-LET α particles, which are known to impart biological damage largely by direct effects. Substantial protection is provided against low-energy β particles emitted by unbound 3H, which cause biological damage primarily by indirect effects. Given the high degree of protection afforded by 10% DMSO in the case of 125IdU and 131IdU, these data suggest that DNA-incorporated 131I and 125I impart lethal damage by a similar mechanism (e.g. indirect). This conclusion is in agreement with that of Rao and colleagues, who showed that DNA-incorporated Auger electron emitters cause lethal damage to spermatogonial cells in mouse testes principally through indirect effects (2, 7–12). This series of in vivo experiments culminated with the work of Goddu et al. (11), which showed that DMSO provides substantial protection against lethal damage from 125IdU (DMF = 4.4 ± 1.0) and cytoplasmically localized 125I (DMF = 3.1 ± 1.0) and offers no protection against lethal damage caused by 5.3 MeV α particles emitted by 210Po citrate (DMF = 1.1 ± 0.2).
Walicka et al. (20) also found that 10% DMSO provided ample protection against the lethal effects of 125IdU in V79 cells (DMF = 5.4 ± 0.6) and reached the same conclusions as Rao and colleagues (2, 7–12) regarding the indirect mechanism of action of DNA-incorporated Auger electron emitters in mammalian cells. Walicka et al. (20) labeled V79 cells with 125IdU for 18 h at 37°C and then stored them on ice for 6 to 48 h for accumulation of decays prior to plating for colony formation. Decays of 125I occur in the cell during the 18-h uptake period, the time during which the cells were stored on ice, and during the colony-forming period. The effect of decays that occurred during the 18-h uptake period and colony-forming period was factored out by using appropriate controls. The resulting surviving fraction, corrected for cryotoxicity and the effects of decays that occurred during the uptake and colony-forming periods, was plotted as a function of intracellular decays that occurred during the storage time on ice. If one examines the number of decays that occur during each period, the correction is substantial. Consider, for example, a cellular uptake of 0.1 mBq/cell after the 18-h uptake period. The intracellular cumulated activity during the uptake period would be 3.2 Bq s (1 Bq s = 1 decay). If the intracellular activity during the colony-forming period decreases with an effective half-time of 12 h, the cumulated activity during this period would be 6.2 decays. Only 2.2 decays would occur in the cells during the period on ice if they were maintained there for 6 h. Therefore, only 20% of the decays occurred in the presence of DMSO, and the effects of the remaining decays had to be factored out by using controls. Hence the surviving fractions were extracted from data that contain high “background noise”. This problem is alleviated somewhat for the later times. When the cells were kept on ice for 24 and 48 h, 48 and 65% of the decays, respectively, occurred in the presence of DMSO. These large corrections may explain in part why Walicka et al. (20) obtained a much higher DMF for 125IdU than for acute 137Cs γ rays. The chronic and acute irradiation protocols for these two radiation sources may also be a factor.
Despite the different experimental models and procedures used to obtain the various data sets on radioprotection against the lethal effects of DNA-incorporated 125I in mammalian cells with radical scavengers and other radioprotectors, most of the results are in relatively good agreement. The early work of Rao and colleagues with mouse testis (11), the recent studies of Walicka et al. (20), and the present studies all strongly support the hypothesis of Rao et al. (7) that indirect mechanisms predominate in the lethal effects of DNA-incorporated Auger electron emitters. Our data for 131IdU indicate that the same can be concluded for DNA-incorporated β-particle emitters. This is expected based on conventional wisdom.
