Abstract
Ubiquitinated aggregates are formed in eukaryotic cells in response to several external stimuli, including exposure to bacterial lipopolysaccharide (LPS). Although Salmonella enterica serovar Typhimurium (S. Typhimurium) LPS has been shown to induce aggresome-like induced structures (ALIS) in macrophages, these have not been described in S. Typhimurium-infected macrophages. Given that LPS is present in infection, this suggests that S. Typhimurium might suppress the formation of ALIS. We found that S. Typhimurium induces the formation of ubiquitinated aggregates in epithelial cells and macrophages, but that their presence is masked by the deubiquitinase (DUB) activity of the S. Typhimurium virulence protein, SseL. SseL deubiquitinates SQSTM1/p62-bound proteins found in S. Typhimurium-induced aggregates and ALIS, and reduces the recruitment of autophagic components. While the functions of ALIS and other ubiquitinated aggregates remain unclear, they serve to sequester cytosolic proteins under a variety of stress conditions and are suggested to be involved in host immune defense. During infection, the deubiquitinase activity of SseL reduces autophagic flux in infected cells and favors bacterial replication. This is a new example of how a bacterial pathogen counteracts the autophagy pathway through the action of a translocated virulence protein.
Keywords: S. Typhimurium, ALIS, DUB, Salmonella, aggregate, autophagy, deubiquitinase, ubiquitin
Ubiquitinated aggregates are likely to have an important role in the immune response to infection. Cytoplasmic aggregates such as ALIS are formed after exposure to several cell stress stimuli, including misfolded protein aggregation, heat stress, protein synthesis inhibition and oxidative stress. They contain defective ribosomal products (DRiPs), but also longer-lived proteins and are characterized as stress-induced protein storage compartments for substrates of both the proteasome and autophagy. Due to their heterogeneous nature, the universal marker for ALIS is ubiquitin, following labeling with an anti-ubiquitin antibody that recognizes both poly- and mono-ubiquitinated substrates.
S. Typhimurium encodes a number of proteins (termed effectors) that are translocated into the host cell cytosol via the Salmonella pathogenicity island (SPI)-2 type 3 secretion system (T3SS). S. Typhimurium, like many other intracellular pathogens, produces several effectors that manipulate the ubiquitin pathway. By studying ubiquitination during infection, we found that intravacuolar S. Typhimurium induce the formation of ubiquitinated aggregates containing autophagic markers in both epithelial cells and macrophages. The presence of these aggregates is greatly enhanced by the deletion of a gene encoding the S. Typhimurium DUB SseL. SseL is a SPI-2 T3SS effector that contributes to delayed macrophage cell death and to virulence in the mouse model of infection.
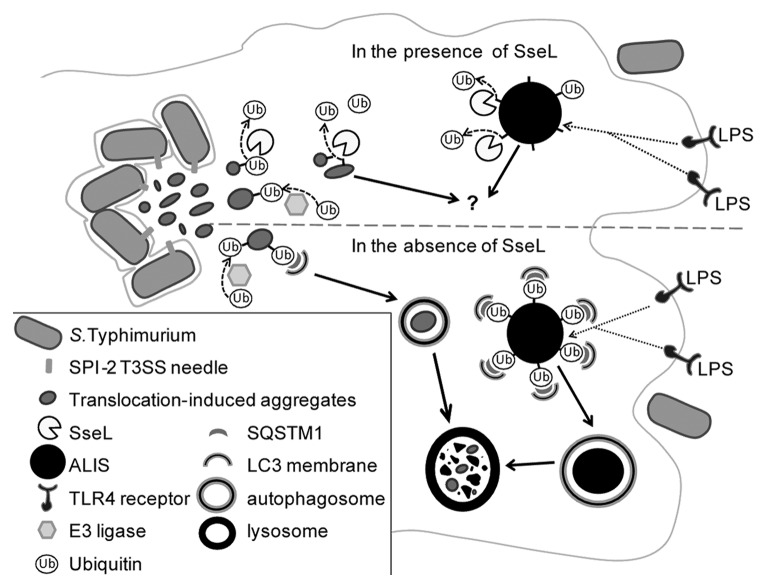
Following infection of epithelial cells, we found diffuse ubiquitin puncta close to SCVs, partially colocalizing with the lysosomal glycoprotein LAMP1 and electron-dense material. Infected macrophages contained similar structures and also larger, dense, more spherical aggregates that resembled ALIS. Although ALIS have not been characterized previously in S. Typhimurium-infected cells, the structures we found in macrophages infected with sseL mutant S. Typhimurium resemble LPS-induced ALIS in their size, shape and recruitment of SQSTM1 and LC3, suggesting that LPS signaling through TLR4 might trigger their formation (Fig. 1). Ubiquitinated aggregates targeted for selective autophagy have been described in cells exposed to other bacterial pathogens, but these have mainly been cytosolic or extracellular bacteria. Expression of the Legionella pneumophila Dot/Icm type IV secretion system in macrophages reduces ALIS formation during infection. Although a Legionella deubiquitinase has not yet been identified, the actions of SseL in S. Typhimurium infection suggest that a Legionella DUB might yet be found.
