Abstract
Ruminant erythrocytes are remarkable for their choline-phospholipid anomalies; namely, low or absent phosphatidylcholine (PC) along with high sphingomyelin levels. Here, we report another anomaly in bovine erythrocytes that affects aminophospholipids: phosphatidylethanolamine (PE) shows an extreme asymmetry, with only 2% of the total present in the outer leaflet. Furthermore, we found that phospholipase A2, an enzyme located on the external surface of the erythrocytes, shows higher activity against PC than against PE. In addition, we observed that acylation of PE is by far the most important biosynthetic event in this system. We propose that deacylation of PE and PC by phospholipase A2 to generate lysocompounds, followed by selective reacylation of lyso-PE in the inner leaflet, can account for the compositional and architectural peculiarities of bovine erythrocyte membranes.
Erythrocyte membranes are important models for studying the structure of natural lipid bilayers (1). In classical studies, lipid asymmetry of the human erythrocyte membrane has been demonstrated by using nonlytic phospholipase treatment, in which phospholipid hydrolysis in the absence of hemolysis was used as indication that only the outer leaflet is affected (1). Using this approach, it was established that the inner leaflet of the human erythrocyte membrane contains all of the phosphatidylserine (PS) and a large fraction of the phosphatidylethanolamine (PE). However, a substantial fraction (20%) of the latter is found in the outer leaflet, which is mainly built of the choline phospholipids sphingomyelin (SM) and phosphatidylcholine (PC) (1, 2). This asymmetric composition was also demonstrated by using the nonpermeating labeling reagent trinitrobenzene sulfonic acid (TNBS), which reacts with amino groups and thus labels externally available PE and PS (3, 4). PS and a large fraction of PE are only labeled after disruption of human erythrocytes, which is consistent with the asymmetry of the aminophospholipids that confines them to the inner leaflet. The differential composition between the inner and outer leaflets in human erythrocytes appears to result from balance between various enzymatic activities, including aminophospholipid translocase (5, 6), which catalyzes the inward movement of aminophospholipids; a phospholipid scramblase, which tends to enhance the movement of all phospholipids in both directions (7–9); and possibly a floppase, which was proposed to counteract the activity of aminophospholipid translocase (10, 11).
In mammals, PC is generally the most abundant erythrocyte membrane phospholipid (1, 12, 13). However, there is a striking deviation in ruminants. Their erythrocyte membranes are particularly deficient in PC, which can be completely absent in some ruminant species. Instead, they contain high levels of SM (13). A second unique aspect of ruminant erythrocyte membranes is the presence of N-acylated aminophospholipids, including N-acyl-PE (NAPE) (14). In contrast, bovine bone marrow cells, from which erythrocytes are derived, display a typical mammalian lipid composition with relatively high PC levels (13).
The mechanism by which the ruminant erythrocyte membrane attains its unique composition is unknown. It has been suggested that endogenous phospholipase A2 (Plase A2) and lysophospholipase activities, acting on PC and lysophosphatidylcholine (LPC), are responsible for the reduction in PC levels (15). However, this previous study was carried out by using ghosts with detergent-disrupted membrane integrity, raising questions regarding its relevance and the relative activities toward different phospholipids in intact erythrocytes. Surprisingly no direct information is available regarding the lipid asymmetry in bovine erythrocytes and its generation and maintenance in the face of a quite different plasma lipid composition. We have undertaken this study to define these aspects of the biology of ruminant erythrocytes.
Materials and Methods
Materials.
Bee venom Plase A2 (1225 units/mg), TNBS, TLC plates, neutral lipid standards, fatty acid-free BSA, palmitic anhydride, dimethylaminopyridine, amylene-stabilized chloroform, and trypsin were purchased from Sigma. [125I] 3-(p-hydroxyphenyl)propionic acid N-hydroxysuccinimide ester [4,400 Ci/mmol (1 Ci = 37 GBq); 125I-labeled Bolton–Hunter reagent (125I-labeled B-H reagent)], 1-palmitoyl-2-[1-14C]arachidonoyl-PE (40–60 μCi/μmol), [1-14C]stearic acid (40–60 μCi/μmol), [1-14C]linoleic acid (18:2, n-6) (40–60 μCi/μmol) were purchased from Amersham Pharmacia and [1-14C]octanoic acid (40–60 μCi/μmol) was obtained from New England Nuclear. Aminocaproyl-(N-4-nitrobenzo-2-oxa-1,3-diazole) (C6-NBD)-PC, C6-NBD-PE, C6-NBD-PS, N-palmitoyl-PE, dioleoyl-PC, and other phospholipids were purchased from Avanti Polar Lipids. Solvents were purchased from Fisher Scientific.
Preparation of Erythrocyte Suspensions.
Erythrocyte suspensions were prepared from freshly drawn blood samples from five different cows (Bos taurus, 10–12 years of age) and three healthy human donors (42–48 years of age). Erythrocytes were washed four times in 20 mM Tris⋅HCl/150 mM NaCl/5.5 mM glucose/1 mM CaCl2/0.1 mM MgCl2, pH 7.4 (TBS-GCM buffer), discarding the buffy coat and the top 2-mm layer in each wash, to minimize leukocyte contamination. They were then resuspended in the same buffer at the indicated concentrations.
Lipid Extraction.
Lipids were extracted according to Rose and Oaklander (16) unless otherwise indicated.
Treatment with Plase A2.