Given that DNA-incorporated Auger electron emitters appear to impart biological damage largely through indirect effects, how is it that they can produce high-LET-type survival curves? Linz and Stöcklin (37) used a supercoiled plasmid DNA model to show that 125I decays cause DSBs hundreds of base pairs from the decay site and that formation of these particular DSBs is strongly influenced by the presence of radical scavengers. In contrast, the presence of radical scavengers had no impact on DSBs formed in the immediate vicinity of the decay site. Furthermore, they found that the 125I decays caused one to three DSBs per decay. Although Linz and Stöcklin argued that the distant DSBs were unimportant compared with the DSBs formed in the immediate vicinity of the decay site, their data show that significant indirect damage occurs at locations distant from the decay site. This latter finding is supported by the theoretical calculations of Terrissol (22), who showed that the convoluted structure of chromatin in the cell nucleus provides an environment whereby radicals resulting from the dense shower of low-energy Auger electrons can cause DSBs hundreds of base pairs distant from the actual site of decay. In fact, Terrissol’s calculations predict that 75% of all DSBs caused by DNA-incorporated 125I are due to indirect effects. Similar to Linz and Stöcklin (37), Walicka et al. (21) have found that DSBs produced by 125IdU in V79 cells can also be protected in part by 10% DMSO and that more than one DSB is produced per decay. Like Terrissol, they invoke a nucleosome model of chromatin structure and suggest that one DSB is due to local effects whereas the additional DSBs are due to interaction of SSBs at distances far from the decay site. This is a plausible hypothesis for explaining the capacity of radioprotectors to mitigate the high-LET-type effects of DNA-incorporated 125I. Interestingly, in 1979, Rao et al.2 hypothesized that the nucleosome was the relevant biological target for DNA-incorporated Auger electron emitters.
Contribution of Direct Effects to Lethality of DNA-Incorporated Radionuclides
Despite the apparent importance of indirect effects in the lethal action of DNA-incorporated Auger electron and β-particle emitters, our data also provide insights into the contribution of direct effects to their lethality. It is interesting to note that the DMFs shown in Table 1 for 10% DMSO are somewhat higher for unbound 3H2O (2.9 ± 0.01) compared to DNA-incorporated 125IdU (2.6 ± 0.2) and 131IdU (2.3 ± 0.5). In addition, our previous communication showed that 5% DMSO provided ample protection against lethal effects caused by unbound 3H2O (DMF = 2.3 ± 0.3) as well as unbound 32P (DMF = 2.6 ± 0.5), which emits energetic β particles (19). However, little or no protection was afforded by 5% DMSO in the case of DNA-incorporated [3H]dThd, 125IdU and 131IdU (19). Therefore, although indirect effects appear to be principally responsible for the lethal damage caused by [3H]dThd, 125IdU and 131IdU, other mechanisms are involved in the damage imparted by DNA-incorporated radionuclides. However, the damage caused by other mechanisms appears to constitute a smaller fraction of the overall damage responsible for cell death.
The portion of the lethal damage to mammalian cells that is imparted by DNA-incorporated radionuclides that is not due to indirect effects can, in principle, be due to direct effects, charge-induced molecular fragmentation (125I) (38, 39), chemical transmutation (40), recoil, and other mechanisms such as long-range energy transfer (41). Consider first the case of the Auger electron emitter 125I. Charge-induced molecular fragmentation as a consequence of the 125I Auger electron cascade is not supported by the data of Hofer et al. (42) or Goddu et al. (11), nor is it supported by the present data. While chemical transmutation cannot be ruled out by the present data, the arguments of Makrigiorgos et al. (43), who compared the effects of 77BrdU, 123IdU and 125IdU, strongly indicate that transmutation is not a significant factor. Recoil obviously is not a significant factor in the case of Auger electron emitters. While long-range energy transfer may be important in the case of 125I (44), it appears that direct effects are the most likely secondary mechanism (11). The presence of a direct component is supported by the data of Kandaiya et al. (15) and Lobachevsky and Martin (16), who found that in the case of 125I-labeled oligonucleotides in vitro, where no cross irradiation from nearby labeled oligonucleotides is present, DMSO does not provide protection against strand breaks within 9 bp of the decay site and provides only limited protection against breaks that occur at more distant locations. Similarly, Adelstein and Kassis (14) found that DMSO protected against damage to plasmid DNA caused by unbound 125I in the solution; however, no protection was observed against damage caused by 125I-labeled Hoechst 33342, which binds to the minor groove of DNA in A-T rich regions. These data suggest that direct effects from 125I decays in the DNA may be responsible for DNA damage within a few base pairs of the decay site.