Figure 1. SseL deubiquitinates cytosolic aggregates destined for autophagy. In the presence of SseL, both ALIS, and aggregates that form in response to S. Typhimurium SPI-2 T3SS effectors, are deubiquitinated. The fate of deubiquitinated cytosolic aggregates is not known. In the absence of SseL, ALIS and SPI-2 T3SS-induced aggregates are ubiquitinated and recruit SQSTM1 and LC3. This leads to engulfment of aggregates by autophagic membranes and their subsequent degradation in lysosomes. Abbreviations: LPS; lipopolysaccharide; Ub, ubiquitin; ALIS, aggresome-like induced structure; TLR, toll-like receptor; SseL, S. Typhimurium secreted effector L; SPI-2, S. Typhimurium pathogenicity island 2; SQSTM1, sequestosome 1; T3SS, Type III secretion system.
Recent studies have shown that eukaryotic DUBs are involved in regulating autophagy. Mutations in the mammalian DUB STAMBP/AMSH (STAM binding protein/associated molecule with the SH3 domain of STAM), cause accumulation of aggregates labeled with ubiquitin and SQSTM1 in neurons, and the DUB TNFAIP3/A20 inhibits LPS-induced autophagic signaling. SseL is the first DUB to be described that directly targets material destined for autophagic degradation, though it is likely that mammalian DUBs also regulate this step. The consequence of SseL deubiquitinating ALIS and other aggregates is a reduction in autophagic flux in infected cells. Autophagy alleviates the cellular stress induced by the presence of protein aggregates; an inability to degrade misfolded or aggregated proteins can lead to cell death, as seen in neurodegenerative disorders. It is possible that the effect of SseL on autophagy accounts for its ability to induce delayed cytotoxicity in macrophages. While the possible functions of ALIS remain unclear, the ubiquitinated proteins in ALIS are considered a source of antigenic peptides for presentation on MHC class I molecules in dendritic cells; the deubiquitination of aggregates by SseL might therefore regulate antigen presentation in infected macrophages and dendritic cells. It will be important to determine the identity and fate of proteins contained within S. Typhimurium-induced aggregates following deubiquitination by SseL. As ubiquitin, and ubiquitin-recruited SQSTM1 and LC3 are currently the only markers of these structures, we were unable to assess whether they persist in cells following their deubiquitination.
Our work has shown that ubiquitinated aggregates and ALIS are formed in S. Typhimurium-infected cells in response to both SPI-2 T3SS-dependent and -independent signals. We found that S. Typhimurium uses the SPI-2 T3SS effector SseL to deubiquitinate proteins in these aggregates, thereby impeding their autophagic degradation. The fate of deubiquitinated protein aggregates is not clear, but reducing the autophagy of these structures is advantageous for intracellular S. Typhimurium replication. Other intracellular pathogens may also encode deubiquitinases that prevent the autophagy of cytosolic aggregates and mask their presence in infected cells—a DUB-ious lack of ALIS in infection suggests that a DUB may be at work!
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/21742