Abstract
Staphylococcus aureus is an intracellular bacterium responsible for serious infectious processes. This pathogen escapes from the phagolysosomal pathway into the cytoplasm, a strategy that allows intracellular bacterial replication and survival with the consequent killing of the eukaryotic host cell and spreading of the infection. S. aureus is able to secrete several virulence factors such as enzymes and toxins. Our recent findings indicate that the main virulence factor of S. aureus, the pore-forming toxin α-hemolysin (Hla), is the secreted factor responsible for the activation of an alternative autophagic pathway. We have demonstrated that this noncanonical autophagic response is inhibited by artificially elevating the intracellular levels of cAMP. This effect is mediated by RAPGEF3/EPAC (Rap guanine nucleotide exchange factor (GEF)3/exchange protein activated by cAMP), a cAMP downstream effector that functions as a GEF for the small GTPase Rap. We have presented evidence that RAPGEF3 and RAP2B, through calpain activation, are the proteins involved in the regulation of Hla and S. aureus-induced autophagy. In addition, we have found that both, RAPGEF3 and RAP2B, are recruited to the S. aureus–containing phagosome. Of note, adding purified α-toxin or infecting the cells with S. aureus leads to a decrease in intracellular cAMP levels, which promotes autophagy induction, a response that favors pathogen intracellular survival, as previously demonstrated. We have identified some key signaling molecules involved in the autophagic response upon infection with a bacterial pathogen, which have important implications in understanding innate immune defense mechanisms.
Keywords: Staphylococcus aureus, LC3, RAP2B, RAPGEF3, autophagy, cAMP, α-hemolysin
Autophagy is a survival mechanism, which is activated by cells in response to different stress situations such as nutrient limitation, high temperatures, oxidative stress and accumulation of damaged organelles, or as a mechanism of innate immunity when the cells are infected with certain pathogens.
Staphylococcus aureus, bacteria responsible for clinically relevant infections, has been classically considered an extracellular bacterial pathogen, but it is also able to invade various types of nonprofessional phagocytic cells. After internalization S. aureus resides temporally in a compartment that shares features of an autophagosomal compartment (i.e., decorated by LC3) where it initiates its replication. It has been shown that the pathogen localizes to autophagosomes, but prevents their maturation and fusion with lysosomes. Afterwards, by disrupting the phagosomal membrane, it escapes into the cytoplasm, where it actively replicates, leading to host cell death and infection of neighboring cells.
Upon infection, S. aureus induces an autophagic response, which is beneficial for bacterial growth. Indeed, this autophagy activation is required for replication, subsequent escape from autophagosomes into the cytoplasm, and S. aureus-induced host cell death. We have previously demonstrated that α-hemolysin, which is a pore-forming toxin secreted by S. aureus, is the factor responsible for the autophagic response induced by this pathogen in the host cell. In addition, we have shown that this autophagic response induced by Hla occurs via a nonclassical pathway, which is independent of BECN1 and PtdIns3K, but requires the autophagy protein ATG5.
We recently investigated the role of cAMP and the calpain pathway in the autophagic activation induced by S. aureus. We first demonstrated, using fluorescence microscopy and western blots assays, that cAMP treatment of cells inhibits autophagy activation induced by both purified Hla addition to the cells and by infection with a S. aureus strain. Additionally, we showed that this autophagy inhibition occurs through a PKA-independent pathway.