The importance of direct effects is also supported by the data of Hofer and Bao (13), who examined the capacity of MEA to protect against lethality and induction of micronuclei in CHO cells maintained at −196°C. A low-LET-type response was observed when cells were synchronized at the G1/S-phase boundary, allowed to progress synchronously into S phase for 30 min, pulse-labeled with 125IdU for 10 min, and then incubated for 30 min in nonradioactive medium prior to freezing. In contrast, a high-LET-type response was observed when the cells were incubated 5 h prior to freezing. Cysteamine provided protection against the effects of 125I during the initial phase of effects characteristic of low-LET radiation, but no protection was seen during the phase of effects characteristic of high-LET radiation. Through comparison with parallel studies with external X rays, Hofer and Bao concluded that the latter phase was indeed of a high-LET nature and suggested that the 125I must be contained within higher-order structures in the cell nucleus to observe this phenomenon. It is difficult to reconcile the absence of protection against their high-LET-type effects with the protection observed for mammalian cells in a liquid environment. One clue to this difference may lie in the lack of protection provided by MEA against the high-LET-type effects. MEA has been shown to provide protection against high-LET radiation (45), so it is curious that it was unable to provide any protection against the high-LET-type effects in the studies of Hofer and Bao. It is possible that 25 mM MEA was sufficient to protect against low-LET-type effects while higher concentrations may be required to protect against high-LET-type effects. Perhaps the frozen conditions may also contribute to the differences observed between the various studies. Livesey has indicated that indirect effects are diminished or eliminated in dry or frozen biological preparations, while hydrogen donation is maintained (46). Accordingly, these conditions have been used by investigators to eliminate radical scavenging to study the hydrogen donation mechanism of radioprotection by thiols such as cysteamine (46). If Hofer and Bao have effectively eliminated radical scavenging and retained hydrogen donation, then the protective mechanisms operative in the liquid environment will be very different from those in the frozen state and therefore will be difficult to compare. If this is so, then perhaps the low-LET-type response observed by Hofer and Bao is modified by hydrogen donation whereas the high-LET-type response is not. The implication for the Auger electron effect is unclear.
Let us now turn our attention to the secondary mechanisms involved in the case of 131IdU. Our data indicate that DNA-incorporated 131I is about three times more lethal than external β particles (e.g. 32P). Furthermore, the differences in protection afforded by both 5 and 10% DMSO against the lethal effects of 3H2O and 131IdU suggest that a significant fraction of the damage imparted by this radiochemical is through mechanisms other than indirect. These findings are in accord with the data of Whaley and Little (47) for 131IdU as well as extensive data on DNA-incorporated 3H and 32P that have been reviewed by Halpern and Stöcklin (41). Halpern and Stöcklin have discussed the many possible mechanisms including direct effects, chemical transmutation, recoil, and other mechanisms such as long-range energy transfer. Whaley and Little have ruled out recoil as a mechanism for the enhanced toxicity of DNA-incorporated 131I due to the very low nuclear recoil energy of the xenon daughter. However, the remaining mechanisms could explain the observed effects. Elucidation of the exact mechanism will require further study.
CONCLUSIONS
The present data support the hypothesis that indirect effects are largely responsible for lethal damage to mammalian cells from DNA-incorporated Auger electron emitters both in vivo (2, 7–12) and in vitro (19–21). Only a small portion of the lethal damage is likely to be due to direct effects. In addition, β-particle emitters are substantially more toxic when incorporated into the DNA of mammalian cells than when they are localized extracellularly.
FIG. 4.
Survival of V79 cells as a function of intracellular activity of 125IdU in the absence (○) and presence (●) of 10% DMSO in the culture medium. The data for two independent experiments are shown in panels A and B. Least-squares fits of the data to a monoexponential function are shown. Standard deviations for individual data points are of the order of the dimensions of the symbols.
APPENDIX
Absorbed Dose From 3H2O
The cells were exposed to 3H2O in 17 × 100-mm Falcon sterile polypropylene culture tubes placed on a rocker-roller. The rocker-roller maintained the cells as a uniform suspension in 2 ml of culture medium. Given that 3H2O diffuses freely into the cells, the mean absorbed dose to the cells is equal to that to the culture medium. The mean absorbed dose to the culture medium per unit cumulated activity of 3H in the culture medium (S) is given by (48):
| (A1) |
where Δ is the mean energy emitter per transition, φ is the absorbed fraction of energy emitted by 3H that is absorbed in the culture medium, and m is the mass of the culture medium (m = 0.002 kg). For 3H, Δ = 9.08 × 10−16 Gy kg Bq−1 s−1 (25); φ = 1 due to the short range of the tritium β particles (mean energy = 5.67 keV). Therefore, S = 4.54 × 10−13 Gy/Bq s for 3H2O. The cumulated activity, Ã, in the tube is given by
| (A2) |
where A(t = 0) is the initial activity in the tube, 𝕵 is the total exposure time, and Tp is the physical half-life. For 3H, is the initial 12-h period at 37°C followed by 72 h at 10.5°C for a total of 84 h. The physical half-life, Tp, is 12.33 years (25). In the absence of DMSO, the mean C0 for the two experiments is 9.7 ± 3.5 MBq/ml (Table 1), therefore, A(t = 0) = 19.5 MBq. Since Tp is very long compared to 84 h, the cumulated activity corresponding to C0 is given by
The mean absorbed dose is given by (49)
| (A3) |
Therefore, the mean absorbed dose delivered to the cells in the absence of DMSO at a 3H2O concentration corresponding to C0 is (5.89 × 1012 Bq s) (4.54 × 10−13 Gy/Bq s) = 2.7 ± 1.0 Gy. Because the dose–response relationship is exponential, this is equal to the dose required to achieve the D37.