Human and bovine erythrocyte suspensions (5% vol/vol) were incubated at 37°C with stirring for the indicated time periods with different concentrations of bee venom Plase A2. Hemolytic effects were assessed by the amount of hemoglobin in the supernatants, after centrifugation of the suspensions, measured spectrophotometrically at 570 nm. Nonlytic conditions, assessed by a release of hemoglobin of <2%, were obtained by using 10 units/ml Plase A2. Total hemolysis of human erythrocytes was observed with 100 units/ml of the enzyme. The reactions were terminated by addition of Na2EDTA (10 mM final conc.) and the lipids were extracted. Lipid extracts were separated by one-dimensional TLC on aluminum-backed Silica gel plates as described (17), to monitor progress of hydrolysis and by two-dimensional TLC for isolation of each lipid class (18). The areas of the plates with the lipids of interest were cut and the lipids eluted with three portions of 2 ml of methanol. The eluates were dried under reduced pressure and the phospholipids were quantitated in triplicate by phosphorus measurements according to Ames (19).
Labeling of Aminophospholipids.
Labeling and quantitation of outer leaflet aminophospholipids in intact erythrocytes and of total aminophospholids available in erythrocytic lipids using TNBS was carried out according to Gordesky et al. (3, 4). The conditions for aminophospholipid labeling with 125I-labeled B-H reagent were essentially as described (20). This reagent has been used before for determination of sidedness of membrane proteins (21). Briefly, intact erythrocytes (30% vol/vol) washed in 20 mM sodium bicarbonate/5.5 mM glucose/0.15 M NaCl, pH 8.1, were exposed to 100 μCi of 125I-labeled B-H reagent for 10–30 s. Total lipid extracts obtained from an equivalent amount of erythrocytes were labeled for 1 min under the same conditions. The reactions were terminated by quenching with 0.2 M Tris⋅HCl, pH 8.1. After an additional 5 min, the lipids were extracted and analyzed on Silica gel G plates with chloroform/methanol/acetic acid (70:30:3, vol/vol). Lipid spots were located by autoradiography and quantitated in a Beckman gamma spectrometer.
Aminophospholipid Translocase Assay.
This activity was measured in washed intact human and bovine erythrocytes according to Connor and Schroit (22) by using C6-NBD-PS and C6-NBD-PE. The method of Calvez et al. (23) was also used with similar results.
Preparation of Labeled Phospholipids.
The radioactively labeled analog of PE, 1-palmitoyl-2-[1-14C]arachidonyl-PE, was acylated with palmitic anhydride in the presence of dimethylaminopyridine by using the conditions described by Gupta et al. (24), except that instead of dry methylene chloride, we used amylene-stabilized chloroform. The acylated forms were purified by TLC, using commercial N-palmitoyl-PE as standard. The final compound gave a single spot with a characteristic pattern and comigrated with commercial N-palmitoyl-dioleoyl-PE. Fluorescent N-palmitoyl-[1-palmitoyl-2-(12-N-NBD-aminododecanoyl)-PE (C6-NBD-NAPE) was obtained by using the same acylating conditions.
Plase A2 Assay in Intact Erythrocytes.
The method will be discussed in detail elsewhere (J.F.-C., M.W., J. Caramelo, M.F.-C., J. M. Delfino, and G.H.P., manuscript in preparation). Briefly, the fluorescent substrates C6-NBD-PC, C6-NBD-PE, and C6-NBD-NAPE were added from 0.6 mM solutions in TBS to a final conc. of 30 μM to 30% (vol/vol) erythrocyte suspensions prepared in TBS-GCM buffer with the addition of 0.1% wt/vol BSA. In the indicated experiments, this buffer was supplemented with 20 mM Na2EDTA as a Plase A2 inhibitor (15), before addition of the substrate. The suspensions were then incubated at 37°C with stirring for up to 2 h. Aliquots of 400 μl were removed and the reactions were terminated by addition of 50 μl of 90 mM Na2EDTA/0.9 M ammonium hydroxide followed by immediate lipid extraction with 1 ml of chloroform/methanol (1:1, vol/vol). After vigorous Vortex mixing and centrifugation, lower and upper phases were separated, evaporated under reduced pressure, and dissolved in 0.1% Triton X-100. With this solvent system, fluorescent fatty acids and lysocompounds partition quantitatively in the upper phase, whereas the nonhydrolyzed fluorescent phospholipid is recovered in the lower phase. Fluorescence in the upper phase was measured spectrofluorometrically (λexc460 − λemm534) for determination of the amount of product formed. Addition of fluorescent phospholipids as well as stirring did not result in measurable hemolysis under the conditions used.
Loading of Erythrocytes with NBD-PC.
Inhibition by EDTA allows loading of the erythrocytes with C6-NBD-PC. In this experiment, the fluorescent phospholipid (30 μM final conc.) was mixed with the erythrocytes (30% vol/vol final conc.) by vigorous Vortex mixing in TBS-CGM/1% BSA/20 mM EDTA, for 30 s. The mixture was then centrifuged (830 × g, 3 min), the lipids of an aliquot of the erythrocytes extracted, and the NBD lipid present fluorometrically determined. Aliquots of the NBD-PC-loaded erythrocytes were then resuspended in either TBS/1% BSA/2 mM CaCl2 or TBS/1% BSA/20 mM EDTA. Hemolysis and lipid hydrolysis were determined in these suspensions as described above.
Treatment of Erythrocytes with Trypsin.
