Abstract
Autophagy-mediated major histocompatibility complex (MHC) class I presentation can follow either the conventional MHC class I pathway or a recently described vacuolar pathway. In the vacuolar pathway, protein degradation is effected by lysosomal proteases, peptide exchange takes place with recirculating MHC complexes and the newly formed peptide-MHC complexes reach the cell surface by the endocytic pathway. This pathway is independent of the proteasome and the transporter associated with antigen processing (TAP) complex, but generates the same, or a similar, epitope as that from the conventional MHC class I pathway. Here, we discuss different mechanisms by which autophagy mediates MHC class I-restricted antigen presentation, which is crucial to its role in the control of intracellular pathogens.
Keywords: autophagy, MHC class I, antigen processing, antigen presentation, human cytomegalovirus
In conventional antigen processing and presentation, endogenous antigens are presented on MHC class I to CD8+ T cells, and exogenous antigens are presented on MHC class II to CD4+ T cells. Endogenous antigens are degraded in the cytosol by the ubiquitin-proteasome system, and the resulting peptides are transferred into the endoplasmic reticulum (ER) by the TAP complex, loaded onto MHC class I molecules, trimmed at the amino termini, and the resulting peptide-MHC complexes traffic to the cell surface by the secretory pathway. In contrast, phagocytosed exogenous antigens are degraded by lysosomal proteases, and the resulting peptides are loaded onto MHC class II molecules and reach the cell surface by the vacuolar pathway.
This link between antigen source and processing pathway is altered when antigens are transferred across compartments. Hence, exogenous antigens taken up by dendritic cells can be retrotranslocated from the phagosome to the cytosol and be cross-presented by the conventional MHC class I pathway. Conversely, autophagy can deliver endogenous antigens, such as the Epstein-Barr virus nuclear antigen 1 (EBNA1), from the cytosol or nucleus into the vacuolar compartment. The proteins are then degraded by lysosomal proteases and the peptides presented via the MHC class II pathway.
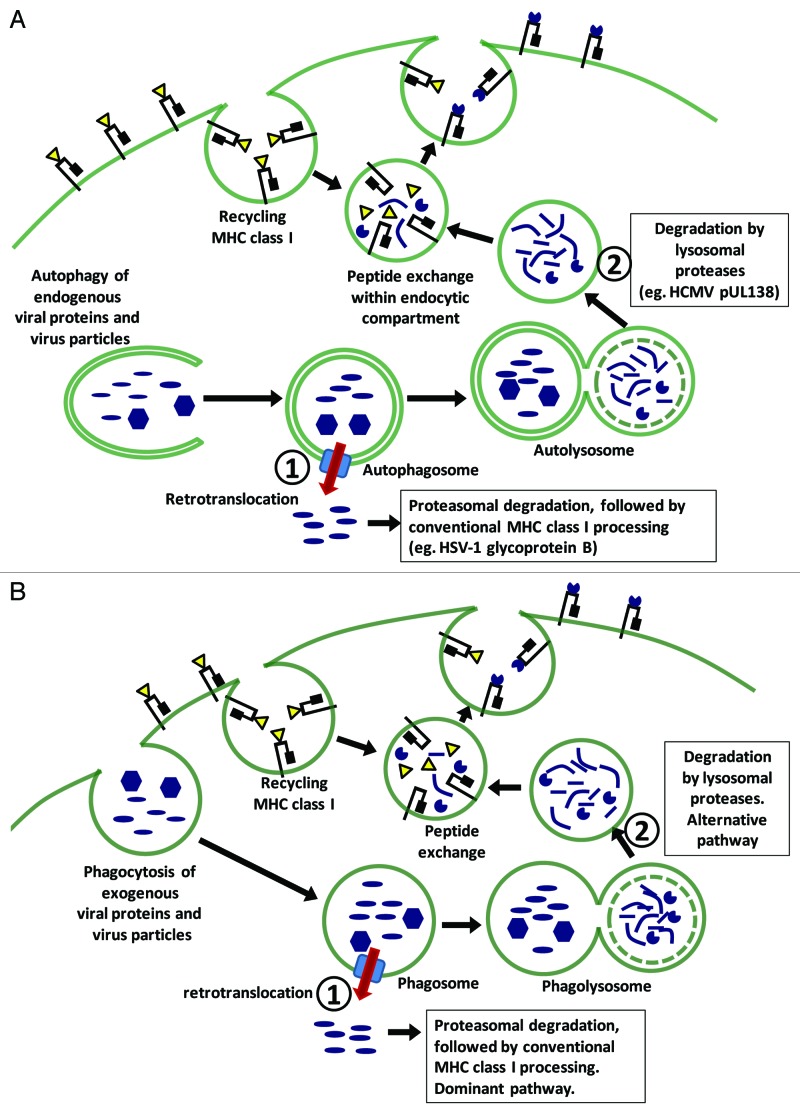
Autophagy can also mediate the MHC class I presentation of endogenous antigens. We recently described the presentation of a human cytomegalovirus (HCMV) protein, pUL138, by an autophagy-mediated pathway. This pathway largely bypasses the conventional MHC class I machinery: it is insensitive to proteasome inhibition by lactacystin and epoxomicin, is TAP-independent and does not involve ER amino-termini trimming. Instead, antigen processing occurs within the vacuolar compartment and is blocked by inhibitors of lysosomal proteases such as chloroquine and leupeptin. Following lysosomal degradation, the peptide epitopes are loaded onto recycling MHC class I molecules and are transported to the cell surface by the vacuolar pathway (Fig. 1A; pathway 2). This is mechanistically similar to the processing of exogenous antigens via the vacuolar route, which is a minor cross-presentation pathway (Fig. 1B; pathway 2). During the constitutive recycling of surface MHC class I molecules, peptide-MHC dissociation occurs when the molecules reach the acidic endocytic environment. This allows peptide exchange to occur during which lysosomal degradation products can be loaded onto MHC class I complexes. Peptide-MHC complexes that are relatively more acid stable eventually traffic though the endocytic pathway and are presented on the cell surface. In support of this, we found that pUL138s colocalizes with internalized surface MHC class I complexes, and the pUL138 peptide-MHC complex was unusually resistant to acid treatment. Hence, the autophagy of endogenous antigens and the phagocytosis of exogenous antigens can lead to a final common pathway in which MHC class I processing and presentation take place within the vacuolar compartment. In the case of pUL138, both the vacuolar pathway and conventional proteasome-ER pathway generate the same, or very similar, peptide epitope, which can be recognized by the same T cells. However, it is possible that lysosomal and proteasomal processing can sometimes give rise to very different epitopes, in which case the autophagy-mediated pathway will broaden the repertoire of presented epitopes. Hypothetically, these epitopes will be presented to effector T cells that arise following cross-priming by the alternative vacuolar route.