Mean Lethal Absorbed Dose and Number of Intracellular Decays from Intracellular 131IdU, 125IdU and 210Po Citrate
The V79 cells were exposed to low concentrations of extracellular 131IdU, 125IdU or 210Po citrate for a short period, washed free of extracellular activity, maintained at 10.5°C for a 72 h, plated for colony formation, and incubated for 7 days, during which the activity per cell decreased as the cells divided to form colonies (4). Following the general formalism for cellular dosimetry given by Eq. 7 of ref. (49), the mean absorbed dose to the cell nucleus, DN, is given by
| (A4) |
| (A5) |
where ÃI, ÃM and ÃCF are the cellular cumulated activities during the periods of incubation for cellular uptake of radioactivity, maintenance at 10.5°C for 72 h, and colony formation, respectively. The quantities fN and fCy are the fraction of cellular activity in the nucleus and cytoplasm, respectively, where fN + fCy = 1. The values of fN are 0.28, 1.0 and 1.0 for 210Po citrate, 131IdU and 125IdU, respectively (4). The quantities S(N←N) and S(N←Cy) are the cellular S values (absorbed dose per unit cumulated activity) for the radionuclide localized in the nucleus and cytoplasm, respectively. The values for S(N←N) are 0.155, 0.00107 and 0.00667 Gy Bq−1 s−1 for 210Po, 131I and 125I when localized in cells with a radius of 5 μm and a nuclear radius of 4 μm (50). These dimensions correspond to those of V79 cells (4). The value for S(N←Cy) for 210Po is 0.0712 Gy Bq−1 s−1 (50).
Since these radiopharmaceuticals are taken up by the cells linearly with time (4), the cellular cumulated activity during the uptake period ÃI is given by
| (A6) |
where AI is the average cellular activity (Bq/cell) at the end of the uptake period and tI is the incubation time during which the radioactivity is taken up by the cells [tI( 210Po) = 0.5 h, tI(125IdU/131IdU) = 12 h]. During a maintenance period, tM, of 72 h, the radioactivity undergoes physical decay only. Therefore, the cumulated activity during this time is given by
| (A7) |
The Tp of 210Po, 131I and 125I is 138, 8 and 60 days, respectively (25). Finally, the cumulated activity during the colony-forming period, tCF, is given by
| (A8) |
where Te,CF is the effective half-time of the radioactivity in the cells during this period. These values are 13.8, 12 and 12 h for 210Po citrate, 131IdU and 125IdU (4).
Column 2 of Table A1 lists the mean A0 based on the values given for two experiments in Table 1. In addition, the cellular cumulated activities during each period assuming a cellular uptake equal to A0 are provided in columns 3–5 of Table A1. The total cumulated activity assuming a cellular uptake of A0 is given by Ã0 in column 6 of Table A1. The D0 is calculated according to Eq. (A4): D0 = Ã0 [fN S(N← N) + fCy S(N← Cy)] and is given in column 7. Since the dose D is directly proportional to the intracellular activity A, the surviving fraction can also be expressed as
| (A9) |
Therefore, the mean absorbed dose to the cell nucleus required to achieve the D37 can be obtained by setting the SF as 0.37 and solving Eq. (A9) for D = D37.
| (A10) |
The mean value of n from two independent experiments is taken from Table 1. The D37 values are given in column 8 of Table A1. Finally, the corresponding number of decays required to achieve 37% survival is equal to Ã37 and is given by N37 in the last column of Table A1.