To determine the sidedness of the Plase A2, the erythrocyte suspensions in TBS-GCM were previously treated for 12 h at 37°C with 1 mg/ml trypsin. These cells and the controls without trypsin were washed four times with TBS before final resuspension in TBS-GCM buffer and assayed for Plase A2 as described above for intact erythrocytes. Trypsin treatment had negligible effects (<0.5%) on erythrocyte suspensions.
Treatment of PE and NAPE with Bovine Erythrocyte Ghost Plase A2.
Ghost suspensions were prepared by lysis of erythrocytes in 20 mM Tris⋅HCl, pH 7.4, followed by centrifugation at 30,000 × g for 20 min at 4°C. The preparations were washed twice in TBS/1 mM CaCl2/0.1 mM MgCl2 and resuspended in the same buffer. The phospholipids (5 nmol) evaporated from chloroform solutions were resuspended in the same buffer and 0.1 ml was added to 0.7 ml of ghost suspensions (1.7 mg of protein per ml). Incubations were extended for 12 h at 37°C, at the end of which 100 μl of 90 mM Na2EDTA were added and the lipids were extracted according to Bligh and Dyer (25). Lipids in the lower phase were dried under nitrogen and separated by TLC using chloroform/methanol/acetic acid (60:30:1, vol/vol) as solvent mixture. Radioactivity was detected by autoradiography.
Incorporation of Radiolabeled Fatty Acids to Erythrocyte Phospholipids.
Control bovine or trypsin-treated erythrocytes prepared as described above were used. After four washes with TBS, the cells were resuspended in TBS-GCM/0.1% (wt/vol) BSA buffer at a 30% (vol/vol) conc. This suspension was incubated with [1-14C]stearic acid or [1-14C]linoleic acid (5 μCi/ml) for 24 h at 37°C in screw-cap glass vials. The fatty acids were previously evaporated in the vials from the toluene stock solutions under nitrogen followed by exposure to high vacuum. The incubations were terminated by addition of 1 vol of methanol and 1 vol of chloroform to 0.9 vol of erythrocyte suspension. After vigorous mixing and centrifugation, the lipids in the lower phase were dried and separated by TLC as described for Plase A2 treatment experiments.
Distribution of Lysophosphatidylethanolamine (LPE) and LPC Between Serum and Erythrocytes.
To investigate how lysophospholipids partition between serum and erythrocytes, washed erythrocytes were resuspended in an equal volume of serum obtained from the same animal. Radiolabeled LPC and LPE were prepared starting from PC and PE purified from metabolically labeled Trypanosoma cruzi cultures incubated with [1-14C]stearic acid in the conditions described before (26, 27). For lysocompound preparations, bee venom Plase A2 (50 units/ml) in TBS 2 mM CaCl2 30 mM sodium borate, pH 8.1, was used. The incubations were extended for 10 h and the lipids were then purified by TLC. The lysophospholipids were resuspended in TBS and added to the 30% erythrocyte (vol/vol)/serum suspensions at a conc. of 105 cpm/ml (≈10 μg/ml). After a 2-h incubation period with stirring at 37°C, the suspensions were centrifuged. The supernatant was separated and the erythrocytes were washed twice with TBS. Lipids were extracted according to Bligh and Dyer (25) from both supernatant and cells, and radioactivity in the extracts was determined by liquid scintillation.
Statistical Analysis.
Numerical data were analyzed by comparison of mean values using Student's t test.
Results and Discussion
The remarkable composition of the bovine erythrocyte membrane, characterized by low levels of PC, high levels of SM, and a unique erythrocyte phospholipid, NAPE (13, 14), poses interesting questions regarding its generation and biological significance (13).
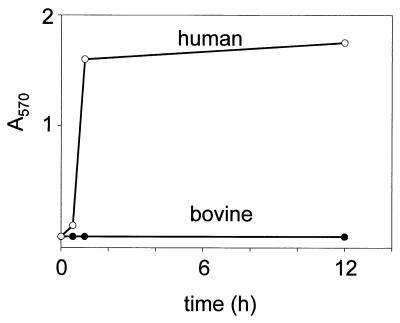
We first addressed these questions examining the action of bee venom Plase A2 on bovine erythrocytes. This enzyme is especially suited for the examination of glycerophospholipid distribution in intact erythrocytes because, in contrast to other Plases A2, it is able to hydrolyze both PC and PE in situ without hemolysis in human erythrocytes (28). Bee venom Plase A2 has not been used previously to analyze the phospholipid distribution in bovine erythrocytes. Our results showed that the enzyme is able to hydrolyze phospholipids without hemolysis in human as well as in bovine erythrocytes at conc. up to 10 units/ml. However, at higher concentrations (100 units/ml) the enzyme rapidly lyses human but not bovine erythrocytes (Fig. 1). In Table 1 we compare the effects of treatment with a nonlytic concentration (10 units/ml) of bee venom Plase A2 on human and bovine erythrocytes. Remarkably, no LPC is formed in bovine erythrocytes whereas PC is extensively hydrolyzed in human erythrocytes. PE is also significantly attacked in the latter. In contrast, the accumulation of LPE in the bovine system is much lower. These results demonstrate a striking resistance of bovine erythrocytes to hydrolysis by Plase A2. They suggest that although PE is sparsely represented in the outer leaflet of bovine erythrocytes, PC is absent or present in too low amounts for detection at this site.
Figure 1.
