Abstract
Killing properties of antitumor drugs can be enhanced by strategies targeting biochemical adaptations of cancer cells. Recently, we reported that depriving cancer cells of glutamine is a feasible approach to enhance antitumor effects of the alkylating analog of pyruvic acid, 3-bromopyruvate, which rely on the induction of autophagic cell death by metabolic-oxidative stress. 3-bromopyruvate chemopotentiation is the result of its increased intracellular uptake mediated by the monocarboxylate transporter 1, whose expression is post-transcriptionally increased upon glutamine withdrawal. Overall, our results identified the metabolic condition able to increase the selectivity of 3-bromopyruvate targets in neoplastic tissues, thereby providing a stage for its use in clinical settings for targeting malignancies and represent a proof of principle that modulation of glutamine availability can influence the delivery of monocarboxylic drugs into tumors.
Keywords: glutamine deprivation, metabolic oxidative stress, MCT-1, chemopotentiation, 3-bromopyruvate
Tumor cells undergo complex alterations in metabolic pathways to support cell mass accumulation and mitotic division. Under aerobic conditions, almost all untransformed cells metabolize glucose to pyruvate, which is further oxidized to CO2 in the Krebs cycle, thus providing electrons to the mitochondrial ATP-generating machinery. By contrast, many cancer cells rely on glycolysis—whose rate is maintained high even in the presence of oxygen (Warburg effect)—to produce energy, as well as precursors of lipids, nucleotides and some amino acids. Glucose addiction is complemented by glutamine, whose uptake and metabolism are strongly enhanced to fulfill ATP needs, to refuel Krebs cycle of metabolites constantly used for anabolic requirements, as well as to support the synthesis of glutathione and NADPH, which are both responsible for the maintenance of the intracellular redox state. Therefore, targeting metabolic adaptations has emerged as a feasible therapeutic strategy against cancer, allowing the improvement of efficacy, selectivity and specificity of different classes of chemotherapeutics.
We found that depriving cancer cells of glutamine enhances the cytotoxic properties of monocarboxylic drugs. Our study began with the observation that glutamine deprivation or pharmacological inhibition of its uptake, sensitizes carcinoma cells, but not their untransformed counterparts, to the antitumor effects of 3-bromopyruvate (3-BrPA), a halogenated and alkylating analog of pyruvic acid, able to compromise ATP synthesis by inhibiting the glycolytic path and mitochondrial complex II (mCII) activity (Fig. 1). We point out that, upon glutamine withdrawal, carcinoma cell death is not executed through apoptosis but has the features of autophagy, as evidenced by the autophagic flux increase and the significant reduction of cell death extent obtained through either pharmacological (3-methyladenine) or genetic (siRNA-mediated ATG5 knockdown) inhibition of the autophagic machinery. We observed that 3-BrPA chemopotentiation depends on the onset of metabolic-oxidative stress, mainly elicited by 3-BrPA-mediated mCII inhibition. Indeed, antioxidant treatment, as well as stable silencing of succinate dehydrogenase complex, subunit A, flavoprotein (SDHA), the subunit responsible for succinate oxidation and electrons entering into the redox core of mCII, reduces oxidative damage, restores ATP levels and renders cancer cells almost insensitive to 3-BrPA-induced autophagic cell death. Strengthening the well-recognized capability of many anticancer drugs to induce autophagic cell death in vivo, our results support the feasible application of therapeutic regimens exploiting autophagy as an effective cancer cell-killing strategy, particularly to target cells with an apoptosis-defective background. Although we did not investigate the molecular pathways responsible for autophagy induction, the engagement of several redox-sensitive proautophagic proteins can be hypothesized. The capability of the mitogen-activated protein kinase 8 (MAPK8/JNK), the AMP-dependent protein kinase (AMPK) and the death-associated protein kinase (DAPK1/DAPK), to elicit oxidative stress-induced cell death, in part through the stimulation of autophagy, makes them putative candidates in mediating 3-BrPA-induced autophagic cell death (Fig. 1).

Figure 1. Scheme of 3-BrPA chemopotentiation induced by glutamine deprivation. SLC16A1 stabilization increases upon glutamine deprivation or L-γ-glutamyl-p-nitroanilide (GPNA)-induced inhibition of SLC1A5-mediated glutamine uptake. This event induces an enhanced 3-BrPA uptake, increases inhibition of mitochondrial complex II (mCII) and results in the generation of metabolic-oxidative stress culminating with cell death, mainly executed by autophagy. Cancer cells with a high basal level of glutamine synthetase (GLUL/GS) are able to replenish the intracellular pool of glutamine upon its withdrawal, thus bypassing 3-BrPA chemopotentiation. GLUL inhibition, achieved either pharmacologically, by methionine sulphoximine (MSO) treatment, or genetically, by overexpressing a GLUL dominant-negative form (DN-GLUL), increases SLC16A1 stability, thereby making cells sensitive to 3-BrPA chemopotentiation. Hypothetical pathways are indicated by dotted lines and squares.