Absorbed Dose from Extracellular 32P
In our earlier publication, we used the same experimental techniques to irradiate the suspended V79 cells with β particles emitted by extracellular 32P (19). This is a good reference radiation for use in calculating RBE values for chronic irradiation by incorporated radionuclides. However, no dosimetry calculations were presented in our earlier work. Therefore, the dosimetry calculations are outlined below for the purpose of determining the D37 for β particles emitted by extracellular 32P. As in the present study, the cells were exposed to the radiochemical in 17 × 100-mm Falcon sterile polypropylene culture tubes placed on a rocker-roller. The rocker-roller maintained the cells as a uniform suspension in 2 ml of culture medium. The absorbed fraction, φ, or the fraction of energy emitted by 32P that is absorbed in the culture medium, depended on the geometry of the solution in the tube. The absorbed fraction is maximum when the culture tube is oriented vertically and minimum in the horizontal position. When placed on the rocker-roller, the geometry oscillates between ones that approximate these two configurations. The average of the absorbed fractions in these two configurations may be used to calculate the mean absorbed dose to the culture medium. Accordingly, the EGS4 Monte Carlo track structure code (50–52) was used to simulate 100,000 32P β-particle histories for each geometry (vertical and horizontal). For the vertical geometry, the 2 ml of culture medium in the tube was taken to have a cylindrical geometry of 1.13 cm in length and a radius of 0.75 cm. The horizontal geometry was taken as a slice along the long axis of the cylindrical tube with dimensions of length 8 cm and a radius of 0.75 cm. This geometry resembles a shallow trough. When the full 32P β-particle spectrum (53) was used in these calculations, the mean absorbed dose per unit cumulated activity (S) was calculated to be 4.64 × 10−11 and 3.37 × 10−11 Gy/Bq s for the vertical and horizontal geometry, respectively. Thus the mean S for the culture medium is 4.00 × 10−11 Gy/Bq s. The cumulated activity, Ã, in the tubes is given by Eq. (A2). For the 32P experiments (19), = 72 h and Tp= 14.2 days (25). Thus Eq. (A2) can be integrated to give à = A(t = 0) · 66.9 h. Finally, the mean absorbed dose delivered to the cultured cells by the 32P β particles is given by
| (A11) |
where A(t = 0) is in Bq. Thus, with the mean value of A0(t = 0) = 2 ml (0.21 ± 0.028 MBq/ml) = 0.42 ± 0.056 MBq from ref. (19), the mean D0 value is 4.0 Gy in the absence of DMSO. Finally, with n = 2.5 (19), Eq. (A10) can be used to calculate the mean D37 = 7.1 ± 0.7 Gy.
Footnotes
D. V. Rao, G. F. Govelitz, K. S. R. Sastry, J. W. Bowers and J. T. Mallams, Radiotoxicity of I-125 deoxyuridine in vivo and microdosimetry of Auger and Coster Kronig electrons. Presented at the Fifth International Conference on Medical Physics, Medical Physics Department, Beilinson Medical Center, Petah Tikva, Israel, 1979.
References
- 1.Hofer KG, Harris CR, Smith JM. Radiotoxicity of intracellular Ga-67, I-125, H-3. Nuclear versus cytoplasmic radiation effects in murine L1210 leukaemia. Int J Radiat Biol. 1975;28:225–241. doi: 10.1080/09553007514550991. [DOI] [PubMed] [Google Scholar]
- 2.Rao DV, Narra VR, Howell RW, Sastry KSR. Biological consequence of nuclear versus cytoplasmic decays of 125I: Cysteamine as a radioprotector against Auger cascades in vivo. Radiat Res. 1990;124:188–193. [PubMed] [Google Scholar]