Bee venom Plase A2 hemolyzes human but not bovine erythrocytes. Human and bovine erythrocyte suspensions were treated with Plase A2 (100 units/ml) and hemolysis was spectrophotometrically determined as described in Materials and Methods.
Table 1.
Effect of bee venom Plase A2 on human and bovine erythrocytes
| Phospholipid class | Human erythrocytes, % of total phospholipids
|
Bovine erythrocytes, % of total phospholipids
|
||
|---|---|---|---|---|
| Control | Treated | Control | Treated | |
| LPC | 1.0 ± 0.1 | 18.7 ± 1.7** | 1.7 ± 0.2 | 1.6 ± 0.2 |
| SM | 25.0 ± 2.0 | 26.9 ± 2.2 | 47.6 ± 3.8 | 46.3 ± 3.1 |
| PC | 29.6 ± 2.6 | 12.2 ± 1.2** | 4.5 ± 0.5 | 4.7 ± 0.4 |
| PI + PS | 14.6 ± 1.7 | 12.7 ± 1.7 | 13.6 ± 1.5 | 14.5 ± 1.3 |
| LPE | 0.8 ± 0.1 | 4.7 ± 0.6** | 0.9 ± 0.1 | 1.5 ± 0.1** |
| PE | 27.4 ± 2.0 | 23.2 ± 1.9* | 29.9 ± 2.8 | 29.4 ± 2.6 |
| PA | 1.6 ± 0.1 | 1.4 ± 0.1 | 1.7 ± 0.2 | 1.9 ± 0.2 |
PI, phosphatidylinositol; PA, phosphatidic acid. Erythrocyte suspensions were washed and treated with Plase A2 (10 units/ml) and their lipids were analyzed as described in Materials and Methods. Results are expressed as percentages of total phospholipids (mean ± SE, n = 3). Significant changes between control (untreated) and Plase A2-treated cells are marked (*, P < 0.01;
, P < 0.001).
A different approach for assessing phospholipid asymmetry is the use of TNBS, a labeling reagent for amino groups that does not permeate cell membranes. Thus, only aminophospholipids exposed on the outer leaflet become covalently labeled in intact cells. In contrast, TNBS treatment of total lipid extracts obtained from an equivalent amount of erythrocytes reveals the total amount of aminophospholipids present, as the lipid extracts do not show the organization of native membranes. Fig. 2a compares the results of treatment with TNBS of intact human and bovine erythrocytes as well as their lipid extracts. Marked contrast is evident. In human erythrocytes, 18.3 ± 1.4% of PE is labeled, whereas in bovine erythrocytes the percentage of PE labeling is only 2.1 ± 0.1%. Consistent results were obtained by using radioiodinated B-H reagent for exposed aminophospholipid detection (Fig. 2b), with PE labeling percentages of 19.2 ± 2.2% and 2.3 ± 0.2% for human and bovine erythrocytes, respectively. These results indicate that although PE in the outer leaflet constitutes 18–19% of the total PE in human erythrocytes, the percentage of PE in the outer leaflet of bovine erythrocytes is only around 2%. Neither intact human nor bovine erythrocytes show labeling of PS, a lipid that is labeled in lipid extracts from both types of cells, confirming that PS is present only in the inner leaflet of the intact cells. It is important to note that the mean volume of bovine erythrocytes is lower than that of their human counterparts (29). Thus, more membrane surface is exposed in the first than in the latter. This emphasizes that the differences in reactivity toward the reagents or the susceptibility to bee venom Plase A2 are due to their composition rather than to differences in surface exposure.
Figure 2.
Differential exposure of aminophospholipids in human and bovine erythrocytes. Intact human (hum) and bovine (bov) erythrocytes and their corresponding lipid extracts were labeled with the nonpermeant reagents: TNBS (a) and 125I-labeled B-H reagent (b) as described in Materials and Methods. TNBS-PE, TNBS-PS, BH-N-PE, and BH-N-PS: TNBS- and B-H-labeled aminophospholipids PE and PS, respectively.
Our results indicate that PE is largely confined to the inner leaflet of bovine erythrocytes. They differ in this respect from their human counterparts where PE is a substantial component of the outer leaflet. It should be noted that the outer leaflet of bovine erythrocytes is mainly composed of SM. This lipid amounts to 46–47% of the total phospholipid (20). Assuming that all of the SM and 2% of the PE are present in the outer leaflet, less than half of the total phospholipid would be accounted for, as required by the notion that inner and outer leaflet contain equimolar amounts of phospholipids. The difference may reflect a contribution of PC not hydrolizable by bee venom Plase A2. It may also indicate that phospholipid from inner and outer leaflets may not be, in fact, equimolar, which is conceivable because the latter accommodates the bulk of the glycolipids (30). The emerging model accounts well for earlier observations (28, 31). Because our model relies not solely on enzymatic action but also on chemical derivations, we conclude that the scarce degradation of PE is due to low amounts of exposed substrate and not only to peculiar requirements of the enzyme used.