In subsequent studies, we rationalized 3-BrPA chemopotentiation, hypothesizing it could result from an increased cellular uptake. Indeed, NMR spectroscopy analyses revealed that the presence of glutamine in cell medium strongly influences the rate of drug internalization. 3-BrPA belongs to the class of monocarboxylic acid drugs whose uptake is mediated by H+-linked (MCTs) and Na+-coupled (SMCTs) monocarboxylate transporters. Our study supports the role of SLC16A1/MCT-1 as the molecular determinant of 3-BrPA uptake and cytotoxicity. Indeed, we provided evidence that (1) SLC16A1 is upregulated upon glutamine deprivation, (2) 3-BrPA uptake is stimulated by SLC16A1 overexpression and (3) pharmacological inhibition of SLC16A1, or its siRNA-mediated knockdown, prevents a 3-BrPA-induced bioenergetic crisis, oxidative stress, autophagy induction and cell death. The tight link between glutamine availability, SLC16A1 levels and 3-BrPA cytotoxicity is further strengthened by the observation that cells expressing high levels of glutamine synthetase and able, in turn, to maintain a high intracellular glutamine load, in the absence of its exogenous supplementation, do not upregulate SLC16A1, thereby bypassing 3-BrPA chemopotentiation.
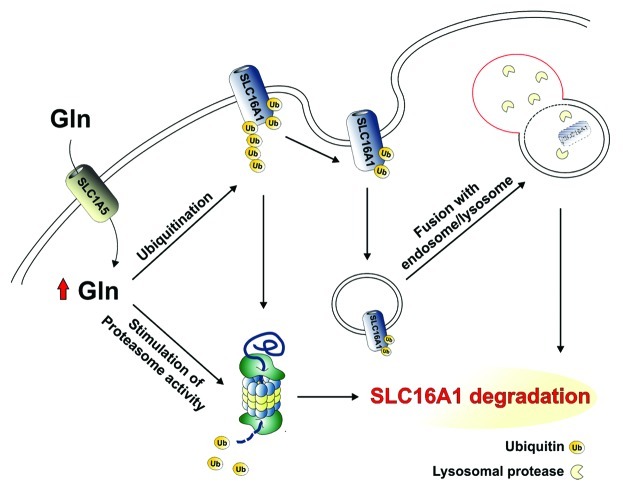
These findings raise several important questions that require further investigation. The mechanism of SLC16A1 upregulation by glutamine deprivation remains unknown. Our findings indicate that glutamine stimulates SLC16A1 degradation. This result finds support in studies documenting the proficiency of this amino acid to modulate protein turnover by stimulating protein ubiquitination. Therefore, on the basis of this knowledge, at least two possible hypotheses can be envisioned: i) glutamine deprivation can directly dampen the activity of the ubiquitin-proteasome system which, in turn, results in a reduced SLC16A1 ubiquitination and/or proteasome-mediated SLC16A1 degradation; ii) membrane protein stability, generally, depends on their translocation from the plasma membrane to endosomal/lysosomal compartments. As ubiquitination facilitates endocytosis of such proteins, it is also reasonable to hypothesize that glutamine might regulate SLC16A1 turnover by triggering its delivery to the endosomal/lysosomal-degradation pathway in a ubiquitin-dependent manner (Fig. 2).
Figure 2. Glutamine-dependent regulation of SLC16A1 stability. Hypothetical pathways regulating SLC16A1 degradation that depended on glutamine availability are shown. Glutamine may stimulate: (1) proteasome activity and/or (2) SLC16A1 ubiquitination. According to the latter hypothesis, ubiquitinated SLC16A1 can be: (1) degraded either by the proteasome or (2) recycled from the plasma membrane and degraded in endosomal/lysosomal compartments.
Although this report is the first documenting SLC16A1 modulation in response to nutrient availability, at this stage, the role of SLC16A1 upregulation in glutamine-deprived cells can be only hypothesized. Reduction in glutamine availability in glutamine-addicted cells induces compensatory mechanisms catalyzed by pyruvate carboxylase, allowing cells to use, for Krebs cycle anaplerosis, glucose-derived pyruvate, rather than glutamine. According to the “stromal-epithelial” lactate shuttle in tumors, SLC16A1 allows the uptake of lactate, ketones and other metabolites released by stromal fibroblasts in the extracellular milieu to refuel epithelial cancer cells within the same tumors. Hence, it is possible to speculate that SLC16A1 upregulation could be part of an adaptive reprogramming allowing glutamine-deprived cancer cells to take up extracellular monocarboxylic compounds (i.e., pyruvate and lactate) to fulfill anaplerotic requirements, by supporting pyruvate carboxylase activity.
Therefore, on the basis of its elevated expression in tumors and modulation in response to glutamine availability, SLC16A1 could be envisaged as a “Trojan horse,” allowing chemotherapeutics structurally related to monocarboxylates to enter into malignant cells and selectively kill them. Our study, indeed, identified the pyruvate dehydrogenase inhibitor dichloroacetate as another anticancer drug chemopotentiated upon glutamine deprivation and able to capitalize on SLC16A1 stabilization. Overall, these results represent proof-of-principle evidence that anticancer effects can be potentiated by combinating monocarboxylic drugs with strategies depriving tumors of glutamine.
Acknowledgments
This work was supported by grants from AIRC (# IG 10636) and from Ministero dell’Università e della Ricerca (MIUR).
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/21795