- 3.Rao DV, Narra VR, Howell RW, Govelitz GF, Sastry KSR. In-vivo radiotoxicity of DNA-incorporated I-125 compared with that of densely ionising alpha-particles. Lancet. 1989;II:650–653. doi: 10.1016/s0140-6736(89)90896-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howell RW, Rao DV, Hou DY, Narra VR, Sastry KSR. The question of relative biological effectiveness and quality factor for Auger emitters incorporated into proliferating mammalian cells. Radiat Res. 1991;128:282–292. [PubMed] [Google Scholar]
- 5.Martin RF, Haseltine WA. Range of radiochemical damage to DNA with decay of iodine-125. Science. 1981;213:896–898. doi: 10.1126/science.7256283. [DOI] [PubMed] [Google Scholar]
- 6.Charlton DE. The range of high-LET effects from 125I decays. Radiat Res. 1986;107:163–171. [PubMed] [Google Scholar]
- 7.Rao DV, Narra VR, Howell RW, Lanka VK, Sastry KSR. Induction of spermhead abnormalities by incorporated radionuclides: Dependence on subcellular distribution, type of radiation, dose rate, and presence of radioprotectors. Radiat Res. 1991;125:89–97. [PMC free article] [PubMed] [Google Scholar]
- 8.Narra VR, Harapanhalli RS, Howell RW, Sastry KSR, Rao DV. Vitamins as radioprotectors in vivo. I. Protection by vitamin C against internal radionuclides in mouse testes: Implications to the mechanism of the Auger effect. Radiat Res. 1994;137:394–399. [PubMed] [Google Scholar]
- 9.Narra VR, Harapanhalli RS, Goddu SM, Howell RW, Rao DV. Radioprotection against biological effects of internal radionuclides in vivo by S-(2-aminoethyl)isothiouronium bromide hydrobromide (AET) J Nucl Med. 1995;36:259–266. [PubMed] [Google Scholar]
- 10.Harapanhalli RS, Narra VR, Yaghmai V, Azure MT, Goddu SM, Howell RW, Rao DV. Vitamins as radioprotectors in vivo. II. Protection by vitamin A and soybean oil against radiation damage caused by internal radionuclides. Radiat Res. 1994;139:115–122. [PubMed] [Google Scholar]
- 11.Goddu SM, Narra VR, Harapanhalli RS, Howell RW, Rao DV. Radioprotection by DMSO against the biological effects of incorporated radionuclidesin vivo. Acta Oncol. 1996;35:901–907. doi: 10.3109/02841869609104044. [DOI] [PubMed] [Google Scholar]
- 12.Narra VR, Harapanhalli RS, Howell RW, Sastry KSR, Rao DV. Chemical protection against radionuclides in vivo: Implications to the mechanism of the Auger effect. In: Howell RW, Narra VR, Sastry KSR, Rao DV, editors. Biophysical Aspects of Auger Processes. American Institute of Physics; Woodbury, NY: 1992. pp. 319–335. [Google Scholar]
- 13.Hofer KG, Bao SP. Low-LET and high-LET radiation action of 125I decays in DNA: Effect of cysteamine on micronucleus formation and cell killing. Radiat Res. 1995;141:183–192. [PubMed] [Google Scholar]
- 14.Adelstein SJ, Kassis AI. Strand breaks in plasmid DNA following positional changes of Auger-electron emitting radionuclides. Acta Oncol. 1996;35:797–801. doi: 10.3109/02841869609104029. [DOI] [PubMed] [Google Scholar]
- 15.Kandaiya IS, Lobachevsky PN, D’Cunha G, Martin RF. DNA strand breakage by 125I-decay in a synthetic oligodeoxynucleotide—1. Fragment distribution and evaluation of DMSO protection effect. Acta Oncol. 1996;35:803–808. doi: 10.3109/02841869609104030. [DOI] [PubMed] [Google Scholar]
- 16.Lobachevsky PN, Martin RF. DNA strand breakage by 125I-decay in a synthetic oligodeoxynucleotide—2. Quantitative analysis of fragment distribution. Acta Oncol. 1996;35:809–815. doi: 10.3109/02841869609104031. [DOI] [PubMed] [Google Scholar]
- 17.Panyutin IG, Neumann RD. Sequence-specific DNA breaks produced by triplex-directed decay of 125I. Acta Oncol. 1996;35:817–823. doi: 10.3109/02841869609104032. [DOI] [PubMed] [Google Scholar]
- 18.Kassis AI, Harapanhalli RS, Adelstein SJ. Comparison of strand breaks in plasmid DNA after positional changes of Auger electron-emitting iodine-125. Radiat Res. 1999;151:167–176. [PubMed] [Google Scholar]