To explore the enzymatic mechanisms accounting for this unique membrane organization, we investigated aminophospholipid translocase and Plase A2 activities in both human and bovine erythrocytes. We found no significant differences in translocase activity between human and bovine erythrocytes (data not shown), a result that confirms previous observations by Connor and Schroit (22). On the other hand, Plase A2 activity was found in bovine erythrocytes by using C6-NBD-labeled phospholipids (Fig. 3) but not in human erythrocytes (data not shown). Importantly, degradation of C6-NBD-PC increases linearly with erythrocyte concentration (Fig. 3a Inset). Our results partially agree with previous ones (15) in which Plase A2 activity against PC was approximately three times higher than that against PE. However, these prior studies were conducted by using bovine erythrocyte ghosts and in the presence of detergent. Our assay of Plase A2 instead was conducted directly on intact erythrocytes, without any detergent addition. It is based on the use of relatively polar short-chain phospholipid analogs, known to rapidly incorporate into cell membranes (32). Thus, we obtain an accurate picture of actual activities as found on the outer leaflet of the erythrocyte membrane. The data show a much higher difference between the activities against PC than against PE (Fig. 3a) than those observed previously using erythrocyte ghosts (15). Thus, bovine erythrocyte Plase A2 cannot only account for the generation of the anomalous overall phospholipid composition, in which Plase A2-resistant SM is highly enriched, but also contribute to explain the remarkable difference between the amounts of PC and PE in these membranes.
Figure 3.
A trypsin- and EDTA-sensitive Plase A2 from bovine erythrocytes has higher activity against fluorescent PC than against PE and is inactive toward NAPE. (a) Bovine erythrocyte suspensions were incubated with C6-NBD analogs of PC, PE, or NAPE and degradation of the substrates was assessed as described in Materials and Methods. (Inset) Linear variation of assay results with erythrocyte concentration. (b) Degradation of fluorescent PC by bovine erythrocyte suspensions that received no treatment (control), were treated with trypsin, or assayed in the presence of 20 mM EDTA.
Two lines of evidence indicate that Plase A2 is present facing the extracellular space. First, overnight treatment of intact bovine erythrocytes with trypsin, which has no hemolytic effects, results in a virtually complete loss of C6-NBD-PC hydrolytic activity (Fig. 3b). Thus, Plase A2 is accessible to the protease, a nonpermeating molecule. Second, Plase A2 activity in intact bovine erythrocytes is fully inhibited by addition of 20 mM EDTA, a polar molecule unlikely to permeate cell membranes (Fig. 3b). Moreover, the inhibition by EDTA was exploited to load the erythrocytes with C6-NBD-PC. Thorough mixing of the lipid with the erythrocytes resulted in a rapid uptake. After 30 s most of the C6-NBD-PC (91 ± 3%) is associated with the erythrocyte pellet upon centrifugation. When these cells were resuspended in buffer containing either 2 mM CaCl2 or 20 mM EDTA and incubated for 30 min at 37°C, the loaded phospholipids underwent very different conversions. In the first case, complete hydrolysis took place that was accompanied by measurable hemolytic effects (5 ± 2%). In the EDTA-incubated cells, on the other hand, most of the phospholipid remained intact and hemolysis was almost absent (0.4 ± 0.1%). Thus, the C6-NBD-PC partitioned into the erythrocyte membranes and became susceptible to endogenous Plase A2 activity in an EDTA-inhibitable fashion. These are evidences emphasizing the role played by Plase A2.
Additional data reveal the action of bovine erythrocyte Plase A2. Recently, using a novel method for distinguishing 1-acyl from 2-acyl LPC, we showed that the LPC found in bovine erythrocyte extracts is predominantly of the type 1-acyl, i.e., the type generated by Plase A2 (33). Furthermore, we found that no changes in lipid composition occurred in erythrocytes incubated in either heparinized plasma or in TBS-GCM buffer at 37°C for up to 48 h (data not shown). These data suggest that bovine erythrocyte Plase A2 is normally almost inactive. However, when the fluorescent phospholipids, which are poorer Plase A2 substrates than natural phospholipids (34), were added to intact cells, the enzyme showed marked activity. Therefore, it appears that, faced with a phospholipid composition perturbation such as that produced by the incorporation of the fluorescent phospholipids into the outer leaflet, Plase A2 becomes activated. Such activation may involve changes in the enzyme conformation that enhance accessibility of the phospholipid substrate to the active site (35). Bovine erythrocyte Plase A2 thus appears to represent a newly identified mechanism maintaining membrane homeostasis.
The presence of NAPE is a unique feature of ruminant erythrocytes. We investigated the susceptibility of synthetic radiolabeled N-palmitoylated-PE to hydrolysis by bovine erythrocyte Plase A2. As shown in Fig. 4, the radiolabeled compound is not hydrolyzed after 18 h of incubation with bovine erythrocyte ghosts, a condition under which the same amount of PE is completely broken down. Erythrocyte ghosts were used in these experiments because long-chain exogenous phospholipids, in contrast to their relatively polar fluorescent counterparts, do not readily react with intact erythrocytes. This is likely because of their lack of diffusibility that does not allow significant partitioning into the cell membranes, preventing the activation of Plase A2.
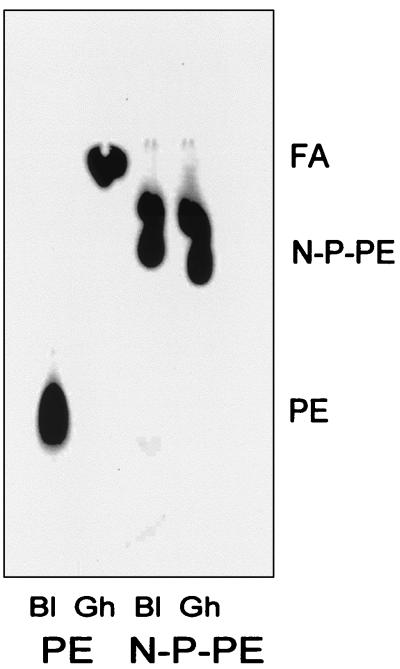
Figure 4.
