Abstract
Breast cancer is one of the most prevalent cancers in women, with more than 240,000 new cases reported in the United States in 2011. Classification of breast cancer based upon hormone and growth factor receptor profiling shows that approximately 70% of all breast cancers express estrogen receptor-α. Thus, drugs that either block estrogen biosynthesis (aromatase inhibitors like Letrozole), or compete with estrogen for estrogen receptor (ER) binding (selective ER modulators including tamoxifen; TAM) and/or cause ER degradation (selective estrogen receptor downregulators such as fulvestrant), are among the most prescribed targeted therapeutics for breast cancer. However, overall clinical benefit from the use of these drugs is often limited by resistance; ER+ breast cancers either fail to respond to endocrine therapies initially (de novo resistance), or they respond and then lose sensitivity over time (acquired resistance). While several preclinical studies postulate how antiestrogen resistance occurs, for the most part, the molecular mechanism(s) of resistance is unknown.
Keywords: breast cancer, antiestrogen resistance, glucose-regulated protein 78, unfolded protein response, autophagy, AMP-activated protein kinase, MTOR
De novo and acquired resistance may arise from different mechanisms, with acquired antiestrogen resistance perhaps reflecting a stress-induced phenotype. One key cellular stress response mechanism, recently implicated in the acquired antiestrogen resistance phenotype, is activation of the endoplasmic reticulum stress response pathway, i.e., the unfolded protein response (UPR). Accumulation of unfolded proteins within the endoplasmic reticulum lumen triggers the release of glucose-regulated protein 78/heat shock 70 kDa protein 5 (HSPA5/GRP78) from the three proteins that normally repress each of the three arms of the UPR signaling pathway resulting in UPR activation: respectively, endoplasmic reticulum to nucleus signaling 1 (ERN1/IRE1), eukaryotic translation initiation factor 2-α kinase 3 (EIF2AK3/PERK), and activating transcription factor 6 (ATF6). Previous work has detailed the role of the downstream ERN1 activation product X-box binding protein 1 (XBP1) in endocrine resistance, clearly implicating UPR function in antiestrogen resistance.
Recently, we showed that elevated levels of HSPA5 can confer TAM and fulvestrant resistance upon ER+ breast cancer cells, suggesting a novel role for UPR in antiestrogen resistance. Our data indicated that HSPA5 expression is increased in tumors with acquired TAM resistance when compared with de novo TAM resistance, further suggesting that these two phenotypes of TAM insensitivity may arise through different mechanisms. Moreover, overexpression of HSPA5 prevents CASP7 activation and increased expression of the antiapoptotic B-cell CLL/lymphoma 2 (BCL2), further supporting a key role for HSPA5 in the cell fate decision machinery.
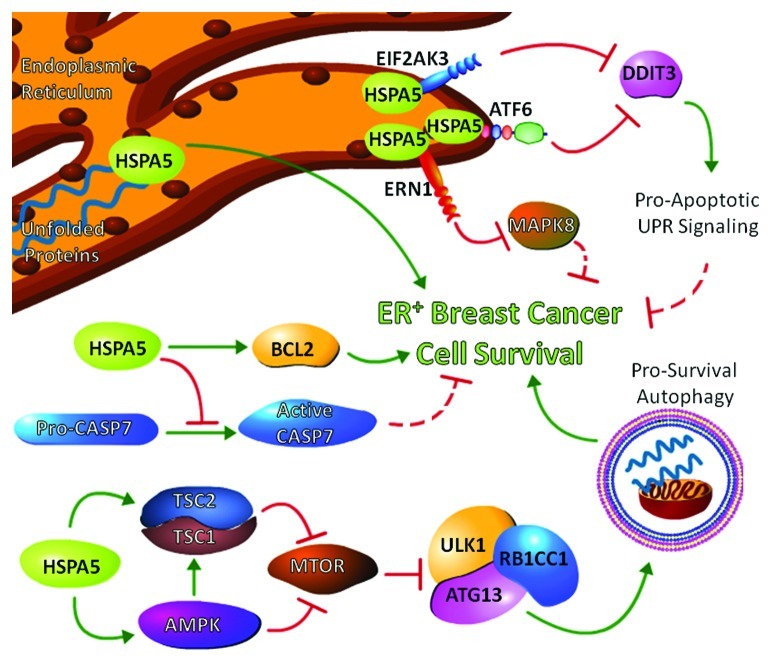
Several interactions occur between autophagy and UPR signaling. EIF2AK3 activation leads to increased ATF4 expression, which in turn stimulates transcription of ATG12 and other autophagy related genes. ERN1 can promote autophagy through its activation of mitogen-activated protein kinase 8 (MAPK8/JNK) and the subsequent disruption of the binding of BCL2 and BECN1 that would otherwise block the ability of BECN1 to initiate autophagy. The recent study illustrates a novel role for HSPA5 in regulating autophagy. While overexpression of HSPA5 is expected to reduce autophagy activation by inhibiting the signaling links between UPR and autophagy, we observed an increase in autophagosome formation when HSPA5 was overexpressed in ER+ breast cancer cells. Indeed, HSPA5 overexpression activated both AMP-activated protein kinase (AMPK) and its phosphorylation of tuberous sclerosis 2 (TSC2). In turn, activation of AMPK and TSC2 inhibit mechanistic target of rapamycin (MTOR), thereby reducing the inhibitory activity of MTOR on the autophagy initiation complex ULK1/ATG1-ATG13-RB1CC1/FIP200. Furthermore, inhibition of autophagosome formation through ATG5 silencing prevents HSPA5-induced antiestrogen resistance, suggesting that HSPA5-mediated autophagy is prosurvival. Figure 1 illustrates the interaction of HSPA5 in controlling crosstalk between UPR, apoptosis, and autophagy to confer antiestrogen resistance upon ER+ breast cancer.