- 19.Howell RW, Goddu SM, Bishayee A, Rao DV. Radioprotection against lethal damage caused by chronic irradiation with radionuclidesin vitro. Radiat Res. 1998;150:391–399. [PMC free article] [PubMed] [Google Scholar]
- 20.Walicka MA, Adelstein SJ, Kassis AI. Indirect mechanisms contribute to biological effects produced by decay of DNA-incorporated iodine-125 in mammalian cells in vitro: Clonogenic survival. Radiat Res. 1998;149:142–146. [PubMed] [Google Scholar]
- 21.Walicka MA, Adelstein SJ, Kassis AI. Indirect mechanisms contribute to biological effects produced by decay of DNA-incorporated iodine-125 in mammalian cells in vitro: Double-strand breaks. Radiat Res. 1998;149:134–141. [PubMed] [Google Scholar]
- 22.Terrissol M. Modelling of radiation damage by 125I on a nucleosome. Int J Radiat Biol. 1994;66:447–451. doi: 10.1080/09553009414551441. [DOI] [PubMed] [Google Scholar]
- 23.Straume T, Carsten AL. Tritium radiobiology and relative biological effectiveness. Health Phys. 1993;65:657–672. doi: 10.1097/00004032-199312000-00005. [DOI] [PubMed] [Google Scholar]
- 24.ICRU. Report 30. International Commission on Radiation Units and Measurements; Bethesda, MD: 1979. Quantitative Concepts and Dosimetry in Radiobiology. [Google Scholar]
- 25.Weber DA, Eckerman KF, Dillman LT, Ryman JC. MIRD: Radionuclide Data and Decay Schemes. Society of Nuclear Medicine; New York: 1989. [Google Scholar]
- 26.Dertinger H, Jung H. Molecular Radiation Biology. Springer Verlag; Heidelberg: 1970. [Google Scholar]
- 27.Roots R, Chatterjee A, Chang P, Lommel L, Blakely EA. Characterization of hydroxyl radical-induced damage after sparsely and densely ionizing irradiation. Int J Radiat Biol. 1985;47:157–166. doi: 10.1080/09553008514550231. [DOI] [PubMed] [Google Scholar]
- 28.Ashwood-Smith MJ. Radioprotective and cryoprotective properties of DMSO. In: Jacob SW, Rosenbaum EE, Wood DC, editors. Dimethyl Sulfoxide. Marcel Dekker; New York: 1971. pp. 147–187. [Google Scholar]
- 29.Reuvers AP, Greenstock CL, Borsa J, Chapman JD. Studies on the mechanism of chemical radioprotection by dimethyl sulfoxide. Int J Radiat Biol. 1973;24:533–536. doi: 10.1080/09553007314551431. [DOI] [PubMed] [Google Scholar]
- 30.Singh DR, Mahajan JM, Krishnan D. Effect of dimethyl sulfoxide (DMSO) on radiation-induced heteroallelic reversion in diploid yeast. Mutat Res. 1976;37:193–200. doi: 10.1016/0027-5107(76)90033-6. [DOI] [PubMed] [Google Scholar]
- 31.Vos O, Kaalen MCAC. Protection of tissue-culture cells against ionizing radiation II. The activity of hypoxia, dimethyl sulphoxide, dimethyl sulphone, glycerol and cysteamine at room temperature and at −196°C. Int J Radiat Biol. 1963;5:609–621. doi: 10.1080/09553006214551251. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe M, Suzuki M, Suzuki K, Hayakawa Y, Miyazaki T. Radioprotective effects of dimethyl sulfoxide in golden hamster embryo cells exposed to γ rays at 77 K: II. Protection from lethal, chromosomal and DNA damage. Radiat Res. 1990;124:73–78. [PubMed] [Google Scholar]
- 33.Hagemann RF, Evans TC, Riley EF. Modification of radiation effect on the eye by topical application of dimethyl sulfoxide. Radiat Res. 1970;44:368–378. [PubMed] [Google Scholar]
- 34.Jones GD, Boswell TV, Lee J, Milligan JR, Ward JF, Weinfeld M. A comparison of DNA damages produced under conditions of direct and indirect action of radiation. Int J Radiat Biol. 1994;66:441–445. doi: 10.1080/09553009414551431. [DOI] [PubMed] [Google Scholar]
- 35.Roberts JC, Koch KE, Detrick SR, Warters RL, Lubec G. Thiazolidine prodrugs of cysteamine and cysteine as radioprotective agents. Radiat Res. 1995;143:203–213. [PubMed] [Google Scholar]