Plase A2 degrades 1-palmitoyl-2-[1-14C]arachidonoyl-PE, but not its N-palmitoylated derivative. Radiolabeled PE and its N-palmitoylated derivative (N-P-PE) were incubated for 12 h with bovine erythrocyte ghost preparations (Gh) (1.7 mg of protein per ml final conc.) or buffer (Bl, blank), and the lipids were extracted, analyzed, and visualized as described in Materials and Methods.
Consistent with the previous results, when fluorescent PE was exposed to intact erythrocytes, it was slowly hydrolyzed, although its N-palmitoylated derivative (C6-NBD-NAPE) was not hydrolyzed at all (Fig. 3a), not even on overnight incubations (data not shown). These results indicate that acylation of the amino group of PE blocks Plase A2 activity, allowing the accumulation of this peculiar erythrocyte lipid. This emphasizes the influence of Plase A2 susceptibility on the lipid repertoire of bovine erythrocyte membranes. Because of its reduced polarity, it can be expected that NAPE can undergo free migration between the inner and outer leaflets and is likely to exist in equilibrium between the two leaflets.
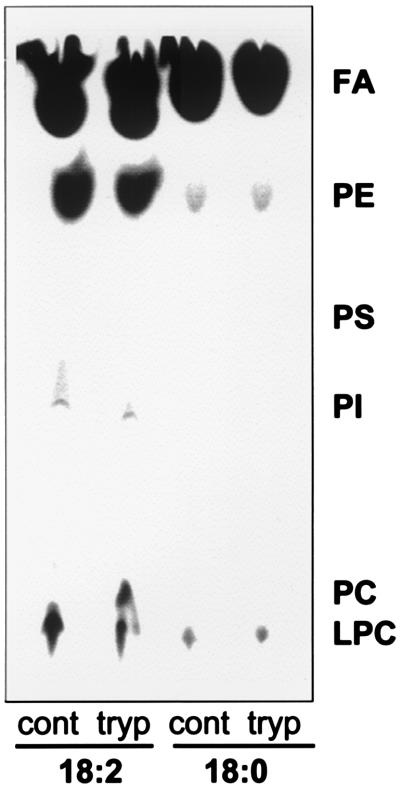
We examined the phospholipid turnover in bovine erythrocytes. Marked differences were found between the incorporations of stearate and linoleic acid into the phospholipids of bovine erythrocytes. In the case of stearate, barely detectable amounts of labeled phospholipids (0.9% PE and 1.1% LPC) were found after a 24-h incubation period. By contrast, when linoleate was used, 23.8% of the radioactivity was recovered in PE, which was the major radiolabeled spot (Fig. 5), followed by LPC (6.4%) and phosphatidylinositol (0.4%). This pattern of phospholipid labeling is highly unusual because incorporation of fatty acids into PC is generally predominant in mammalian cells (17, 36). However, these observations in bovine erythrocytes are consistent with their anomalous phospholipid composition. Also, the difference between stearate and linoleate labeling indicates that position sn-2, usually occupied by unsaturated fatty acids like linoleate, is the one that turns over, whereas position sn-1, the main destination of stearate, shows little, if any, turnover. Finally, control and trypsin-treated cells show similar labeling of PE, indicating that acylation is a process that takes place in the inner leaflet of the erythrocyte membrane (Fig. 5). Trypsin-treated cells appear to accumulate small amounts of PC. This may reflect the absence of Plase A2 allowing building up of PC. We conclude that both specific deacylation by Plase A2, as well as selective acylation of PE and low acylation of PC appear to act in concert to generate and maintain an atypical membrane phospholipid composition and distribution in these cells.
Figure 5.
Bovine erythrocytes preferentially incorporate unsaturated fatty acids into PE. Erythrocytes were labeled for 24 h with either 5 μCi/ml stearic acid (18:0) or linoleic acid (18:2, n-6). Control (cont) and trypsin-treated (tryp) erythrocytes were prepared in TBS-GCM buffer and phospholipids were extracted and analyzed by TLC and autoradiography as described in Materials and Methods.
We examined the partition of exogenous radiolabeled LPC and LPE between serum and erythrocytes. The proportion of radioactivity recovered from serum and erythrocytes were, respectively, 4.2:1 for LPC and 2.3:1 for LPE. Both lipids partition favorably in the serum but LPC to a higher extent, which is consistent with the fact that LPC has a more polar nature than LPE.
Based on the information gathered here, we suggest that Plase A2-generated lysocompounds have different fates according to their polar heads: whereas LPC tends to be lost to the plasma, LPE has a higher tendency to remain associated with the erythrocyte membrane. At this site, it can undergo translocation to the inner leaflet as a result of aminophospholipid translocase activity. Once there, it can be acylated to PE. This can explain how the PC concentration decreases to eventually reach sufficiently low levels at which it is no longer hydrolyzed by Plase A2, whereas PE remains a stable component. Fig. 6 shows a schematic diagram depicting the proposed enzymatic events that can lead to the generation of bovine erythrocyte composition.
Figure 6.
