Abstract
Hepatocellular carcinoma (HCC), the most common primary malignant liver tumor, is the third leading cause of cancer deaths. The pathogenesis of HCC is closely associated with chronic liver inflammation fired by a variety of stimulates such as virus infection and metabolic stress. Recent work indicates that autophagy, a homeostatic self-degradation process, which decides cell survival or death upon stress, acts as an effector machinery of immune systems in defending microbial invasion and carcinogenesis. SQSTM1 is a selective target and receptor of autophagy, and the protein content of SQSTM1 reflects the level of autophagic flux in cells. Through degrading SQSTM1, decreasing SQSTM1 aggregates, and therefore interrupting the positive feedback between SQSTM1 aggregates and ROS production, autophagy plays a protective role against hepatocellular carcinoma. Indeed, our studies indicate that toll-like receptor 2 (TLR2)-mediated immune activities in the genotoxic carcinogen diethylnitrosamine (DEN)-injured liver tissue provide essential nutrient stimulates to induce intracellular senescence, which can ensure the activation and maturation of autophagy in liver cells. Loss of TLR2-mediated immune activity and senescence leads to the attenuation of autophagic flux, which cannot eliminate SQSTM1 aggregates, ROS accumulation, and DNA damage, and facilitates the development and progression of HCC.
Keywords: DEN, SQSTM1, autophagic flux, hepatocellular carcinoma, immune network, senescence, toll-like receptor 2
Autophagy is an essential, homeostatic self-degradation process helping cells survive during nutrient starvation. Recent studies indicate autophagy functions as an effector mechanism of the immune system. For instance, autophagy acts as an effector machinery of immunity in defending against pathogenic bacteria in macrophages and dendritic cells through the engulfment of intracellular microbes. Autophagy is required for phagolysosomal maturation during microbial invasion and antigen presentation. Autophagy also helps immune signals to keep the balance between activation and inactivation of certain effector responses. As key effector machinery for the immune system, autophagy is subjected to regulation by various immune molecules. Several immune signals, such as pathogen- or damage-associated molecular patterns (PAMPs or DAMPs), T helper 1 (Th1) cytokines IFNG/IFNγ, TNFA/TNFα, and inflammatory signaling molecules MAPK8/JNK and NFKB1/NFκB, can initiate and maintain autophagy. Stimulation of toll-like receptors (TLRs) 3, 4, 7, and 9 also activates autophagy in several experimental systems. However, immunosuppressive factors such as Th2 cytokines Interleukin 4 (IL4), IL10 and IL13 suppress autophagy. We recently demonstrated that Th17 cytokine IL17A attenuates autophagy to promote pulmonary fibrosis. Even so, how these immune molecules exactly decide the ultimate dynamic autophagy function or autophagic flux is still obscure.
Activation of autophagy has previously been shown to protect against HCC, although it may act in a contrary way in other cancers, due to its complicated roles in the regulation of cell survival and cell death. Autophagy plays a protective role against HCC mainly through its powerful clearing function of SQSTM1. SQSTM1 is an adaptor protein of autophagy and acts as a cargo in autophagic clearing. Through the binding action to both LC3 and ubiquitin, SQSTM1 participates in the delivery process of ubiquitin-modified proteins into autophagosomes. Thus, SQSTM1 is a target and receptor for selective autophagy, and the protein level of SQSTM1 reflects the level of autophagic flux. Higher autophagic activity always results in a decrease in the content of SQSTM1, whereas defects in autophagic flux always lead to high SQSTM1 content in cells. Accumulated SQSTM1 is aggregated as insoluble inclusions and is toxic for many organs, especially for liver. The accumulation of SQSTM1 aggregates forms a positive-feedback loop with ROS, which increases genome instability and finally increases the risk of liver carcinogenesis. Thus, through eliminating the accumulation of SQSTM1 aggregates, autophagy serves as a defensive barrier against HCC.
Cellular senescence is a unique state of stable cell arrest with active metabolism. It can be initiated by many stimuli such as dysfunctional telomeres and/or other types of DNA damage stress. Senescent cells express and secret senescence-associated secretory proteins (SASP), leading to a robust increase in cytokines, chemokines, growth factors and proteases. These primordial characters make senescence prevent runaway replication of damaged cells and form a physiological barrier against carcinogenesis and tumor progression. Autophagy and senescence are different cellular responses to stress, and both are machinery for tumor suppression. Studies have shown the relationship between autophagy and senescence. Both autophagy and senescence are regulated by the TP53/p53 tumor suppression pathway. TP53 is one of the two major onset pathways for cellular senescence. And TP53 also participates in the induction of autophagy. The impairment of autophagy can initiate senescence in a ROS-TP53-dependent manner because of the failure of dysfunctional mitochondria elimination. Otherwise, autophagy can be induced during the process of senescence. It facilitates the senescence process, acts as one of the effector mechanisms of senescence, and mediates the acquisition of the senescence phenotype.