Figure 1. Autophagy of endogenous viral antigens can mediate MHC class I presentation through two distinct pathways, each with a counterpart in the cross-presentation of exogenous antigens. (A) Endogenous virus particles or viral antigens that are engulfed by autophagosomes can be retrotranslocated into the cytosol where they are degraded by the proteasome and processed by the conventional MHC class I machinery (pathway 1). Alternatively, they can remain within the vacuolar compartment where they are degraded by lysosomal proteases, and the resulting peptides loaded onto recycling MHC class I by means of peptide exchange (pathway 2). It is presently unclear which pathway is dominant. (B) Phagocytosed exogenous virus particles or viral antigens can also be channeled into the conventional MHC class I pathway following retrotranslocation; or be processed within the vacuolar compartment by lysosomal proteases. Here, pathway 1, which involves retrotranslocation and conventional processing, is dominant.
What is the significance of this autophagy-mediated pathway? It does not appear to be essential since the pUL138 epitope is presented very efficiently by the dominant conventional MHC class I pathway. However, in virus-infected cells, alternative presentation pathways may have heightened significance because they may circumvent viral immune evasion strategies which largely target the conventional MHC class I pathway. For example, the TAP-independent vacuolar pathway can circumvent TAP inhibition by HCMV-encoded US6. Indeed, the autophagy-mediated vacuolar pathway is not detectable in TAP-sufficient cells infected with recombinant adenovirus, which encodes a minimum of immune evasion genes, but is readily detected in HCMV-infected cells, where a multitude of immune evasion genes are operational.
Autophagy also mediates another form of endogenous antigen presentation, which uses the conventional MHC class I pathway. This process is described in the presentation of the herpes simplex virus-1 (HSV-1) glycoprotein B. Following the engulfment of viral particles or unenveloped viral capsids, the viral antigens are channeled into the conventional MHC class I pathway, which by inference, involves the retrotranslocation of viral antigens or viral particles from an autophagosome into the cytosol (Fig. 1A; pathway 1). While this pathway will not circumvent viral immune evasion, linking antigen presentation with autophagy, which is itself linked to multiple innate and stress sensing pathways, may be important for the early immune control of intracellular infection.
Not all endogenous viral antigens are well presented by virus-infected cells and not all antigens can access the autophagy-mediated pathways. The requirements for autophagy-mediated presentation are unknown. We hypothesize that the autophagy-mediated conventional pathway, which involves the engulfment of viral particles and viral capsids followed by retrotranslocation by an undefined mechanism, is potentially restricted to structural proteins, such as glycoprotein B. On the other hand, the autophagy-mediated vacuolar pathway will be accessible to nonstructural as well as structural proteins, but will be restricted to epitopes that can be processed by lysosomal degradation and form peptide-MHC interactions that are relatively acid stable. The timing of viral antigen expression in relation to autophagy activation and suppression may also be important. In the case of pUL138, both antigen expression and autophagy induction occur within the first few hours of HCMV infection, making the timing optimal for autophagy-mediated antigen presentation. There remain important unanswered questions regarding the role, magnitude and conditions under which autophagy-mediated MHC class I presentation is operational. However, it is clear that enhancing antigen presentation is an important means by which autophagy combats intracellular microbes and counteracts the effect of viral immune evasion strategies.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/21860