Diagram of the proposed events leading to the acquisition of the bovine erythrocyte phospholipid composition and architecture. Our results support the view that different processes operate on PE (a) and PC (b). PC is degraded by Plase A2 in the outer leaflet and LPC is lost to the plasma. PE is also deacylated at the outer surface but the resulting LPE is translocated to the inner leaflet via aminophospholipid translocase, where it is finally reacylated to PE. This diagram explains how PC contents decrease while PE is maintained and confined to the inner leaflet. 1, Plase A2; 2, aminophospholipid translocase; 3, acyltransferase; and 4, diffusion and various protein-catalyzed translocations.
What is the biological significance of bovine erythrocyte lipid anomalies? Results from our laboratory have revealed that normal bovine serum contains antiphospholipid antibodies with cytolytic activity on erythrocytes from nonruminant mammals. These Abs are likely to arise in response to the entrance of aminoethylphosphonolipids, the phosphono analogs of PE, synthesized by rumen ciliates (37). Phosphonolipids are extremely resistant to enzymatic hydrolysis, which results in their uptake as intact molecules (38). Bovine antiphospholipid Abs, which crossreact with PE and PC, are involved in the killing of ruminal and free living ciliates (J.F.-C., C. E. Suarea, M.F.-C., W.C.B., T.F.M., and G.H.P., manuscript in preparation). Thus, in this group of mammals, the harboring of a ciliate fauna implies exposure to phosphonolipids and, as a consequence, production of antiphospholipid Abs. In light of these findings, the erythrocyte lipid composition appears as a coevolutionary adaptation allowing ruminants to harbor abundant rumen ciliates and to produce antiphospholipid Abs without hemolytic effects.
The results presented here are also relevant to the biology of the babesial parasites, Babesia bigemina, Babesia bovis, and Babesia divergens, that invade and replicate in mature bovine erythrocytes (39). First, the low amounts of glycerophospholipids on the surface of the erythrocytes make it unlikely that a phospholipase A activity acting on erythrocyte phospholipids can play a significant role in cell invasion. Second, in the case of Babesia sp. parasites, rich in PC (20), antiphospholipid Abs appear as a highly selective means to target parasites without affecting the erythrocytes. Thus, a new avenue in the immunoprophylaxis of bovine babesiosis is open.
In conclusion, it is shown here that in addition to the marked deviations in choline phospholipid composition, bovine erythrocyte membranes have striking organizational features in which SM is vastly predominant and PE has an extremely asymmetrical distribution. Cell surface Plase A2 as well as selective acylation of PE, acting as membrane composition determinants, may account for both the generation and maintenance of the unique phospholipid composition and peculiar architecture of the erythrocytes from this group of animals.
Acknowledgments
The skillful assistance of Kim Kegerreis, Debbie Alperin, and Emma Karel and support from U.S. Agency for International Development Grant PCE-G-0098-00043-00 and Consejo Nacionel de Investigaciones Científicas y Técnicas de Argentina (PICT 0124 and PICT 98/08-3838) are acknowledged.
Abbreviations
- PS
phosphatidylserine
- 125I-labeled B-H reagent
3-(p-hydroxyphenyl)propionic acid N-hydroxysuccinimide ester
- NAPE
N-acyl-phosphatidylethanolamine
- C6-NBD
aminocaproyl-(N-4-nitrobenzo-2-oxa-1,3-diazole)
- LPC
lysophosphatidylcholine
- LPE
lysophosphatidylethanolamine
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- Plase A2
phospholipase A2
- SM
sphingomyelin
- TNBS
trinitrobenzene sulfonic acid
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Roelofsen B, Zwaal R A. Methods Membr Biol. 1976;7:147–177. [Google Scholar]
- 2.Schroit A J, Zwaal R F A. Biochim Biophys Acta. 1991;1071:313–329. doi: 10.1016/0304-4157(91)90019-s. [DOI] [PubMed] [Google Scholar]
- 3.Gordesky S E, Marinetti G V. Biochem Biophys Res Commun. 1973;50:1027–1031. doi: 10.1016/0006-291x(73)91509-x. [DOI] [PubMed] [Google Scholar]
- 4.Gordesky S E, Marinetti G V, Segel G B. Biochem Biophys Res Commun. 1972;47:1004–1009. doi: 10.1016/0006-291x(72)90932-1. [DOI] [PubMed] [Google Scholar]
- 5.Selgneuret M, Devaux P F. Proc Natl Acad Sci USA. 1984;81:3751–3755. doi: 10.1073/pnas.81.12.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connor J, Schroit A J. Biochemistry. 1987;26:5099–5105. doi: 10.1021/bi00390a031. [DOI] [PubMed] [Google Scholar]
