Abstract
Alzheimer disease (AD) is sometimes referred to as type III diabetes because of the shared risk factors for the two disorders. Insulin resistance, one of the major components of type II diabetes mellitus (T2DM), is a known risk factor for AD. Insulin resistance increases amyloid-β peptide (Aβ) generation, but the exact mechanism underlying the linkage of insulin resistance to increased Aβ generation in the brain is unknown. In this study, we investigated the effect of insulin resistance on amyloid β (A4) precursor protein (APP) processing in mice fed a high-fat diet (HFD), and diabetic db/db mice. We found that insulin resistance promotes Aβ generation in the brain via altered insulin signal transduction, increased BACE1/β-secretase and γ-secretase activities, and accumulation of autophagosomes. Using an in vitro model of insulin resistance, we found that defects in insulin signal transduction affect autophagic flux by inhibiting the mechanistic target of rapamycin (MTOR) pathway. The insulin resistance-induced autophagosome accumulation resulted in alteration of APP processing through enrichment of secretase proteins in autophagosomes. We speculate that the insulin resistance that underlies the pathogenesis of T2DM might alter APP processing through autophagy activation, which might be involved in the pathogenesis of AD. Therefore, we propose that insulin resistance-induced autophagosome accumulation becomes a potential linker between AD and T2DM.
Keywords: APP processing, Alzheimer disease, Aβ, autophagosome, diabetes, insulin resistance
Alzheimer disease (AD), the most common form of dementia, is characterized by senile plaques, neurofibrillary tangles and neuronal loss. Senile plaques are extracellular deposits of Aβ; the deposits are associated with AD-related neurodegeneration. Aβ is a peptide of 40- or 42-amino-acids derived predominantly from APP upon sequential cleavage by BACE1 and the γ-secretase complex. BACE1 and γ-secretase reside predominantly in intracellular membrane compartments of the vacuolar apparatus, including autophagic vacuoles (AVs). Previous reports have shown that AVs exist in the brains of both AD model mice and AD patients, and that they colocalize intimately with the γ-secretase complex, APP and β-secretase-derived C-terminal fragment (β-CTF), suggesting that autophagosomes may be one of the generation sites for Aβ, one of the major toxic peptides in AD pathology.
Several epidemiological studies have identified a relationship between AD and T2DM, and the two disorders have several shared risk factors including insulin degrading enzyme (IDE) activity, mitochondrial dysfunction, inflammation, and oxidative stress. Recent studies have demonstrated that expression of insulin receptor (INSR), insulin-like growth factor-1 receptor (IGF1R), and insulin receptor substrate (IRS1) proteins is downregulated in brains from AD patients, and defective brain insulin signaling has been suggested to contribute to the cognitive deficits in AD patients. However, the molecular mechanisms underlying this co-morbidity are not fully understood. In this study, we used two diabetic animal models, mice fed a high-fat diet (HFD) and db/db mice, which carry a homozygous mutation in the leptin receptor and suffer severe glucose intolerance, obesity and hyperinsulinemia. We found altered insulin signaling, an enhanced amyloidogenic pathway (increased BACE1 and γ-secretase activities and Aβ generation), and autophagosome accumulation in the brains of HFD mouse and db/db mouse.
To investigate the relationship among diet- or genetically induced insulin resistance, autophagosome accumulation and alteration in APP processing, we established a cell culture model of insulin resistance in human neuroblastoma SH-SY5Y cells and primary cortical neurons. Cells were subjected to prolonged exposure to high levels of insulin, leading to cellular insulin resistance, and then by an in vitro model of insulin resistance, we found that insulin resistance induces autophagosome accumulation via inhibition of the AKT1-MTOR pathway. In addition, we analyzed whether insulin resistance-induced accumulation of autophagosomes was caused by activation of the induction for autophagosome formation or by defects in autolysosome maturation. Using mRFP-GFP tandem fluorescence-tagged LC3 (TfLC3), autolysosomes are formed normally under insulin-resistant conditions. These results were confirmed by double-labeling with GFP-LC3 and LysoTracker. Western blot analysis also showed that insulin-resistant SH-SY5Y cells in the presence of chloroquine (CQ), a lysosomal inhibiting agent, result in a significantly higher level of LC3-II–to–LC3-I ratio than with insulin alone. We also checked the levels of BECN1, a component of the autophagosome initiation complex. BECN1 expression is increased under insulin-resistant conditions. These results support the notion that insulin resistance can induce autophagosome formation without defects in autolysosome maturation.