Figure 1. Signaling crosstalk among the unfolded protein response (UPR), apoptosis, and autophagy can be controlled by heat shock 70 kDa protein 5 (HSPA5). The figure represents signaling to regulate estrogen receptor positive (ER+) breast cancer cell survival. Elevated expression of HSPA5 increases the cellular stress tolerance threshold by affecting protein folding while maintaining inactivation of proapoptotic UPR signaling. For example, HSPA5 inactivation of eukaryotic translation initiation factor 2-α kinase 3 (EIF2AK3) and activating transcription factor 6 (ATF6) blocks the induction of DNA-damage-inducible transcript 3 (DDIT3/CHOP), and/or prevents endoplasmic reticulum to nucleus signaling 1 (ERN1) driven activation of mitogen-activated protein kinase 8 (MAPK8/JNK). HSPA5 also inhibits CASP7 activation and increases expression of anti-apoptotic B-cell CLL/lymphoma 2 (BCL2) family members thereby promoting cell survival. Increased HSPA5 expression activates AMPK and its phosphorylation of tuberous sclerous 2 (TSC2) to promote mechanistic target of rapamycin (MTOR) inhibition and enable the initiation of prosurvival autophagy.
The discovery that HSPA5 promotes AMPK activity leads to several new implications between UPR and the control of cellular metabolism. Originally associated with glucose regulation, hence the name glucose-regulated protein 78 (GRP78/HSPA5), it is logical that overexpression of a glucose-sensing protein such as HSPA5 could result in AMPK activation to help restore glucose homeostasis. Moreover, low intracellular glucose concentrations can trigger the accumulation of misfolded or unfolded proteins within the endoplasmic reticulum, thereby stimulating UPR signaling and increasing transcription of HSPA5. Thus, the UPR has a built-in mechanism to stimulate glucose/energy metabolism to help restore homeostasis, presumably to help facilitate the highly energy-dependent process of folding misfolded/unfolded proteins. Stimulation of autophagy by HSPA5 would also support the restoration of energy balance and metabolism by the recycling of intermediate metabolites recovered from the digestion of cellular contents. These observations show that the UPR is elegantly designed to stimulate AMPK signaling to affect both autophagy and metabolism to promote cell survival.
The study illustrates a basic principle that regulation of homeostatic pathways, such as UPR and/or its master regulator/sensor HSPA5, can determine cell fate by affecting the balance of apoptotic and/or autophagy signaling. This raises a provocative paradigm: is this signaling more widely applicable and therefore represents a major stress response enabling many different cell types to survive diverse stressors including multiple antineoplastic interventions? Future development of HSPA5 inhibitors to target UPR signaling may be useful to broadly affect many different types of cancers and restore therapeutic sensitivity to reduce tumor burden.
Acknowledgments
K.C. is supported by a DOD Breast Cancer Research Program Postdoctoral Fellowship (BC112023). This research was also supported in part by awards from the U.S. Department of Health and Human Services (R01-CA131465 and U54-CA149147) to R.C.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/21765