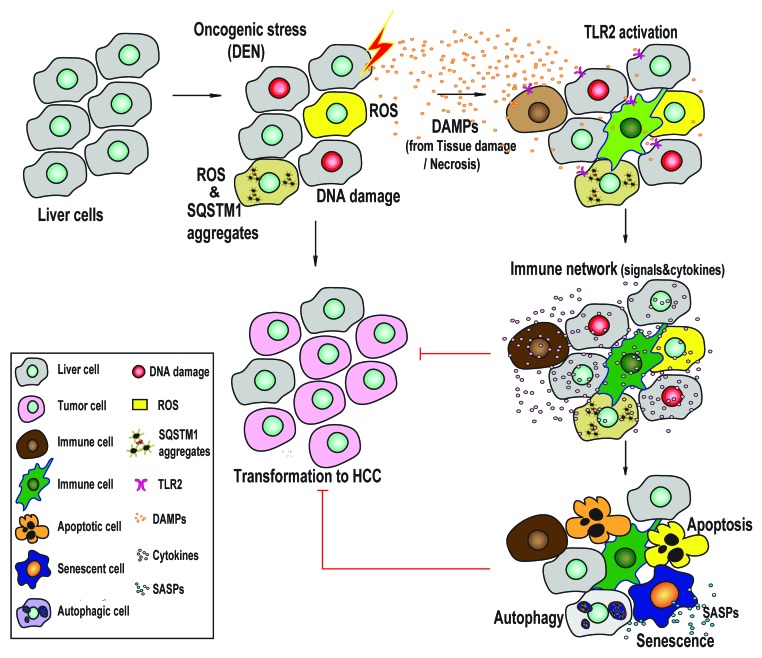
HCC, the most common primary malignant liver tumor, is the third leading cause of cancer deaths. The pathogenesis of HCC is closely associated with chronic liver inflammation induced by a variety of stimulates such as virus infection and excessive alcohol intake. Multiple mechanisms also participate in the transformation and progression of HCC: increasing cellular stresses, losing the integrity of the immunity-monitor-system, cell death and/or senescent failure, and nonfunctional autophagy. Each of these factors eases the formation of HCC. Our recent work has shown that genetic knockout of TLR2 favors the development and progression of HCC in response to diethylnitrosamine, a genotoxic carcinogen. Together with the collapse of the immune network, the failure of cell death and senescence, accumulation of ROS, and enhanced activation of autophagic signals associated with a suppressive autophagic flux, as indicated by accumulated SQSTM1 aggregates, are observed in the DEN-injured liver tissue in TLR2-deficient mouse.
How to explain the paradoxical facts regarding the activated autophagic signals associated with an impaired autophagy flux in TLR2-deficient liver tissue? It is well known that two signaling pathways, a MYD88-dependent MAPK (p38)-/NFKB1, and a MYD88-independent PI3K-AKT1-MTOR, are utilized to transduce TLR2 signals. The MYD88-dependent pathway activates, whereas the PI3K-AKT1-MTOR pathway suppresses, autophagy. Genetic inhibition of TLR2 results in losing both of the signaling pathways. A balanced but suppressed autophagy activity can be observed in TLR2-deficient liver tissue. Thus, DEN stress can stimulate autophagic signals in PI3K-AKT1-MTOR-defective liver tissue, but defects in the MYD88-dependent response interfere with a final procession of autophagy-associated lysosome degradation. We therefore conclude that the collapse of the immune network impairs the autophagic flux, as well as cell death and cellular senescence. Then, the impaired autophagic flux fails to clear unfolded proteins and accumulated ROS, which strongly stimulates autophagy. Indeed, administration of IFNG/IFNγ, a Th1 cytokine and positive modulator of autophagy, rescues the autophagic flux, cell death and cellular senescence, and finally ameliorates HCC development in the TLR2-deficient mouse. Thus, TLR2-mediated immunity provides an essentially driving force to stimulate cellular senescence and activate autophagy (Fig. 1).
Figure 1. Model of the TLR2-mediated immune network in the regulation of DEN-induced HCC. In the process of DEN-induced HCC transformation, the genotoxic carcinogen DEN induces protective activities such as cellular senescence, autophagy and apoptosis in liver cells. These protective mechanisms decrease the generation of ROS and SQSTM1 aggregates, and interrupt the feedback loop of ROS production and SQSTM1 aggregation. Genetic inhibition of TLR2 activity results in a loss of the immune network-supported protective activities and enhances HCC development and progression.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/22094