- 36.Howell RW. Radiation spectra for Auger-electron emitting radionuclides: Report No. 2 of AAPM Nuclear Medicine Task Group No. 6. Med Phys. 1992;19:1371–1383. doi: 10.1118/1.596927. [DOI] [PubMed] [Google Scholar]
- 37.Linz U, Stöcklin G. Chemical and biological consequences of the radioactive decay of iodine-125 in plasmid DNA. Radiat Res. 1985;101:262–278. [PubMed] [Google Scholar]
- 38.Ertl HH, Feinendegen LE, Heiniger HJ. Iodine-125, a tracer in cell biology: Physical properties and biological aspects. Phys Med Biol. 1970;15:447–456. doi: 10.1088/0031-9155/15/3/005. [DOI] [PubMed] [Google Scholar]
- 39.Burki HJ, Roots R, Feinendegen LE, Bond VP. Inactivation of mammalian cells after disintegrations of H-3 or I-125 in cell DNA at −196°C. Int J Radiat Biol. 1973;24:363–375. doi: 10.1080/09553007314551221. [DOI] [PubMed] [Google Scholar]
- 40.Apelgot S, Coppey J, Gaudemer A, Grisvard J, Guille E, Sasaki I, Sissoeff I. Similar lethal effect in mammalian cells for two radioisotopes of copper with different decay schemes, Cu-64 and Cu-67. Int J Radiat Biol. 1989;55:365–384. doi: 10.1080/09553008914550421. [DOI] [PubMed] [Google Scholar]
- 41.Halpern A, Stöcklin G. Chemical and biological consequences of beta-decay Part 2. Radiat Environ Biophys. 1977;14:257–274. doi: 10.1007/BF01325241. [DOI] [PubMed] [Google Scholar]
- 42.Hofer KG, Keough G, Smith JM. Biological toxicity of Auger emitters: Molecular fragmentation versus electron irradiation. Curr Top Radiat Res Q. 1977;12:335–354. [PubMed] [Google Scholar]
- 43.Makrigiorgos GM, Kassis AI, Baranowska-Kortylewicz J, McElvany KD, Welch MJ, Sastry KSR, Adelstein SJ. Radiotoxicity of 5-[123I]iodo-2′-deoxyuridine in V79 cells: A comparison with 5-[125I]iodo-2′-deoxyuridine. Radiat Res. 1989;118:532–544. [PubMed] [Google Scholar]
- 44.Baverstock KF, Cundall RB. Solitons and energy transfer in DNA. Nature. 1988;332:312–313. doi: 10.1038/332312b0. [DOI] [PubMed] [Google Scholar]
- 45.Bird RP. Cysteamine as a protective agent with high-LET radiations. Radiat Res. 1980;82:290–296. [PubMed] [Google Scholar]
- 46.Livesey JC, Reed DJ. Chemical protection against ionizing radiation. Adv Radiat Biol. 1987;13:285–340. [Google Scholar]
- 47.Whaley JM, Little JB. Efficient mutation induction by 125I and 131I decays in DNA of human cells. Radiat Res. 1990;123:68–74. [PubMed] [Google Scholar]
- 48.Loevinger R, Budinger TF, Watson EE. MIRD Primer for Absorbed Dose Calculations. The Society of Nuclear Medicine; New York: 1991. [Google Scholar]
- 49.Goddu SM, Howell RW, Bouchet LG, Bolch WE, Rao DV. MIRD Cellular S values: Self-absorbed dose per unit cumulated activity for selected radionuclides and monoenergetic electron and alpha particle emitters incorporated into different cell compartments. Society of Nuclear Medicine; Reston, VA: 1997. [Google Scholar]
- 50.Nelson WR, Hirayama RH, Roger DWO. SLAC Report 265. Stanford Linear Accelerator Center; Palo Alto, CA: 1985. The EGS4 Code System. [Google Scholar]
- 51.Bolch WE, Bouchet LG, Robertson JS, Wessels BW, Siegel JA, Howell RW, Erdi AK, Aydogan B, Costes S, Watson EE. MIRD Pamphlet No. 17: The dosimetry of nonuniform activity distributions—radionuclide S values at the voxel level. J Nucl Med. 1999;40:11S–36S. [PubMed] [Google Scholar]
- 52.Bouchet LG, Bolch WE, Weber DA, Atkins HL, Poston JW., Sr MIRD Pamphlet No. 15: Radionuclide S values in a revised model of the adult head and brain. J Nucl Med. 1999;40:62S–101S. [PubMed] [Google Scholar]
- 53.Eckerman KF, Westfall RJ, Ryman JC, Cristy M. Nuclear Decay Data Files of the Dosimetry Research Group. Oak Ridge National Laboratory; Oak Ridge, TN: 1993. Report ORNL/TM-12350. [Google Scholar]