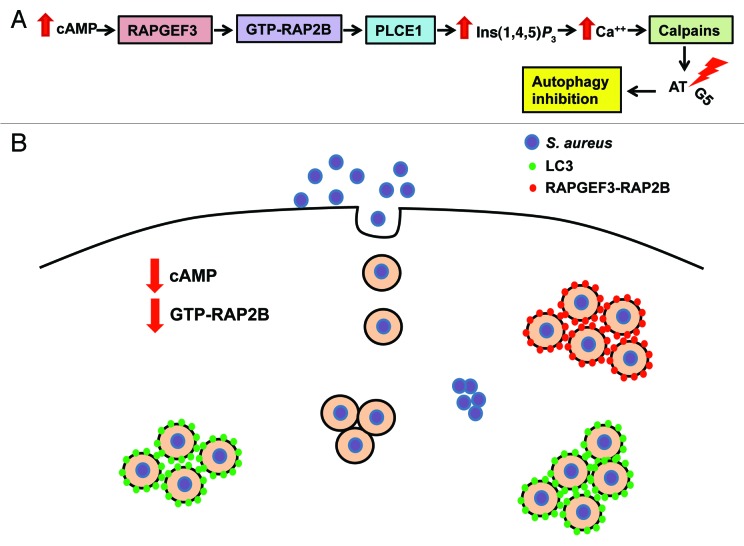
Then we demonstrated that RAPGEF3, a cAMP-modulated effector that functions by activating GDP/GTP exchange in the small GTPase RAP, negatively regulates the autophagy induced by Hla and S. aureus. Furthermore, in order to corroborate whether the RAPGEF3 pathway is responsible for the regulation of autophagy activation induced by Hla and S. aureus, we analyzed the role of a downstream component of this pathway, the small GTPase RAP2B. By overexpressing wild-type RAP2B or its negative mutant (GDP inactive form) and by depleting RAP2B with siRNA, we demonstrated that RAP2B is a key participant in the regulation of autophagy induced by Hla and S. aureus. Our results indicate that this small GTPase needs to be inactivated to allow the autophagic response. Indeed, by a pull-down assay we have determined that not only the purified toxin, but also infection with S. aureus leads to a decrease in the levels of active RAP2B. In addition, we have shown that the intracellular levels of cAMP are downregulated by S. aureus infection. These data suggest that the α-toxin decreases the amount of active RAP2B, likely by decreasing the levels of intracellular cAMP, to allow autophagy induction, which in turn, favors pathogen intracellular survival.
RAP2B produces an increase in cytoplasmic calcium through a rise in Ins(1,4,5)P3. Calpains are a family of cysteine proteases that are activated by intracellular calcium. When calpains are activated, they are able to cleave ATG5, inhibiting autophagy. Using a calpain inhibitor, we showed that the inhibition of calpains allows Hla-induced autophagy activation, indicating that the activation of calpains by cAMP prevents the autophagy induced by the toxin. Therefore, calpains might act as negative regulators of this particular autophagic response.
Additionally, we demonstrated that endogenous RAPGEF3 and RAP2B are recruited to a subset of the bacteria-containing phagosomes. However, the vacuoles that contain either RAPGEF3 or RAP2B associate with the phagosomal surface do not colocalize with LC3, suggesting that the population of bacteria that recruit RAPGEF3- RAP2B does not recruit LC3, or that both proteins associate with different kinetics, depending perhaps on the damage state of the phagosomal limiting membrane.
Together, ours results clearly confirm that the identified molecular components involved in the autophagic pathway induced by the purified toxin also participate in the autophagic response upon infection with S. aureus, negatively regulating the autophagic response exerted by this pathogen. We showed that, through a decrease in the intracellular cAMP levels, S. aureus prevents RAPGEF3-RAP2B activation, inhibiting calpain action and promoting autophagy activation (Fig. 1). The association of RAPGEF3-RAP2B with the pathogen-containing phagosomes remains enigmatic and a more comprehensive analysis will be necessary to fully understand the role of these molecules in the bacteria-containing phagosomes. However, given the fact that S. aureus is a microorganism that causes serious diseases, we believe that dissecting the signal transduction mechanisms leading to this “noncanonical” autophagic response, which enhances S. aureus intracellular survival, is of seminal importance.
Figure 1. Model showing the RAPGEF3-dependent pathway involved in the control of autophagy and the molecules involved in the autophagic response induced by Staphylococcus aureus. (A) Bacterial infections may modulate the levels of intracellular cAMP. When the levels of cAMP are increased, this can activate RAPGEF3, which through RAP2B and PLCE1/PLCε activation induces an increase in Ins(1,4,5)P3. In turn, this Ins(1,4,5)P3 increase leads to calcium release from the ER, which activates the calpain cysteine-proteases. These calpains are able to cleave ATG5 inhibiting the autophagic pathway. Thus, cAMP via calpains activation negatively regulates autophagy. (B) S. aureus is incorporated into the cell by phagocytosis. After the infection, S. aureus produces a decrease in the amount of active RAP2B, likely through the decline in the intracellular cAMP levels activating the autophagic pathway. The damage caused by the α-toxin Hla to the phagosomal membrane is likely responsible for the recruitment of LC3 to the phagosomal membrane of a subset of phagosomal-containing bacteria. Afterwards, the pathogen escapes into the cytoplasm to finally cause cell death, and spread to neighboring cells. Another group of bacteria recruits RAPGEF3 and/or RAP2B to the phagosome membrane.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/22161