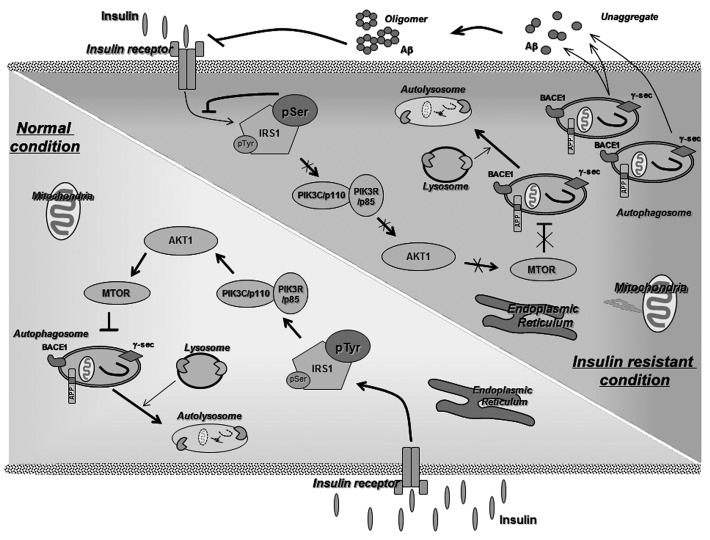
So, is insulin resistance-induced autophagosome accumulation associated with Aβ peptide generation? We found that accumulation of autophagosomes under insulin-resistant conditions provides for greater Aβ peptide generation, resulting in accelerated Aβ generation by modulation of BACE1 and γ-secretase activities. Treatment with rosiglitazone, a well-known insulin-sensitizing agent, or 3-MA, an autophagy inhibitor, under insulin-resistant conditions reverses the changes induced by insulin resistance, indicating that exacerbation of Aβ generation under insulin resistant conditions may be mediated by autophagosome accumulation (Fig. 1).
Figure 1. The underlying link between AD and T2DM. Insulin resistance might increase Aβ generation by triggering accumulation of autophagosomes via the inhibition of the mechanistic target of rapamycin (MTOR) pathway.
We next explored the mechanisms through which insulin resistance-induced autophagosome accumulation alters APP processing. Previous studies have demonstrated that the increase in cholesterol enhances the levels of membrane lipid rafts, which are implicated in Aβ production. Because insulin resistance affects lipid metabolism, we examined the effect of membrane lipid rafts in insulin resistant cells on Aβ generation. With insulin-resistant cells, treatment with simvastatin or methyl-β-cyclodextrin (MβCD), cholesterol-lowering drugs, has no effect on Aβ generation. These results indicate that autophagosome accumulation, not cholesterol or lipid raft, is a major factor in Aβ generation, at least in our cell culture system. Recent studies have shown that autophagosomes are highly enriched in the components and activities of γ-secretase complexes, APP and β-CTF. Finally, we found that secretase proteins enriched predominantly in insulin resistance-induced autophagosomes, thus increasing Aβ generation.
The present study demonstrates that hyperinsulinemia and insulin resistance induced by genetic or dietary factors enhances Aβ generation through autophagosome accumulation (Fig. 1). Our findings suggest that insulin resistance-induced autophagosome accumulation might be a potential link between AD and T2DM, and therapeutic strategies focused on modulating autophagy, i.e., development of autophagy-modulating drugs, may decrease the risk of AD in patients with T2DM.
Acknowledgment
This work was supported by grants from the National Research Foundation (2012R1A2A1A01002881, 2008-05943), the Medical Research Council (2011-0030738), the World Class University (R32-10084), and the Korea National Institute of Health ROAD R&D Program Project (A092058) to I.M.J., and by the Hi Seoul Science/Humanities Fellowship from Seoul Scholarship Foundation to S.S.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/21861