- 7.Bevers E M, Comfurius P, van Rijn J L, Hemker H C, Zwaal R F A. Eur J Biochem. 1982;122:429–436. doi: 10.1111/j.1432-1033.1982.tb05898.x. [DOI] [PubMed] [Google Scholar]
- 8.Bevers E M, Comfurius P, Zwaal R F A. Biochim Biophys Acta. 1983;736:57–66. doi: 10.1016/0005-2736(83)90169-4. [DOI] [PubMed] [Google Scholar]
- 9.Zwaal R F A, Comfurius P, Bevers E M. Biochem Soc Trans. 1993;21:248–254. doi: 10.1042/bst0210248. [DOI] [PubMed] [Google Scholar]
- 10.Connor J, Gillum K, Schroit A J. Biochim Biophys Acta. 1990;1025:82–86. doi: 10.1016/0005-2736(90)90193-r. [DOI] [PubMed] [Google Scholar]
- 11.Dekkers D W C, Comfurius P, Schroit A J, Bevers E M, Zwaal R F A. Biochemistry. 1998;37:14833–14837. doi: 10.1021/bi981011w. [DOI] [PubMed] [Google Scholar]
- 12.Hadley N F. The Adaptive Role of Lipids in Biological Systems. New York: Wiley; 1985. [Google Scholar]
- 13.Christie W W. In: Lipid Metabolism in Ruminant Animals. Christie W W, editor. Oxford: Pergamon; 1981. pp. 95–191. [Google Scholar]
- 14.Matsumoto M, Miwa W. Biochim Biophys Acta. 1973;296:350–364. doi: 10.1016/0005-2760(73)90093-3. [DOI] [PubMed] [Google Scholar]
- 15.Zwaal R F A, Fluckiger R, Moser S, Zahler P. Biochim Biophys Acta. 1974;373:416–424. doi: 10.1016/0005-2736(74)90021-2. [DOI] [PubMed] [Google Scholar]
- 16.Rose H G, Oaklander M. J Lipid Res. 1965;6:428–431. [PubMed] [Google Scholar]
- 17.Florin-Christensen J, Florin-Christensen M, Delfino J M, Stegmann T, Rasmussen H. J Biol Chem. 1992;267:14783–14789. [PubMed] [Google Scholar]
- 18.Broekhuyse R M. Clin Chim Acta. 1969;23:457–459. doi: 10.1016/0009-8981(69)90349-0. [DOI] [PubMed] [Google Scholar]
- 19.Ames B N. Methods Enzymol. 1966;8:515–518. [Google Scholar]
- 20.Florin-Christensen J, Suarez C E, Florin-Christensen M, Hines S A, McElwain T F, Palmer G H. Mol Biochem Parasitol. 2000;106:147–156. doi: 10.1016/s0166-6851(99)00209-1. [DOI] [PubMed] [Google Scholar]
- 21.Dzhandzhugazyan K N, Jorgenesen P L. Biochim Biophys Acta. 1985;817:165–173. doi: 10.1016/0005-2736(85)90079-3. [DOI] [PubMed] [Google Scholar]
- 22.Connor J, Schroit A J. Biochemistry. 1989;28:9680–9685. doi: 10.1021/bi00451a021. [DOI] [PubMed] [Google Scholar]
- 23.Calvez J-Y, Zachowski A, Hermann A, Morrot G, Devaux P F. Biochemistry. 1988;27:5666–5670. doi: 10.1021/bi00415a041. [DOI] [PubMed] [Google Scholar]
- 24.Gupta C M, Radhakrishnan R, Khorana H G. Proc Natl Acad Sci USA. 1977;74:4315–4319. doi: 10.1073/pnas.74.10.4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bligh E G, Dyer W J. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 26.Florin-Christensen M, Florin-Christensen J, Isola E D, Lammel E, Meinardi E, Brenner R R, Rasmussen L. Mol Biochem Parasitol. 1997;88:25–33. doi: 10.1016/s0166-6851(97)00056-x. [DOI] [PubMed] [Google Scholar]
- 27.Wainszelbaum M, Isola E, Wilkowsky S, Cannata J J B, Florin-Christensen J, Florin-Christensen M. Biochem J. 2001;355:765–770. doi: 10.1042/bj3550765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colley C M, Zwaal R F A, Roelofsen B, van Deenen L L M. Biochim Biophys Acta. 1973;307:74–82. doi: 10.1016/0005-2736(73)90026-6. [DOI] [PubMed] [Google Scholar]
- 29.Barman B N, Ashwood E R, Giddings J C. Anal Biochem. 1993;212:35–42. doi: 10.1006/abio.1993.1287. [DOI] [PubMed] [Google Scholar]
- 30.Rothman J E, Lenard J. Science. 1977;195:743–753. doi: 10.1126/science.402030. [DOI] [PubMed] [Google Scholar]
- 31.Taguchi R, Ikezawa H. Arch Biochem Biophys. 1976;173:538–545. doi: 10.1016/0003-9861(76)90290-3. [DOI] [PubMed] [Google Scholar]
- 32.Pagano R E, Sleight R G. Science. 1985;229:1051–1057. doi: 10.1126/science.4035344. [DOI] [PubMed] [Google Scholar]
- 33.Florin-Christensen J, Narvaez-Vazquez J, Florin-Christensen M, Ryan C A. Analyt Biochem. 1999;276:13–17. doi: 10.1006/abio.1999.4322. [DOI] [PubMed] [Google Scholar]
- 34.Moreau R A. Lipids. 1989;24:691–699. [Google Scholar]
- 35.Caramelo J J, Florin-Christensen J, Florin-Christensen M, Delfino J M. Biochem J. 2000;346:679–690. [PMC free article] [PubMed] [Google Scholar]
- 36.Florin-Christensen J, Florin-Christensen M, Delfino J M, Rasmussen H. Biochem J. 1993;289:783–788. doi: 10.1042/bj2890783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogimoto K, Imai S. Atlas of Rumen Microbiology. Tokyo: Japan Scientific Societies Press; 1981. [Google Scholar]
- 38.Tamari M, Kametaka M. Agric Biol Chem. 1980;44:1957–1959. [Google Scholar]
- 39.Purnell R E. In: Babesiosis. Ristic M, Kreier J P, editors. New York: Academic; 1981. pp. 25–64. [Google Scholar]